

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B2 bradykinin receptor (RAT) | BDBM50370083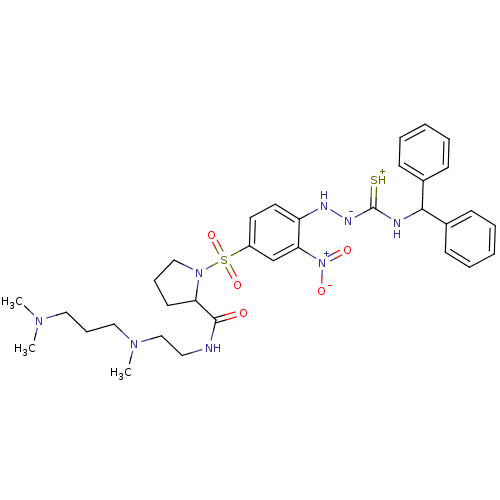 (CHEMBL1907651) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50366620 (RESINIFERATOXIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Binding affinity against Vanilloid receptor in dorsal Root Ganglion (DRG) membranes using [3H]RTX binding assay. | J Med Chem 39: 2939-52 (1996) Article DOI: 10.1021/jm960139d BindingDB Entry DOI: 10.7270/Q2CV4JD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50366620 (RESINIFERATOXIN) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Binding affinity against Vanilloid receptor in dorsal Root Ganglion (DRG) membranes using [3H]RTX binding assay. | J Med Chem 39: 2939-52 (1996) Article DOI: 10.1021/jm960139d BindingDB Entry DOI: 10.7270/Q2CV4JD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052327 (6-hydroxy-15-isopropenyl-4,13,17-trimethyl-5-oxo-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Binding affinity against Vanilloid receptor in dorsal Root Ganglion (DRG) membranes using [3H]RTX binding assay. | J Med Chem 39: 2939-52 (1996) Article DOI: 10.1021/jm960139d BindingDB Entry DOI: 10.7270/Q2CV4JD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50370077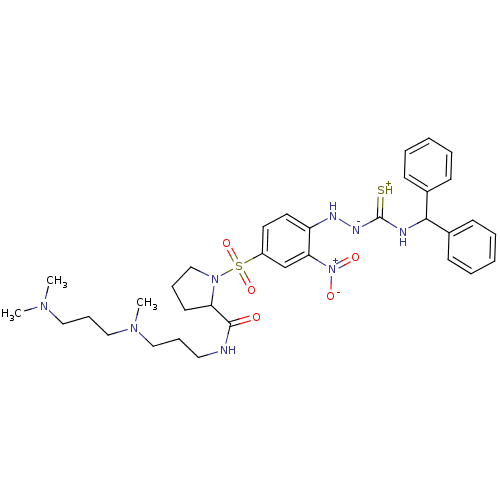 (CHEMBL1907652) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50113263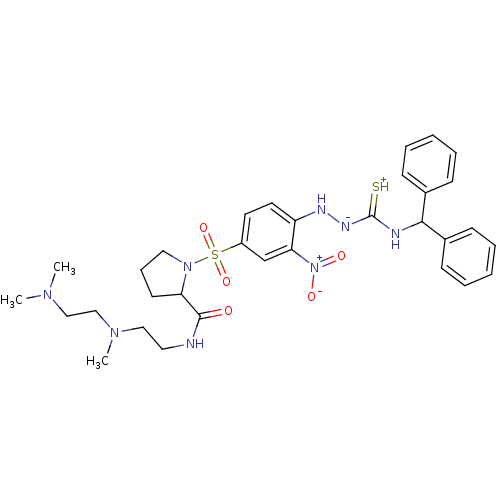 ((S)-1-[4-(4-benzhydrylthiosemicarbazido)-3-nitrobe...