 Found 316 hits with Last Name = 'rivault' and Initial = 'f'
Found 316 hits with Last Name = 'rivault' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Fe(3+)-pyochelin receptor
(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...) | BDBM50444965
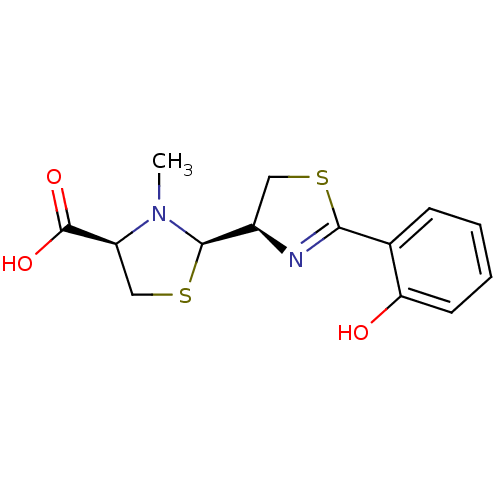
(CHEMBL3099904)Show SMILES CN1[C@H](SC[C@H]1C(O)=O)[C@H]1CSC(=N1)c1ccccc1O |r,c:13| Show InChI InChI=1S/C14H16N2O3S2/c1-16-10(14(18)19)7-21-13(16)9-6-20-12(15-9)8-4-2-3-5-11(8)17/h2-5,9-10,13,17H,6-7H2,1H3,(H,18,19)/t9-,10+,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 7242 CNRS-Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of Pch-55Fe(III) from Pseudomonas aeruginosa PAD07 FptA after 1 hr in presence of CCCP |
Bioorg Med Chem Lett 24: 132-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.054
BindingDB Entry DOI: 10.7270/Q2J38V1F |
More data for this
Ligand-Target Pair | |
Fe(3+)-pyochelin receptor
(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...) | BDBM50444963
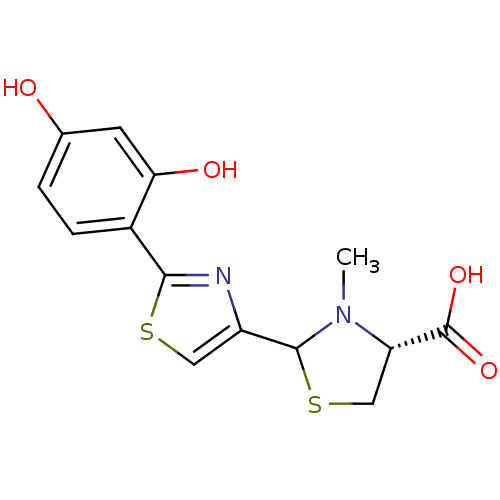
(CHEMBL3099907)Show SMILES CN1[C@@H](CSC1c1csc(n1)-c1ccc(O)cc1O)C(O)=O |r| Show InChI InChI=1S/C14H14N2O4S2/c1-16-10(14(19)20)6-22-13(16)9-5-21-12(15-9)8-3-2-7(17)4-11(8)18/h2-5,10,13,17-18H,6H2,1H3,(H,19,20)/t10-,13?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 7242 CNRS-Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of Pch-55Fe(III) from Pseudomonas aeruginosa PAD07 FptA after 1 hr in presence of CCCP |
Bioorg Med Chem Lett 24: 132-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.054
BindingDB Entry DOI: 10.7270/Q2J38V1F |
More data for this
Ligand-Target Pair | |
Fe(3+)-pyochelin receptor
(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...) | BDBM50444964
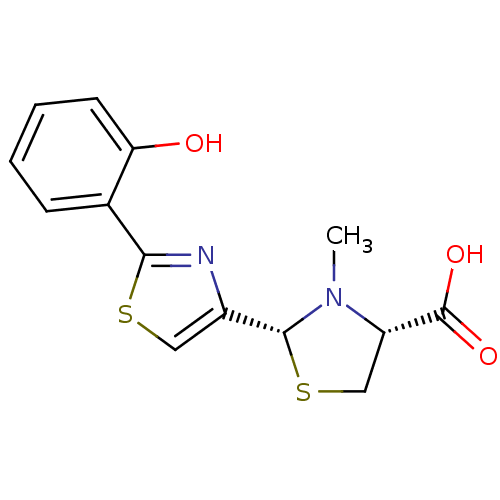
(CHEMBL3099905)Show SMILES CN1[C@@H](CS[C@@H]1c1csc(n1)-c1ccccc1O)C(O)=O |r| Show InChI InChI=1S/C14H14N2O3S2/c1-16-10(14(18)19)7-21-13(16)9-6-20-12(15-9)8-4-2-3-5-11(8)17/h2-6,10,13,17H,7H2,1H3,(H,18,19)/t10-,13+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 7242 CNRS-Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of Pch-55Fe(III) from Pseudomonas aeruginosa PAD07 FptA after 1 hr in presence of CCCP |
Bioorg Med Chem Lett 24: 132-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.054
BindingDB Entry DOI: 10.7270/Q2J38V1F |
More data for this
Ligand-Target Pair | |
Fe(3+)-pyochelin receptor
(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...) | BDBM50444962
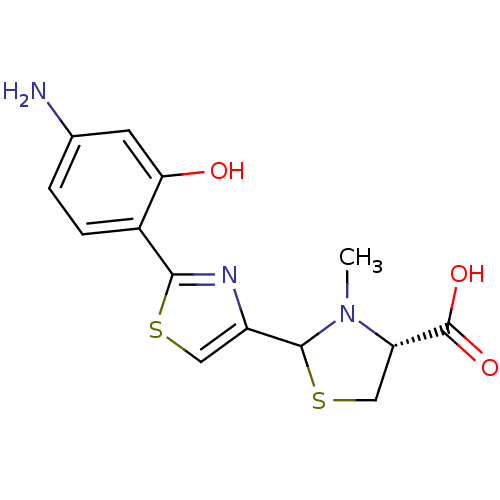
(CHEMBL3099909)Show SMILES CN1[C@@H](CSC1c1csc(n1)-c1ccc(N)cc1O)C(O)=O |r| Show InChI InChI=1S/C14H15N3O3S2/c1-17-10(14(19)20)6-22-13(17)9-5-21-12(16-9)8-3-2-7(15)4-11(8)18/h2-5,10,13,18H,6,15H2,1H3,(H,19,20)/t10-,13?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 7242 CNRS-Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of Pch-55Fe(III) from Pseudomonas aeruginosa PAD07 FptA after 1 hr in presence of CCCP |
Bioorg Med Chem Lett 24: 132-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.054
BindingDB Entry DOI: 10.7270/Q2J38V1F |
More data for this
Ligand-Target Pair | |
Fe(3+)-pyochelin receptor
(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...) | BDBM50444961
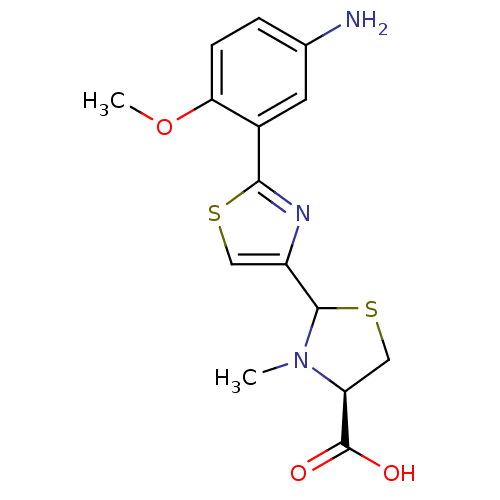
(CHEMBL3099911)Show SMILES COc1ccc(N)cc1-c1nc(cs1)C1SC[C@H](N1C)C(O)=O |r| Show InChI InChI=1S/C15H17N3O3S2/c1-18-11(15(19)20)7-23-14(18)10-6-22-13(17-10)9-5-8(16)3-4-12(9)21-2/h3-6,11,14H,7,16H2,1-2H3,(H,19,20)/t11-,14?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 7242 CNRS-Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of Pch-55Fe(III) from Pseudomonas aeruginosa PAD07 FptA after 1 hr in presence of CCCP |
Bioorg Med Chem Lett 24: 132-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.054
BindingDB Entry DOI: 10.7270/Q2J38V1F |
More data for this
Ligand-Target Pair | |
Fe(3+)-pyochelin receptor
(Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 /...) | BDBM50444960
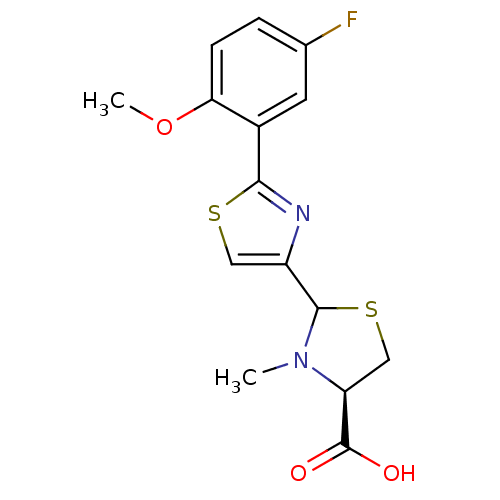
(CHEMBL3099912)Show SMILES COc1ccc(F)cc1-c1nc(cs1)C1SC[C@H](N1C)C(O)=O |r| Show InChI InChI=1S/C15H15FN2O3S2/c1-18-11(15(19)20)7-23-14(18)10-6-22-13(17-10)9-5-8(16)3-4-12(9)21-2/h3-6,11,14H,7H2,1-2H3,(H,19,20)/t11-,14?/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 7242 CNRS-Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Displacement of Pch-55Fe(III) from Pseudomonas aeruginosa PAD07 FptA after 1 hr in presence of CCCP |
Bioorg Med Chem Lett 24: 132-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.054
BindingDB Entry DOI: 10.7270/Q2J38V1F |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205450
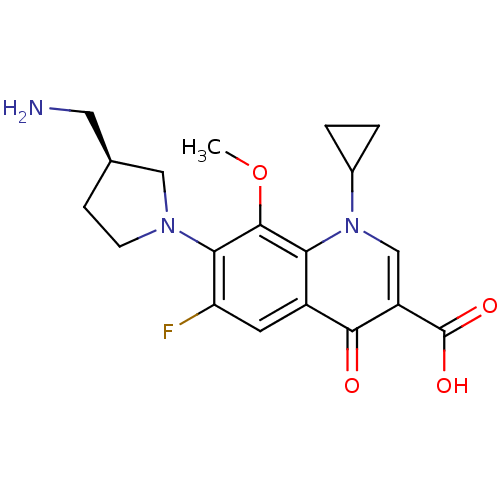
((S)-7-(3-(aminomethyl)pyrrolidin-1-yl)-1-cycloprop...)Show SMILES COc1c(N2CC[C@@H](CN)C2)c(F)cc2c1n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C19H22FN3O4/c1-27-18-15-12(6-14(20)16(18)22-5-4-10(7-21)8-22)17(24)13(19(25)26)9-23(15)11-2-3-11/h6,9-11H,2-5,7-8,21H2,1H3,(H,25,26)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205452
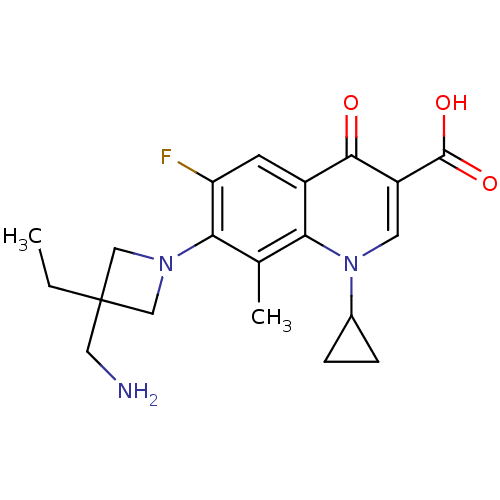
(7-(3-(aminomethyl)-3-ethylazetidin-1-yl)-1-cyclopr...)Show SMILES CCC1(CN)CN(C1)c1c(F)cc2c(c1C)n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C20H24FN3O3/c1-3-20(8-22)9-23(10-20)17-11(2)16-13(6-15(17)21)18(25)14(19(26)27)7-24(16)12-4-5-12/h6-7,12H,3-5,8-10,22H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205465
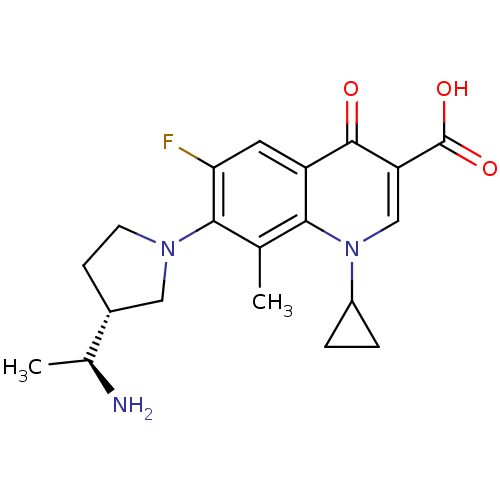
(7-((R)-3-((S)-1-aminoethyl)pyrrolidin-1-yl)-1-cycl...)Show SMILES C[C@H](N)[C@@H]1CCN(C1)c1c(F)cc2c(c1C)n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C20H24FN3O3/c1-10-17-14(19(25)15(20(26)27)9-24(17)13-3-4-13)7-16(21)18(10)23-6-5-12(8-23)11(2)22/h7,9,11-13H,3-6,8,22H2,1-2H3,(H,26,27)/t11-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205451
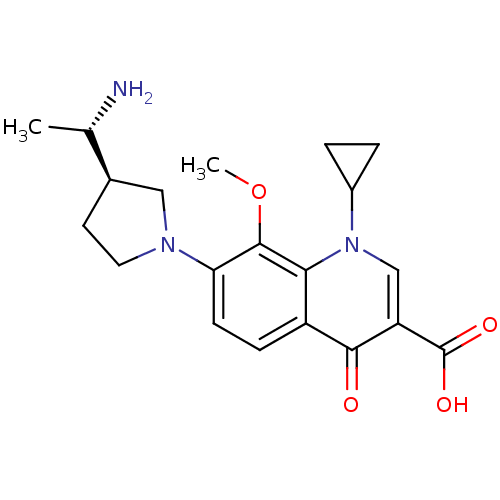
(7-((R)-3-((S)-1-aminoethyl)pyrrolidin-1-yl)-1-cycl...)Show SMILES COc1c(ccc2c1n(cc(C(O)=O)c2=O)C1CC1)N1CC[C@H](C1)[C@H](C)N Show InChI InChI=1S/C20H25N3O4/c1-11(21)12-7-8-22(9-12)16-6-5-14-17(19(16)27-2)23(13-3-4-13)10-15(18(14)24)20(25)26/h5-6,10-13H,3-4,7-9,21H2,1-2H3,(H,25,26)/t11-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205466