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50409120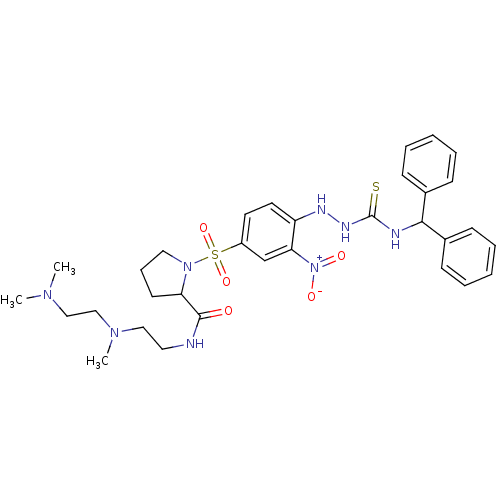 (CHEMBL2112044 | CHEMBL2112937) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-BK to rat bradykinin B2 receptor expressed in NG108-15 neuroblastoma-glioma hybrid cell membranes | J Med Chem 43: 769-71 (2000) BindingDB Entry DOI: 10.7270/Q2XD10W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50113263 ((S)-1-[4-(4-benzhydrylthiosemicarbazido)-3-nitrobe...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50409120 (CHEMBL2112044 | CHEMBL2112937) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-BK to rat bradykinin B2 receptor expressed in NG108-15 neuroblastoma-glioma hybrid cell membranes | J Med Chem 43: 769-71 (2000) BindingDB Entry DOI: 10.7270/Q2XD10W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50409527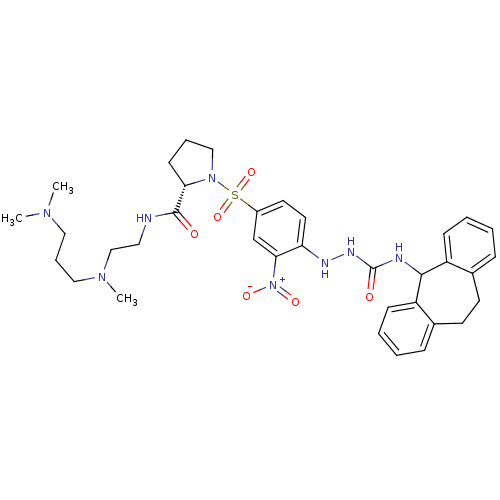 (CHEMBL2112283) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052320 (13-benzyl-6-hydroxy-15-isopropenyl-4,17-dimethyl-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Binding affinity against Vanilloid receptor in dorsal Root Ganglion (DRG) membranes using [3H]RTX binding assay. | J Med Chem 39: 2939-52 (1996) Article DOI: 10.1021/jm960139d BindingDB Entry DOI: 10.7270/Q2CV4JD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50151885 (1-[4-(2,2-Diphenyl-ethylamino)-3-(morpholine-4-car...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells | J Med Chem 47: 4642-4 (2004) Article DOI: 10.1021/jm049747g BindingDB Entry DOI: 10.7270/Q2J965V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50409529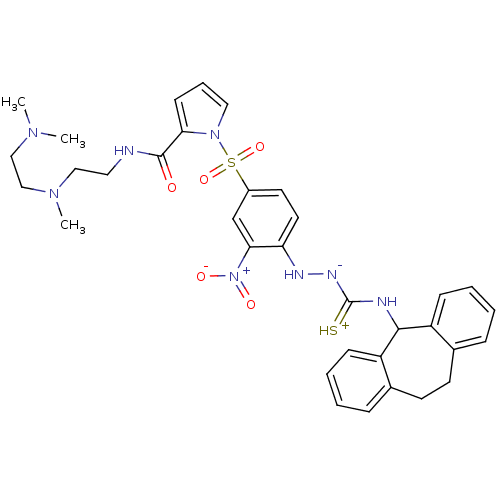 (CHEMBL2112221) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50085685 (4-benzhydrylamino(thioxo)methylhydrazine-3-nitrobe...