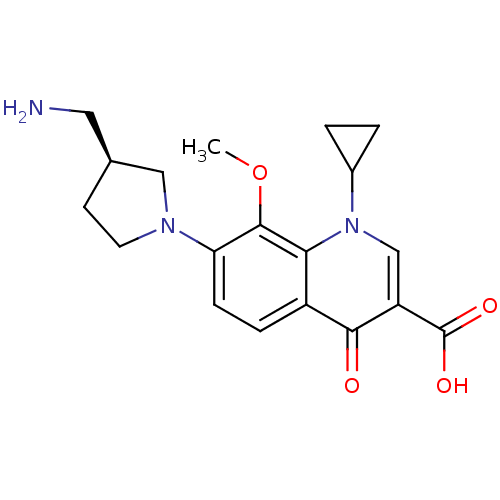
((S)-7-(3-(aminomethyl)pyrrolidin-1-yl)-1-cycloprop...)Show SMILES COc1c(ccc2c1n(cc(C(O)=O)c2=O)C1CC1)N1CC[C@@H](CN)C1 Show InChI InChI=1S/C19H23N3O4/c1-26-18-15(21-7-6-11(8-20)9-21)5-4-13-16(18)22(12-2-3-12)10-14(17(13)23)19(24)25/h4-5,10-12H,2-3,6-9,20H2,1H3,(H,24,25)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205457

(7-((R)-3-((S)-1-(2-cyanoethylamino)ethyl)pyrrolidi...)Show SMILES C[C@H](NCCC#N)[C@@H]1CCN(C1)c1c(F)cc2c(c1C)n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C23H27FN4O3/c1-13-20-17(22(29)18(23(30)31)12-28(20)16-4-5-16)10-19(24)21(13)27-9-6-15(11-27)14(2)26-8-3-7-25/h10,12,14-16,26H,3-6,8-9,11H2,1-2H3,(H,30,31)/t14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205453
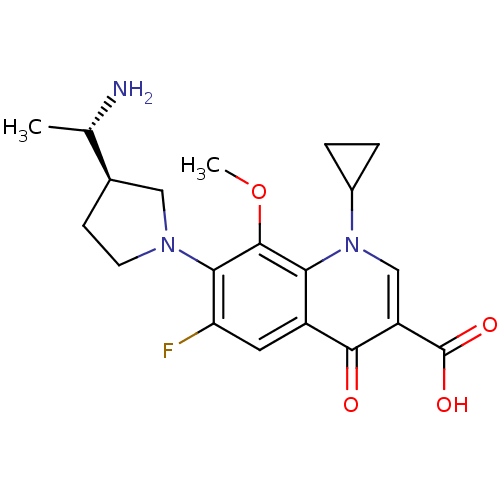
(7-((R)-3-((S)-1-aminoethyl)pyrrolidin-1-yl)-1-cycl...)Show SMILES COc1c(N2CC[C@H](C2)[C@H](C)N)c(F)cc2c1n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C20H24FN3O4/c1-10(22)11-5-6-23(8-11)17-15(21)7-13-16(19(17)28-2)24(12-3-4-12)9-14(18(13)25)20(26)27/h7,9-12H,3-6,8,22H2,1-2H3,(H,26,27)/t10-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205446
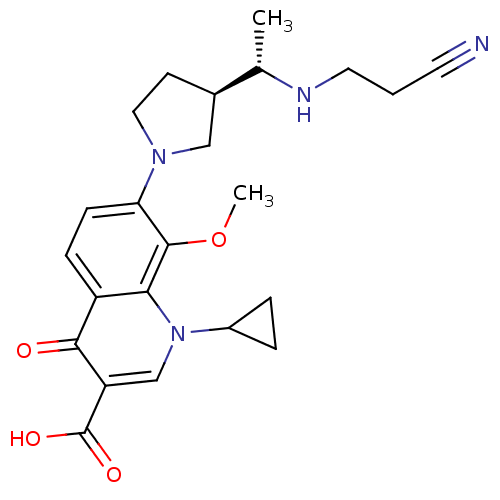
(7-((R)-3-((S)-1-(2-cyanoethylamino)ethyl)pyrrolidi...)Show SMILES COc1c(ccc2c1n(cc(C(O)=O)c2=O)C1CC1)N1CC[C@H](C1)[C@H](C)NCCC#N Show InChI InChI=1S/C23H28N4O4/c1-14(25-10-3-9-24)15-8-11-26(12-15)19-7-6-17-20(22(19)31-2)27(16-4-5-16)13-18(21(17)28)23(29)30/h6-7,13-16,25H,3-5,8,10-12H2,1-2H3,(H,29,30)/t14-,15+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205447

(10-((R)-3-((S)-1-aminoethyl)pyrrolidin-1-yl)-9-flu...)Show SMILES C[C@H](N)[C@@H]1CCN(C1)c1c(F)cc2c3c1OCC(C)n3cc(C(O)=O)c2=O |w:17.19| Show InChI InChI=1S/C19H22FN3O4/c1-9-8-27-18-15-12(17(24)13(19(25)26)7-23(9)15)5-14(20)16(18)22-4-3-11(6-22)10(2)21/h5,7,9-11H,3-4,6,8,21H2,1-2H3,(H,25,26)/t9?,10-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM21690

(1-cyclopropyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,...)Show InChI InChI=1S/C17H18FN3O3/c18-13-7-11-14(8-15(13)20-5-3-19-4-6-20)21(10-1-2-10)9-12(16(11)22)17(23)24/h7-10,19H,1-6H2,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205461
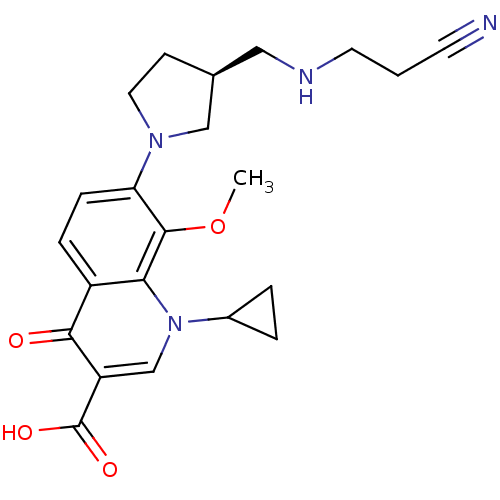
((S)-7-(3-((2-cyanoethylamino)methyl)pyrrolidin-1-y...)Show SMILES COc1c(ccc2c1n(cc(C(O)=O)c2=O)C1CC1)N1CC[C@@H](CNCCC#N)C1 Show InChI InChI=1S/C22H26N4O4/c1-30-21-18(25-10-7-14(12-25)11-24-9-2-8-23)6-5-16-19(21)26(15-3-4-15)13-17(20(16)27)22(28)29/h5-6,13-15,24H,2-4,7,9-12H2,1H3,(H,28,29)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205459
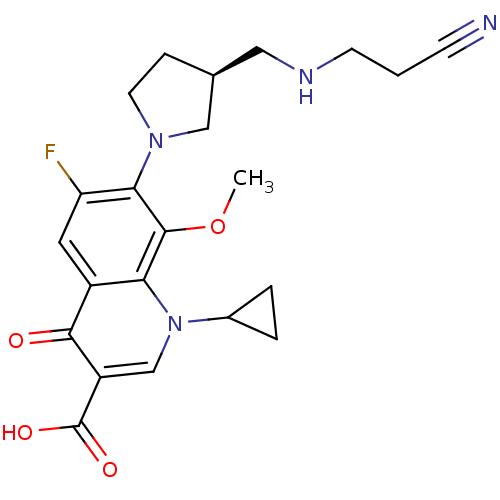
((S)-7-(3-(aminomethyl)pyrrolidin-1-yl)-1-cycloprop...)Show SMILES COc1c(N2CC[C@@H](CNCCC#N)C2)c(F)cc2c1n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C22H25FN4O4/c1-31-21-18-15(20(28)16(22(29)30)12-27(18)14-3-4-14)9-17(23)19(21)26-8-5-13(11-26)10-25-7-2-6-24/h9,12-14,25H,2-5,7-8,10-11H2,1H3,(H,29,30)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205455