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-BK to rat bradykinin B2 receptor expressed in NG108-15 neuroblastoma-glioma hybrid cell membranes | J Med Chem 43: 769-71 (2000) BindingDB Entry DOI: 10.7270/Q2XD10W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50409528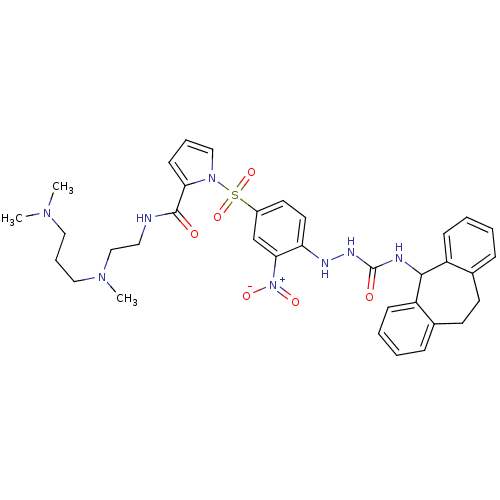 (CHEMBL2112220) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052323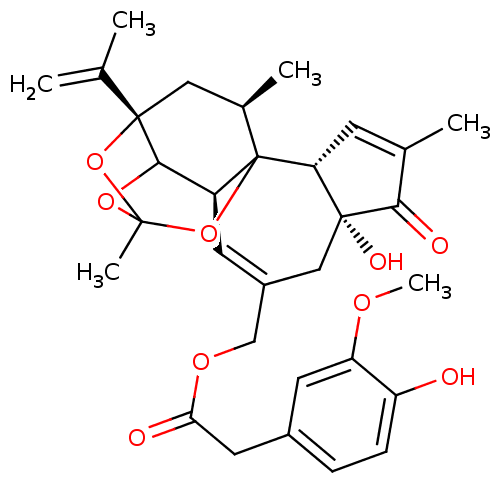 ((4-Hydroxy-3-methoxy-phenyl)-acetic acid 4a,7b-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Binding affinity against Vanilloid receptor in dorsal Root Ganglion (DRG) membranes using [3H]RTX binding assay. | J Med Chem 39: 2939-52 (1996) Article DOI: 10.1021/jm960139d BindingDB Entry DOI: 10.7270/Q2CV4JD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50085684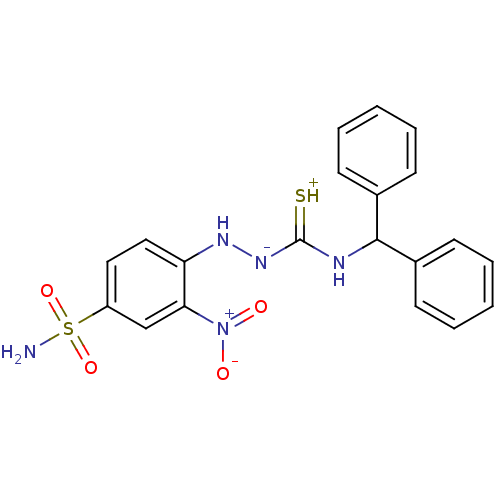 (4-benzhydrylamino(thioxo)methylhydrazine-3-nitro-1...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-BK to rat bradykinin B2 receptor expressed in NG108-15 neuroblastoma-glioma hybrid cell membranes | J Med Chem 43: 769-71 (2000) BindingDB Entry DOI: 10.7270/Q2XD10W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052333 (13-benzyl-6-hydroxy-15-isopropenyl-4,17-dimethyl-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Binding affinity against Vanilloid receptor in dorsal Root Ganglion (DRG) membranes using [3H]RTX binding assay. | J Med Chem 39: 2939-52 (1996) Article DOI: 10.1021/jm960139d BindingDB Entry DOI: 