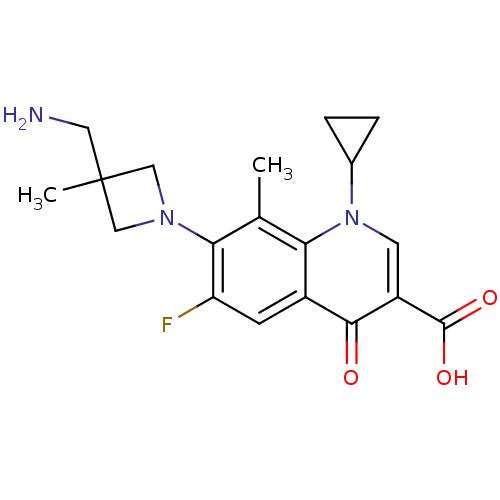
(7-(3-(aminomethyl)-3-methylazetidin-1-yl)-1-cyclop...)Show SMILES Cc1c(N2CC(C)(CN)C2)c(F)cc2c1n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C19H22FN3O3/c1-10-15-12(5-14(20)16(10)22-8-19(2,7-21)9-22)17(24)13(18(25)26)6-23(15)11-3-4-11/h5-6,11H,3-4,7-9,21H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50131445
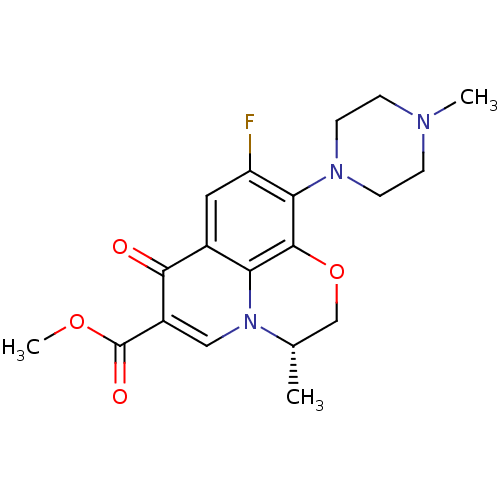
((3S)-9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)...)Show SMILES COC(=O)c1cn2[C@@H](C)COc3c(N4CCN(C)CC4)c(F)cc(c23)c1=O Show InChI InChI=1S/C19H22FN3O4/c1-11-10-27-18-15-12(17(24)13(9-23(11)15)19(25)26-3)8-14(20)16(18)22-6-4-21(2)5-7-22/h8-9,11H,4-7,10H2,1-3H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205467
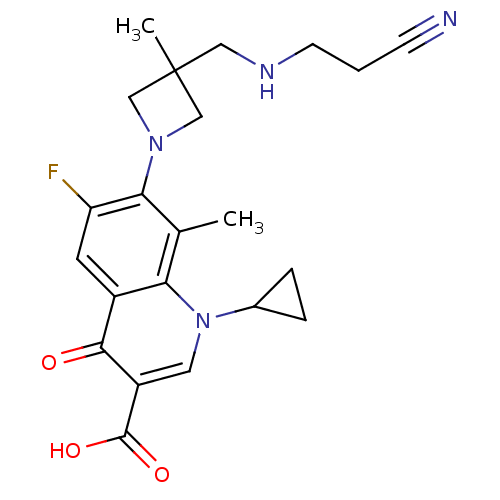
(7-(3-((2-cyanoethylamino)methyl)-3-methylazetidin-...)Show SMILES Cc1c(N2CC(C)(CNCCC#N)C2)c(F)cc2c1n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C22H25FN4O3/c1-13-18-15(20(28)16(21(29)30)9-27(18)14-4-5-14)8-17(23)19(13)26-11-22(2,12-26)10-25-7-3-6-24/h8-9,14,25H,3-5,7,10-12H2,1-2H3,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205456
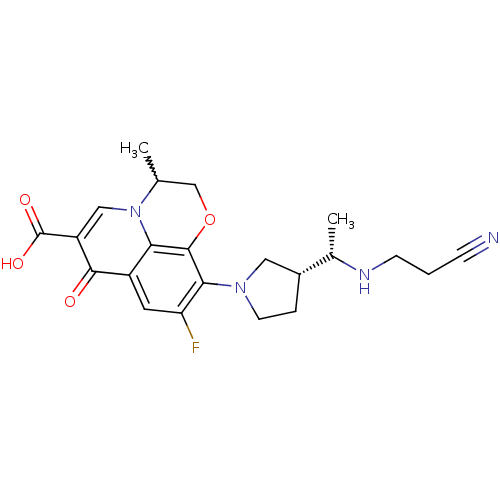
(10-((R)-3-((S)-1-(2-cyanoethylamino)ethyl)pyrrolid...)Show SMILES C[C@H](NCCC#N)[C@@H]1CCN(C1)c1c(F)cc2c3c1OCC(C)n3cc(C(O)=O)c2=O |w:21.23| Show InChI InChI=1S/C22H25FN4O4/c1-12-11-31-21-18-15(20(28)16(22(29)30)10-27(12)18)8-17(23)19(21)26-7-4-14(9-26)13(2)25-6-3-5-24/h8,10,12-14,25H,3-4,6-7,9,11H2,1-2H3,(H,29,30)/t12?,13-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205463
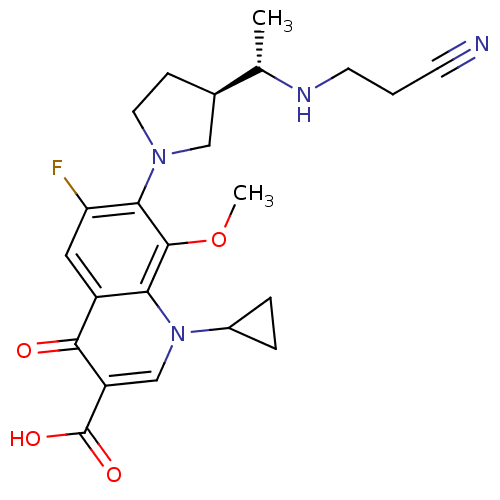
(7-((R)-3-((S)-1-(2-cyanoethylamino)ethyl)pyrrolidi...)Show SMILES COc1c(N2CC[C@H](C2)[C@H](C)NCCC#N)c(F)cc2c1n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C23H27FN4O4/c1-13(26-8-3-7-25)14-6-9-27(11-14)20-18(24)10-16-19(22(20)32-2)28(15-4-5-15)12-17(21(16)29)23(30)31/h10,12-15,26H,3-6,8-9,11H2,1-2H3,(H,30,31)/t13-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205454
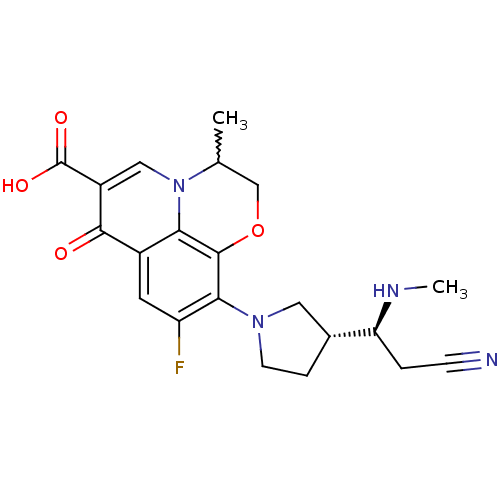
(10-((R)-3-((S)-2-cyano-1-(methylamino)ethyl)pyrrol...)Show SMILES CN[C@@H](CC#N)[C@@H]1CCN(C1)c1c(F)cc2c3c1OCC(C)n3cc(C(O)=O)c2=O |w:20.22| Show InChI InChI=1S/C21H23FN4O4/c1-11-10-30-20-17-13(19(27)14(21(28)29)9-26(11)17)7-15(22)18(20)25-6-4-12(8-25)16(24-2)3-5-23/h7,9,11-12,16,24H,3-4,6,8,10H2,1-2H3,(H,28,29)/t11?,12-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205449
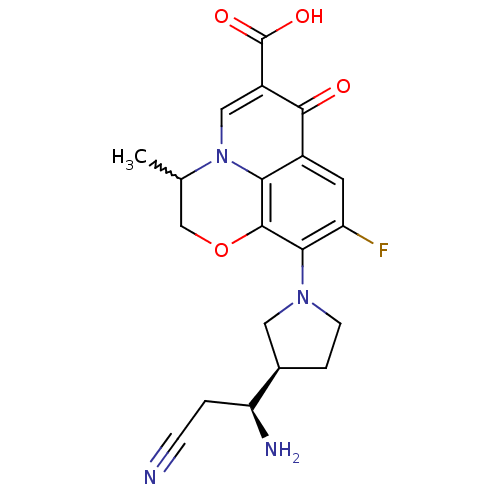
(10-((R)-3-((S)-1-amino-2-cyanoethyl)pyrrolidin-1-y...)Show SMILES CC1COc2c(N3CC[C@H](C3)[C@@H](N)CC#N)c(F)cc3c2n1cc(C(O)=O)c3=O |w:1.0| Show InChI InChI=1S/C20H21FN4O4/c1-10-9-29-19-16-12(18(26)13(20(27)28)8-25(10)16)6-14(21)17(19)24-5-3-11(7-24)15(23)2-4-22/h6,8,10-11,15H,2-3,5,7,9,23H2,1H3,(H,27,28)/t10?,11-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205458
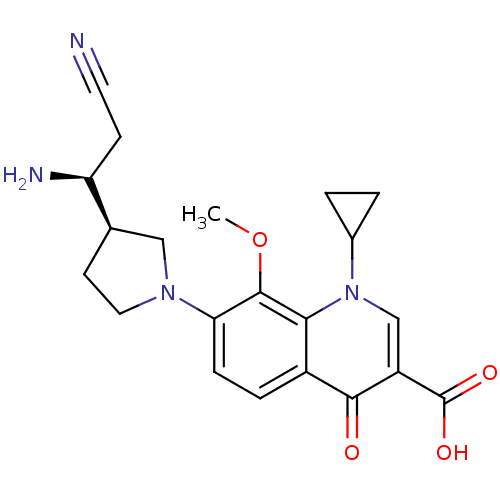
(7-((R)-3-((S)-1-amino-2-cyanoethyl)pyrrolidin-1-yl...)Show SMILES COc1c(ccc2c1n(cc(C(O)=O)c2=O)C1CC1)N1CC[C@H](C1)[C@@H](N)CC#N Show InChI InChI=1S/C21H24N4O4/c1-29-20-17(24-9-7-12(10-24)16(23)6-8-22)5-4-14-18(20)25(13-2-3-13)11-15(19(14)26)21(27)28/h4-5,11-13,16H,2-3,6-7,9-10,23H2,1H3,(H,27,28)/t12-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205462
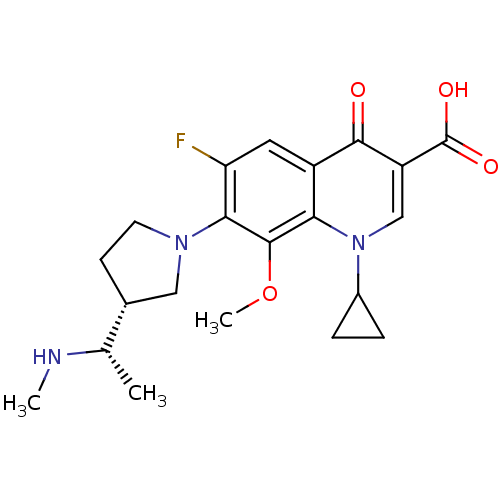
(1-cyclopropyl-6-fluoro-8-methoxy-7-((R)-3-((S)-1-(...)Show SMILES CN[C@@H](C)[C@@H]1CCN(C1)c1c(F)cc2c(c1OC)n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C21H26FN3O4/c1-11(23-2)12-6-7-24(9-12)18-16(22)8-14-17(20(18)29-3)25(13-4-5-13)10-15(19(14)26)21(27)28/h8,10-13,23H,4-7,9H2,1-3H3,(H,27,28)/t11-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205464
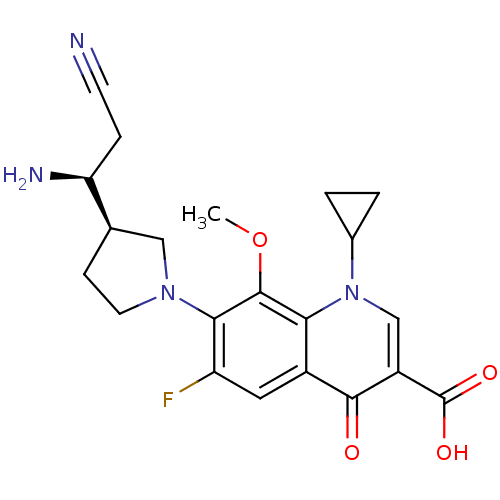
(7-((R)-3-((S)-1-amino-2-cyanoethyl)pyrrolidin-1-yl...)Show SMILES COc1c(N2CC[C@H](C2)[C@@H](N)CC#N)c(F)cc2c1n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C21H23FN4O4/c1-30-20-17-13(19(27)14(21(28)29)10-26(17)12-2-3-12)8-15(22)18(20)25-7-5-11(9-25)16(24)4-6-23/h8,10-12,16H,2-5,7,9,24H2,1H3,(H,28,29)/t11-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205460
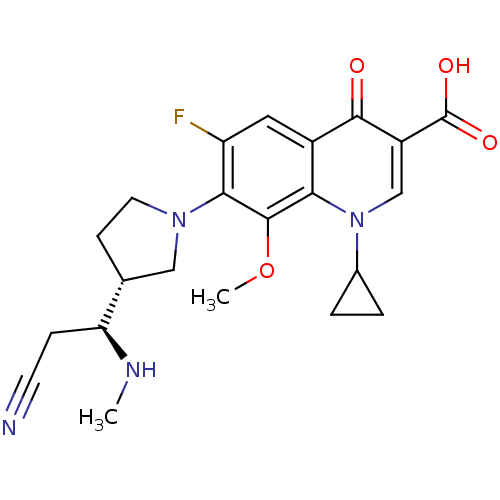
(7-((R)-3-((S)-2-cyano-1-(methylamino)ethyl)pyrroli...)Show SMILES CN[C@@H](CC#N)[C@@H]1CCN(C1)c1c(F)cc2c(c1OC)n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C22H25FN4O4/c1-25-17(5-7-24)12-6-8-26(10-12)19-16(23)9-14-18(21(19)31-2)27(13-3-4-13)11-15(20(14)28)22(29)30/h9,11-13,17,25H,3-6,8,10H2,1-2H3,(H,29,30)/t12-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50205448
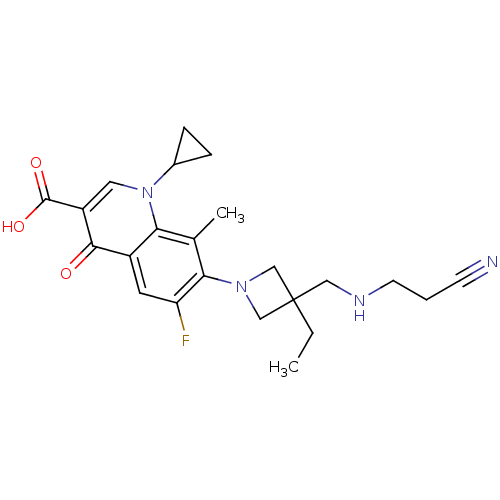
(7-(3-((2-cyanoethylamino)methyl)-3-ethylazetidin-1...)Show SMILES CCC1(CNCCC#N)CN(C1)c1c(F)cc2c(c1C)n(cc(C(O)=O)c2=O)C1CC1 Show InChI InChI=1S/C23H27FN4O3/c1-3-23(11-26-8-4-7-25)12-27(13-23)20-14(2)19-16(9-18(20)24)21(29)17(22(30)31)10-28(19)15-5-6-15/h9-10,15,26H,3-6,8,11-13H2,1-2H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG by fliter binding assay |
Bioorg Med Chem Lett 17: 2150-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.090
BindingDB Entry DOI: 10.7270/Q2GM86Z7 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM50191486
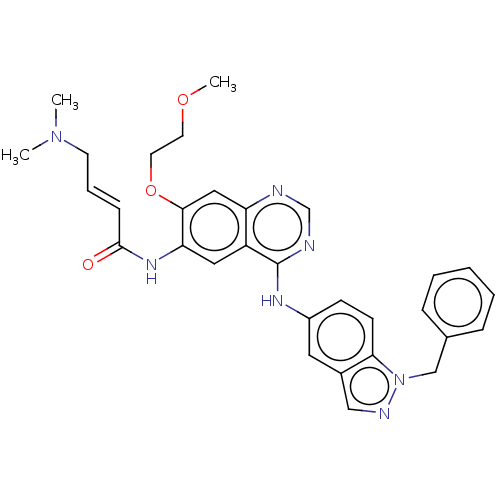
(CHEMBL3959248)Show SMILES COCCOc1cc2ncnc(Nc3ccc4n(Cc5ccccc5)ncc4c3)c2cc1NC(=O)\C=C\CN(C)C Show InChI InChI=1S/C31H33N7O3/c1-37(2)13-7-10-30(39)36-27-17-25-26(18-29(27)41-15-14-40-3)32-21-33-31(25)35-24-11-12-28-23(16-24)19-34-38(28)20-22-8-5-4-6-9-22/h4-12,16-19,21H,13-15,20H2,1-3H3,(H,36,39)(H,32,33,35)/b10-7+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Irreversible inhibition of GST-tagged ERBB4 (unknown origin) (Gly-259 to Gly-690 residues) expressed in baculovirus infected Sf9 insect cells assesse... |
J Med Chem 59: 8103-24 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00883
BindingDB Entry DOI: 10.7270/Q2FF3VB3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50191587
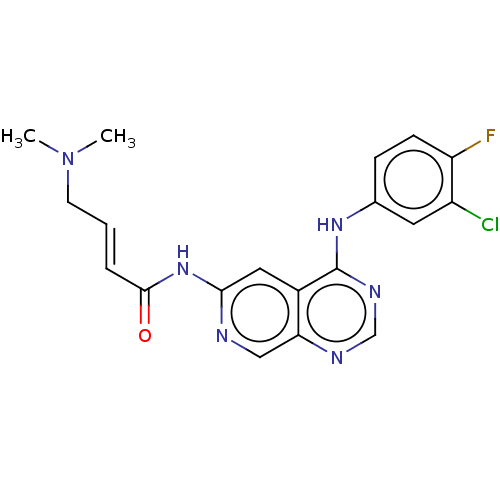
(CHEMBL3968417)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cn1 Show InChI InChI=1S/C19H18ClFN6O/c1-27(2)7-3-4-18(28)26-17-9-13-16(10-22-17)23-11-24-19(13)25-12-5-6-15(21)14(20)8-12/h3-6,8-11H,7H2,1-2H3,(H,22,26,28)(H,23,24,25)/b4-3+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Irreversible inhibition of full length human ERBB1 autophosphorylation transfected in EGF-stimulated mouse NIH/3T3 cells incubated for 2 hrs followed... |