10.7270/Q2CV4JD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50370080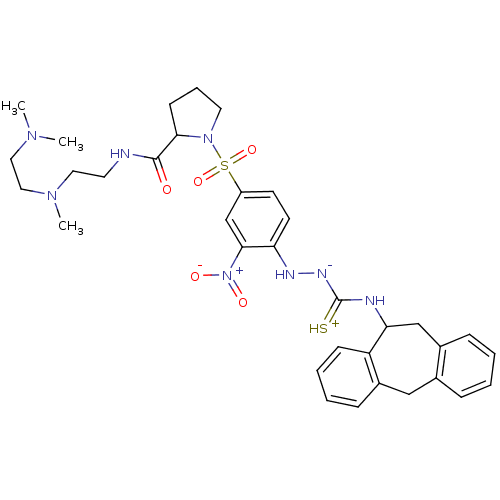 (CHEMBL1907656) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50370081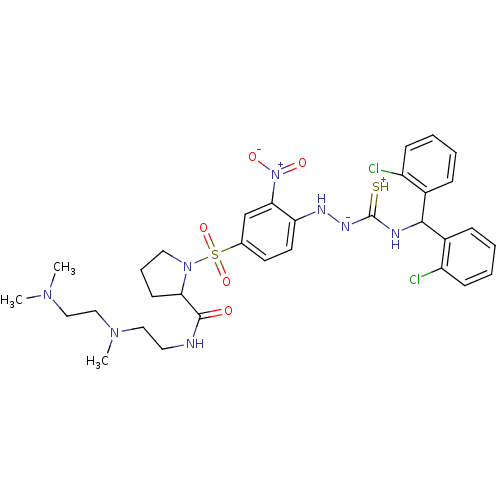 (CHEMBL1907654) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50370078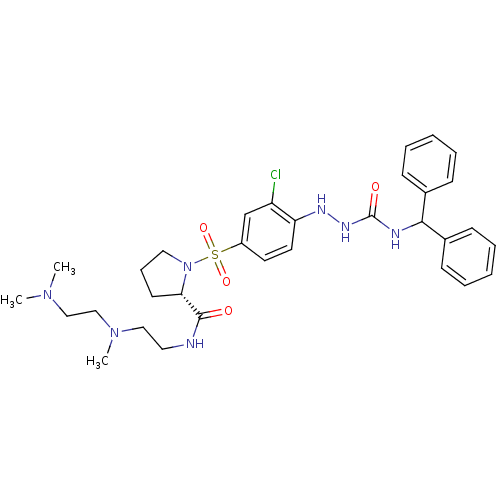 (CHEMBL1907653) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052328 (13-benzyl-6-hydroxy-15-isopropenyl-4,17-dimethyl-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Binding affinity against Vanilloid receptor in dorsal Root Ganglion (DRG) membranes using [3H]RTX binding assay. | J Med Chem 39: 2939-52 (1996) Article DOI: 10.1021/jm960139d BindingDB Entry DOI: 10.7270/Q2CV4JD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50151891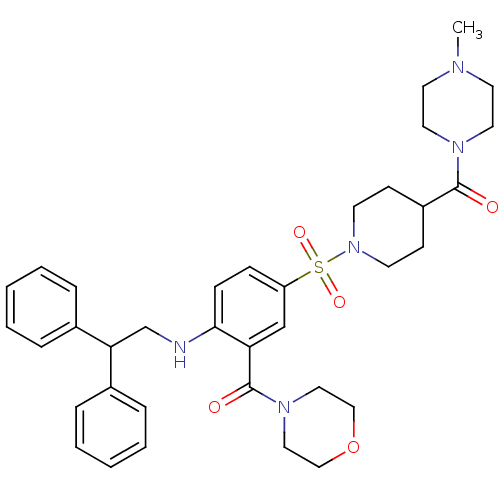 (CHEMBL273869 | {2-(2,2-Diphenyl-ethylamino)-5-[4-(...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells | J Med Chem 47: 4642-4 (2004) Article DOI: 10.1021/jm049747g BindingDB Entry DOI: 10.7270/Q2J965V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50151886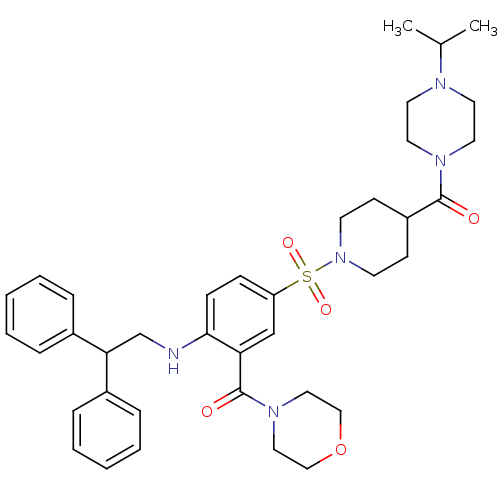 (CHEMBL181695 | {2-(2,2-Diphenyl-ethylamino)-5-[4-(...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells | J Med Chem 47: 4642-4 (2004) Article DOI: 10.1021/jm049747g BindingDB Entry DOI: 10.7270/Q2J965V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50151884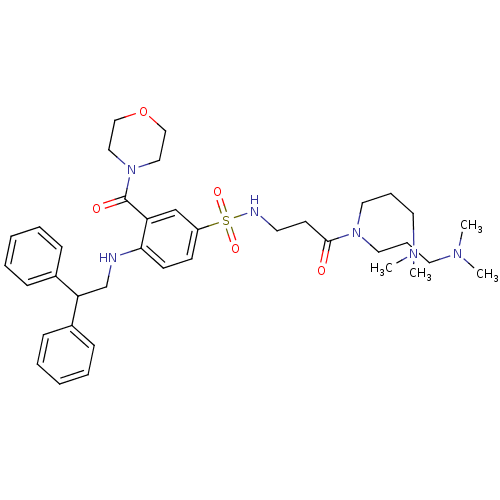 (CHEMBL185551 | N,N-Bis-(3-dimethylamino-propyl)-3-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells | J Med Chem 47: 4642-4 (2004) Article DOI: 10.1021/jm049747g BindingDB Entry DOI: 10.7270/Q2J965V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50151894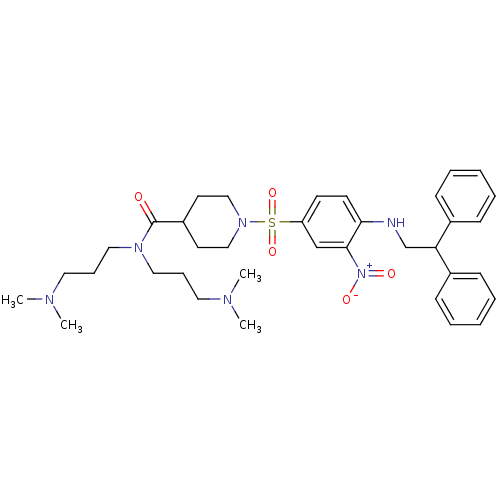 (1-[4-(2,2-Diphenyl-ethylamino)-3-nitro-benzenesulf...