J Med Chem 59: 8103-24 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00883
BindingDB Entry DOI: 10.7270/Q2FF3VB3 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM50191587

(CHEMBL3968417)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cn1 Show InChI InChI=1S/C19H18ClFN6O/c1-27(2)7-3-4-18(28)26-17-9-13-16(10-22-17)23-11-24-19(13)25-12-5-6-15(21)14(20)8-12/h3-6,8-11H,7H2,1-2H3,(H,22,26,28)(H,23,24,25)/b4-3+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Irreversible inhibition of GST-tagged ERBB2 (unknown origin) (Ile-675 to Val-1256 residues) expressed in baculovirus infected Sf9 insect cells assess... |
J Med Chem 59: 8103-24 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00883
BindingDB Entry DOI: 10.7270/Q2FF3VB3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50191640
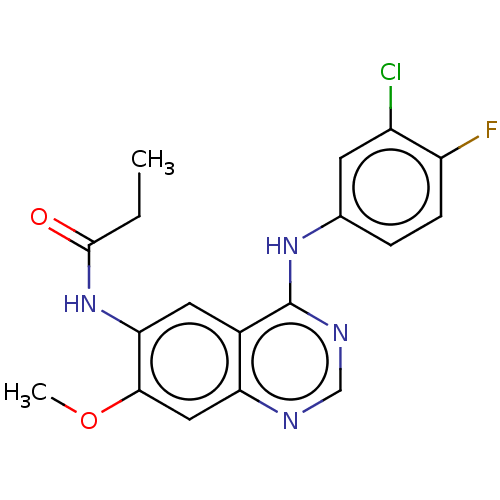
(CHEMBL3930316)Show InChI InChI=1S/C18H16ClFN4O2/c1-3-17(25)24-15-7-11-14(8-16(15)26-2)21-9-22-18(11)23-10-4-5-13(20)12(19)6-10/h4-9H,3H2,1-2H3,(H,24,25)(H,21,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Irreversible inhibition of GST-tagged ERBB1 (unknown origin) (Met-668 to Ala-1211 residues) expressed in baculovirus infected Sf9 insect cells assess... |
J Med Chem 59: 8103-24 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00883
BindingDB Entry DOI: 10.7270/Q2FF3VB3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Irreversible inhibition of GST-tagged ERBB1 (unknown origin) (Met-668 to Ala-1211 residues) expressed in baculovirus infected Sf9 insect cells assess... |
J Med Chem 59: 8103-24 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00883
BindingDB Entry DOI: 10.7270/Q2FF3VB3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50191668
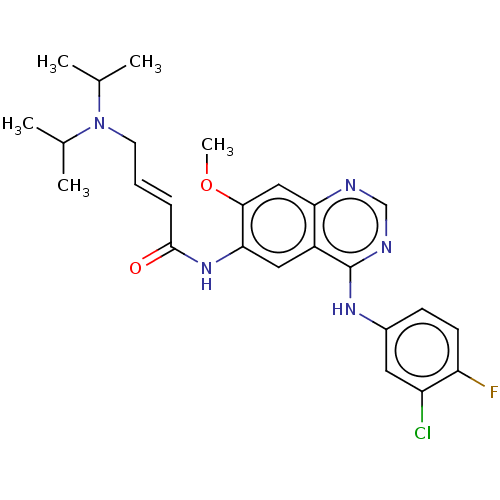
(CHEMBL3950549)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1NC(=O)\C=C\CN(C(C)C)C(C)C Show InChI InChI=1S/C25H29ClFN5O2/c1-15(2)32(16(3)4)10-6-7-24(33)31-22-12-18-21(13-23(22)34-5)28-14-29-25(18)30-17-8-9-20(27)19(26)11-17/h6-9,11-16H,10H2,1-5H3,(H,31,33)(H,28,29,30)/b7-6+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Irreversible inhibition of GST-tagged ERBB1 (unknown origin) (Met-668 to Ala-1211 residues) expressed in baculovirus infected Sf9 insect cells assess... |
J Med Chem 59: 8103-24 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00883
BindingDB Entry DOI: 10.7270/Q2FF3VB3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50191587

(CHEMBL3968417)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(F)c(Cl)c3)ncnc2cn1 Show InChI InChI=1S/C19H18ClFN6O/c1-27(2)7-3-4-18(28)26-17-9-13-16(10-22-17)23-11-24-19(13)25-12-5-6-15(21)14(20)8-12/h3-6,8-11H,7H2,1-2H3,(H,22,26,28)(H,23,24,25)/b4-3+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Irreversible inhibition of GST-tagged ERBB1 (unknown origin) (Met-668 to Ala-1211 residues) expressed in baculovirus infected Sf9 insect cells assess... |
J Med Chem 59: 8103-24 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00883
BindingDB Entry DOI: 10.7270/Q2FF3VB3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM4779

(CHEMBL31965 | CHEMBL545315 | CI-1033 | Canertinib ...)Show SMILES Fc1ccc(Nc2ncnc3cc(OCCCN4CCOCC4)c(NC(=O)C=C)cc23)cc1Cl Show InChI InChI=1S/C24H25ClFN5O3/c1-2-23(32)30-21-13-17-20(14-22(21)34-9-3-6-31-7-10-33-11-8-31)27-15-28-24(17)29-16-4-5-19(26)18(25)12-16/h2,4-5,12-15H,1,3,6-11H2,(H,30,32)(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Irreversible inhibition of full length human ERBB1 autophosphorylation transfected in EGF-stimulated mouse NIH/3T3 cells incubated for 2 hrs followed... |
J Med Chem 59: 8103-24 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00883
BindingDB Entry DOI: 10.7270/Q2FF3VB3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50191489
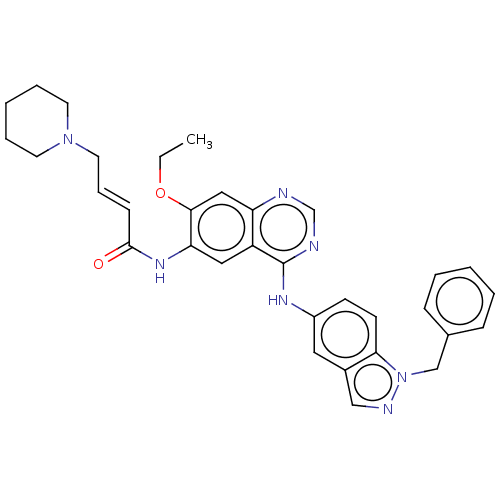
(CHEMBL3942228)Show SMILES CCOc1cc2ncnc(Nc3ccc4n(Cc5ccccc5)ncc4c3)c2cc1NC(=O)\C=C\CN1CCCCC1 Show InChI InChI=1S/C33H35N7O2/c1-2-42-31-20-28-27(19-29(31)38-32(41)12-9-17-39-15-7-4-8-16-39)33(35-23-34-28)37-26-13-14-30-25(18-26)21-36-40(30)22-24-10-5-3-6-11-24/h3,5-6,9-14,18-21,23H,2,4,7-8,15-17,22H2,1H3,(H,38,41)(H,34,35,37)/b12-9+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Irreversible inhibition of full length human ERBB1 autophosphorylation transfected in EGF-stimulated mouse NIH/3T3 cells incubated for 2 hrs followed... |
J Med Chem 59: 8103-24 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00883
BindingDB Entry DOI: 10.7270/Q2FF3VB3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50191488
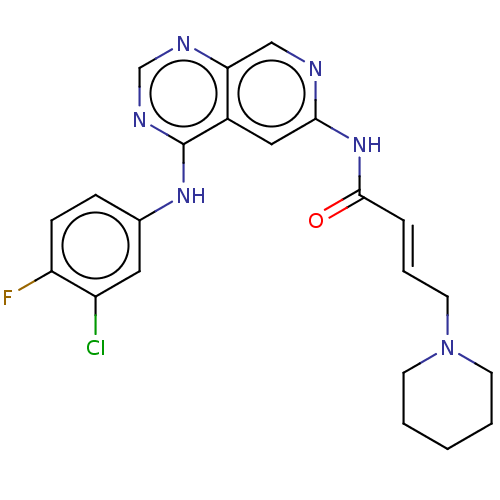
(CHEMBL3930506)Show SMILES Fc1ccc(Nc2ncnc3cnc(NC(=O)\C=C\CN4CCCCC4)cc23)cc1Cl Show InChI InChI=1S/C22H22ClFN6O/c23-17-11-15(6-7-18(17)24)28-22-16-12-20(25-13-19(16)26-14-27-22)29-21(31)5-4-10-30-8-2-1-3-9-30/h4-7,11-14H,1-3,8-10H2,(H,25,29,31)(H,26,27,28)/b5-4+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Irreversible inhibition of full length human ERBB1 autophosphorylation transfected in EGF-stimulated mouse NIH/3T3 cells incubated for 2 hrs followed... |
J Med Chem 59: 8103-24 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00883
BindingDB Entry DOI: 10.7270/Q2FF3VB3 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM50191661
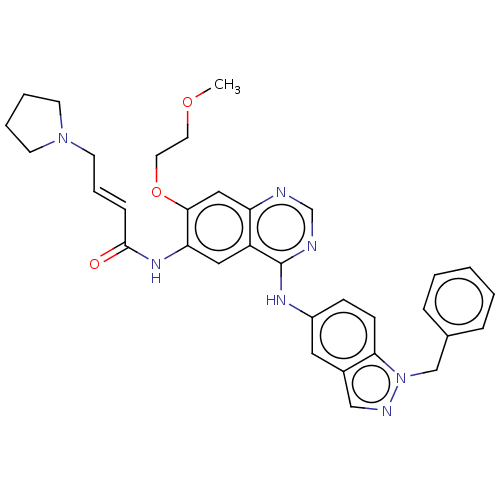
(CHEMBL3890186)Show SMILES COCCOc1cc2ncnc(Nc3ccc4n(Cc5ccccc5)ncc4c3)c2cc1NC(=O)\C=C\CN1CCCC1 Show InChI InChI=1S/C33H35N7O3/c1-42-16-17-43-31-20-28-27(19-29(31)38-32(41)10-7-15-39-13-5-6-14-39)33(35-23-34-28)37-26-11-12-30-25(18-26)21-36-40(30)22-24-8-3-2-4-9-24/h2-4,7-12,18-21,23H,5-6,13-17,22H2,1H3,(H,38,41)(H,34,35,37)/b10-7+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Irreversible inhibition of GST-tagged ERBB4 (unknown origin) (Gly-259 to Gly-690 residues) expressed in baculovirus infected Sf9 insect cells assesse... |
J Med Chem 59: 8103-24 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00883