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells | J Med Chem 47: 4642-4 (2004) Article DOI: 10.1021/jm049747g BindingDB Entry DOI: 10.7270/Q2J965V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50409527 (CHEMBL2112283) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254456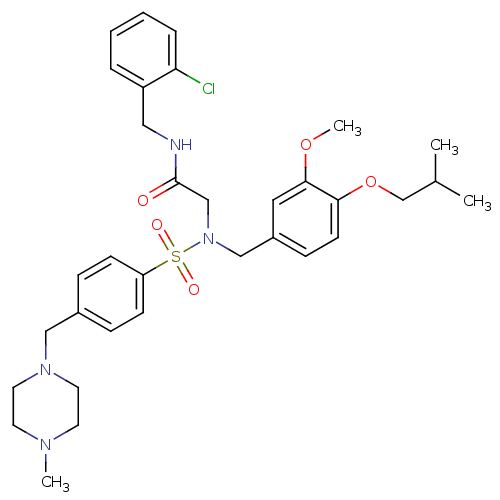 (CHEMBL447392 | N-(2-chlorobenzyl)-2-(N-(4-isobutox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Displacement of [3H]desArg from human B1 in human WI 38 cells | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM50052330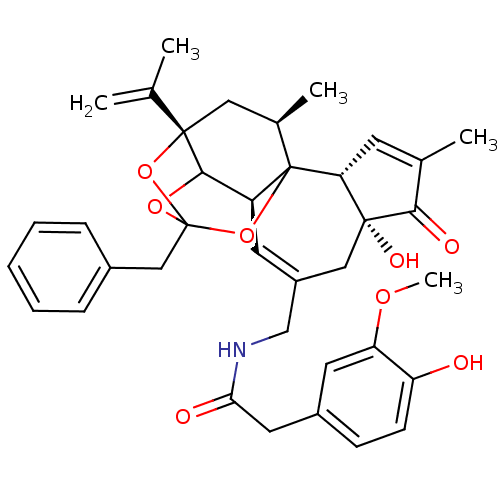 (13-benzyl-5,6-dihydroxy-15-isopropenyl-4,17-dimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Medical Research Curated by ChEMBL | Assay Description Binding affinity against Vanilloid receptor in dorsal Root Ganglion (DRG) membranes using [3H]RTX binding assay. | J Med Chem 39: 2939-52 (1996) Article DOI: 10.1021/jm960139d BindingDB Entry DOI: 10.7270/Q2CV4JD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50370080 (CHEMBL1907656) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254457 (CHEMBL443207 | N-(2-chlorobenzyl)-2-(N-(3-chloro-4...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Displacement of [3H]desArg from human B1 in human WI 38 cells | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254458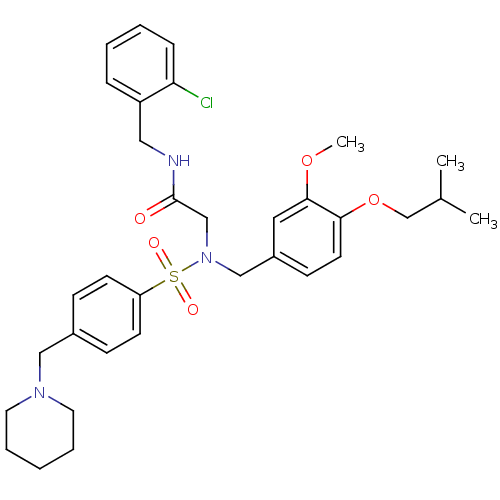 (CHEMBL452238 | N-(2-chlorobenzyl)-2-(N-(4-isobutox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Displacement of [3H]desArg from human B1 in human WI 38 cells | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50370081 (CHEMBL1907654) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 34.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254456 (CHEMBL447392 | N-(2-chlorobenzyl)-2-(N-(4-isobutox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Antagonist activity at human cloned B1 receptor expressed in african green monkey COS7 cells by calcium mobilization assay | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50085698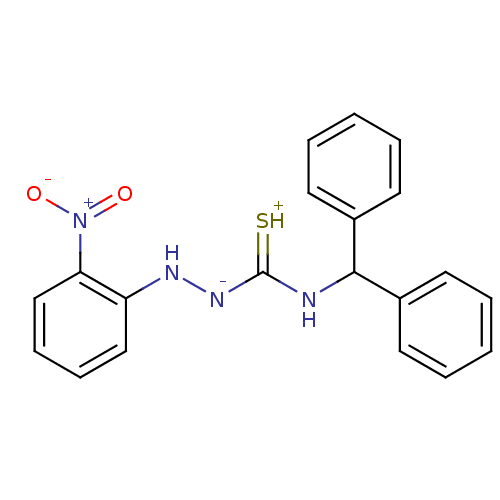 (CHEMBL164636 | benzhydrylamino-2-nitrobenzylhydraz...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-BK to rat bradykinin B2 receptor expressed in NG108-15 neuroblastoma-glioma hybrid cell membranes | J Med Chem 43: 769-71 (2000) BindingDB Entry DOI: 10.7270/Q2XD10W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50409529 (CHEMBL2112221) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 64.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254459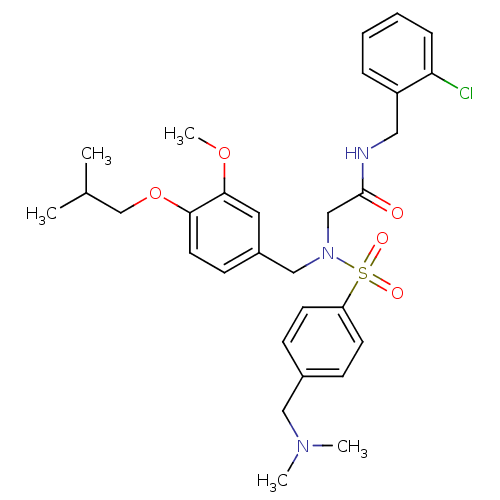 (CHEMBL512111 | N-(2-chlorobenzyl)-2-(N-(4-isobutox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Displacement of [3H]desArg from human B1 in human WI 38 cells | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50370077 (CHEMBL1907652) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254460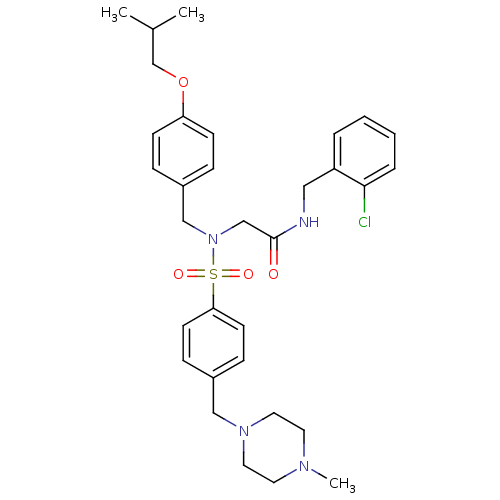 (CHEMBL499999 | N-(2-chlorobenzyl)-2-(N-(4-isobutox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Displacement of [3H]desArg from human B1 in human WI 38 cells | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50370083 (CHEMBL1907651) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50151888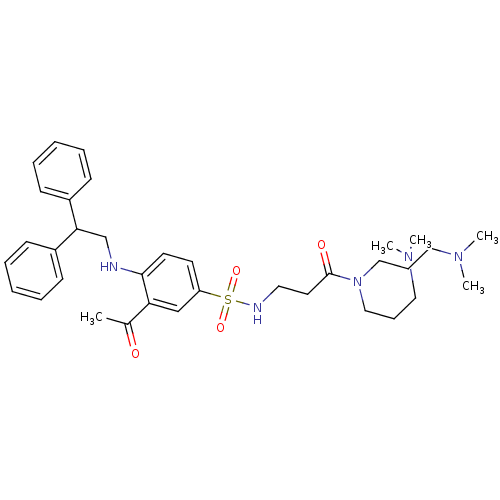 (3-[3-Acetyl-4-(2,2-diphenyl-ethylamino)-benzenesul...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells | J Med Chem 47: 4642-4 (2004) Article DOI: 10.1021/jm049747g BindingDB Entry DOI: 10.7270/Q2J965V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50151889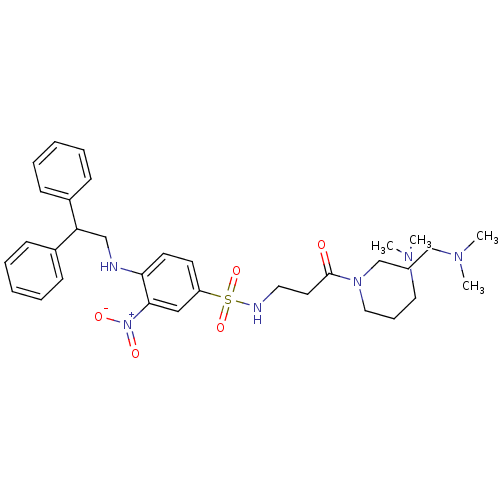 (CHEMBL180143 | N,N-Bis-(3-dimethylamino-propyl)-3-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells | J Med Chem 47: 4642-4 (2004) Article DOI: 10.1021/jm049747g BindingDB Entry DOI: 10.7270/Q2J965V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50085696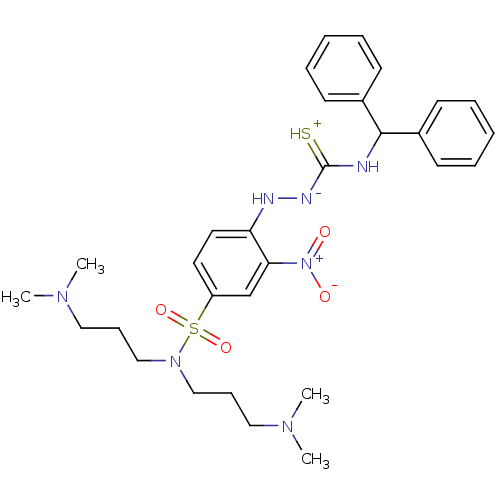 (1N,1N-di(3-dimethylaminopropyl)-4-benzhydrylamino(...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-BK to rat bradykinin B2 receptor expressed in NG108-15 neuroblastoma-glioma hybrid cell membranes | J Med Chem 43: 769-71 (2000) BindingDB Entry DOI: 10.7270/Q2XD10W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254461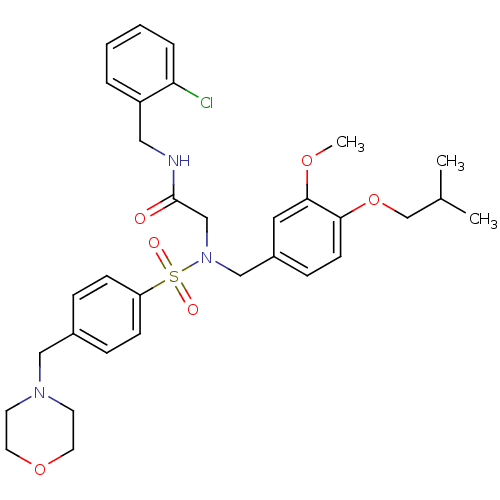 (CHEMBL507546 | N-(2-chlorobenzyl)-2-(N-(4-isobutox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Displacement of [3H]desArg from human B1 in human WI 38 cells | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50409528 (CHEMBL2112220) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 176 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254457 (CHEMBL443207 | N-(2-chlorobenzyl)-2-(N-(3-chloro-4...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Antagonist activity at human cloned B1 receptor expressed in african green monkey COS7 cells by calcium mobilization assay | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50254462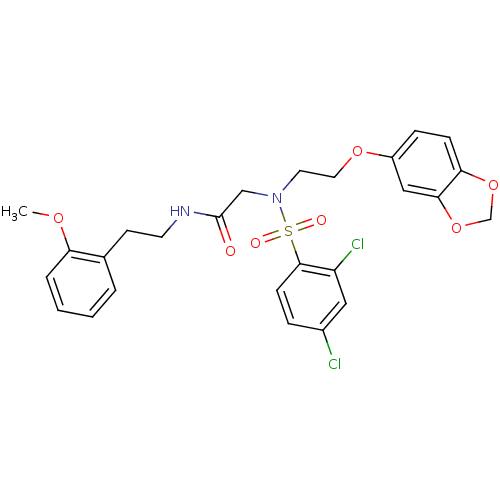 (CHEMBL468492 | N-(2-methoxyphenethyl)-2-(N-(2-(ben...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Inc Curated by ChEMBL | Assay Description Displacement of [3H]desArg from human B1 in human WI 38 cells | Bioorg Med Chem Lett 19: 119-22 (2008) Article DOI: 10.1016/j.bmcl.2008.11.005 BindingDB Entry DOI: 10.7270/Q2KH0N6M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50151890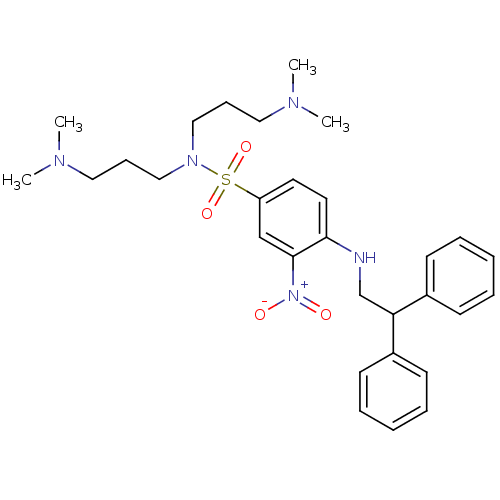 (CHEMBL181098 | N,N-Bis-(3-dimethylamino-propyl)-4-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells | J Med Chem 47: 4642-4 (2004) Article DOI: 10.1021/jm049747g BindingDB Entry DOI: 10.7270/Q2J965V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50113264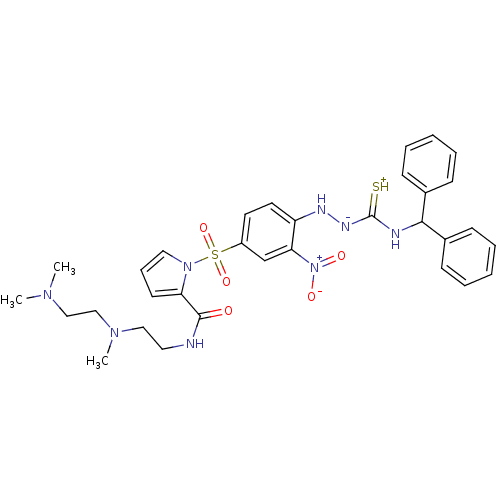 (1-[4-(4-benzhydrylthiosemicarbazido)-3-nitrobenzen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 237 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50151893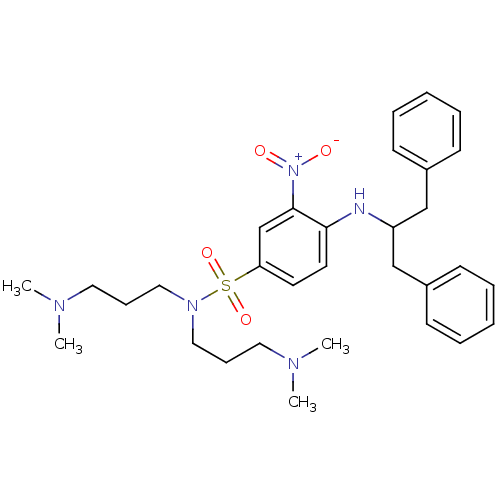 (4-(1-Benzyl-2-phenyl-ethylamino)-N,N-bis-(3-dimeth...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells | J Med Chem 47: 4642-4 (2004) Article DOI: 10.1021/jm049747g BindingDB Entry DOI: 10.7270/Q2J965V7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 90 total ) | Next | Last >> |