BindingDB Entry DOI: 10.7270/Q2FF3VB3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50191654
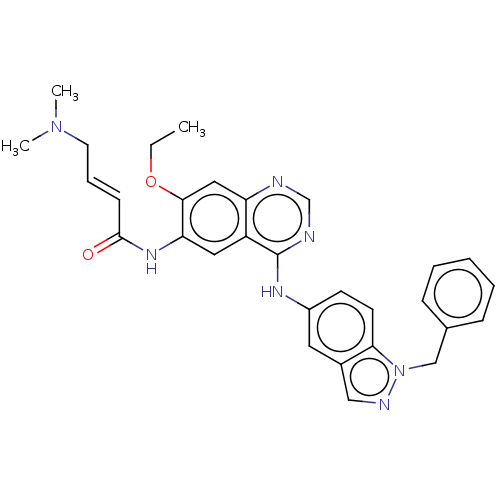
(CHEMBL3932176)Show SMILES CCOc1cc2ncnc(Nc3ccc4n(Cc5ccccc5)ncc4c3)c2cc1NC(=O)\C=C\CN(C)C Show InChI InChI=1S/C30H31N7O2/c1-4-39-28-17-25-24(16-26(28)35-29(38)11-8-14-36(2)3)30(32-20-31-25)34-23-12-13-27-22(15-23)18-33-37(27)19-21-9-6-5-7-10-21/h5-13,15-18,20H,4,14,19H2,1-3H3,(H,35,38)(H,31,32,34)/b11-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Irreversible inhibition of full length human ERBB1 autophosphorylation transfected in EGF-stimulated mouse NIH/3T3 cells incubated for 2 hrs followed... |
J Med Chem 59: 8103-24 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00883
BindingDB Entry DOI: 10.7270/Q2FF3VB3 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM50191495
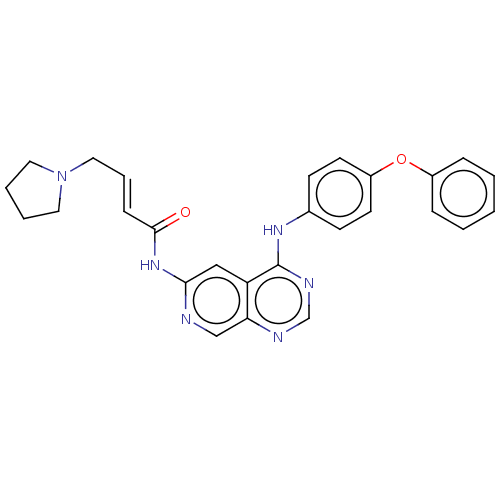
(CHEMBL3896779)Show SMILES O=C(Nc1cc2c(Nc3ccc(Oc4ccccc4)cc3)ncnc2cn1)\C=C\CN1CCCC1 Show InChI InChI=1S/C27H26N6O2/c34-26(9-6-16-33-14-4-5-15-33)32-25-17-23-24(18-28-25)29-19-30-27(23)31-20-10-12-22(13-11-20)35-21-7-2-1-3-8-21/h1-3,6-13,17-19H,4-5,14-16H2,(H,28,32,34)(H,29,30,31)/b9-6+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Irreversible inhibition of GST-tagged ERBB4 (unknown origin) (Gly-259 to Gly-690 residues) expressed in baculovirus infected Sf9 insect cells assesse... |
J Med Chem 59: 8103-24 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00883
BindingDB Entry DOI: 10.7270/Q2FF3VB3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50191664
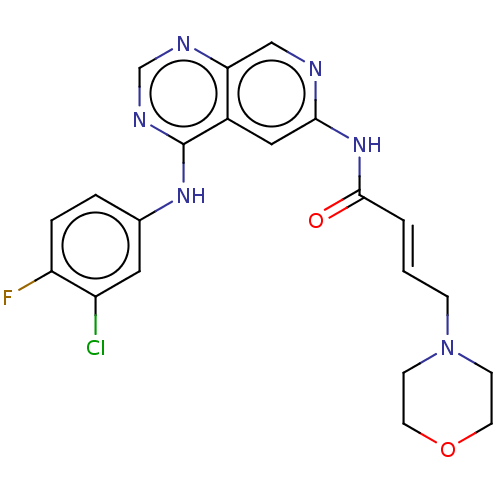
(CHEMBL3958006)Show SMILES Fc1ccc(Nc2ncnc3cnc(NC(=O)\C=C\CN4CCOCC4)cc23)cc1Cl Show InChI InChI=1S/C21H20ClFN6O2/c22-16-10-14(3-4-17(16)23)27-21-15-11-19(24-12-18(15)25-13-26-21)28-20(30)2-1-5-29-6-8-31-9-7-29/h1-4,10-13H,5-9H2,(H,24,28,30)(H,25,26,27)/b2-1+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Irreversible inhibition of full length human ERBB1 autophosphorylation transfected in EGF-stimulated mouse NIH/3T3 cells incubated for 2 hrs followed... |
J Med Chem 59: 8103-24 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00883
BindingDB Entry DOI: 10.7270/Q2FF3VB3 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM50191479
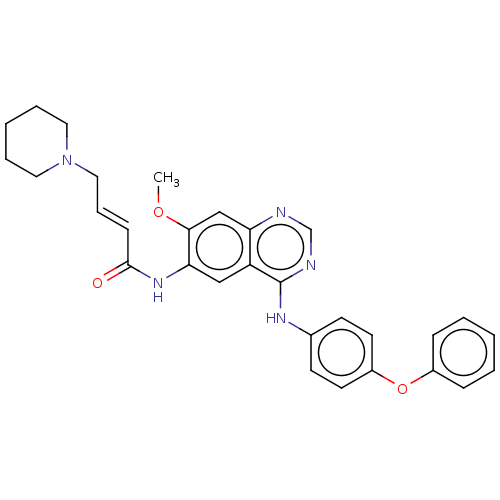
(CHEMBL3958482)Show SMILES COc1cc2ncnc(Nc3ccc(Oc4ccccc4)cc3)c2cc1NC(=O)\C=C\CN1CCCCC1 Show InChI InChI=1S/C30H31N5O3/c1-37-28-20-26-25(19-27(28)34-29(36)11-8-18-35-16-6-3-7-17-35)30(32-21-31-26)33-22-12-14-24(15-13-22)38-23-9-4-2-5-10-23/h2,4-5,8-15,19-21H,3,6-7,16-18H2,1H3,(H,34,36)(H,31,32,33)/b11-8+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Irreversible inhibition of GST-tagged ERBB4 (unknown origin) (Gly-259 to Gly-690 residues) expressed in baculovirus infected Sf9 insect cells assesse... |
J Med Chem 59: 8103-24 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00883
BindingDB Entry DOI: 10.7270/Q2FF3VB3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50191646
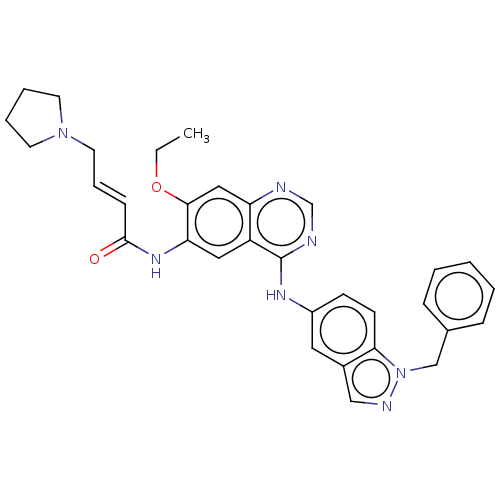
(CHEMBL3923228)Show SMILES CCOc1cc2ncnc(Nc3ccc4n(Cc5ccccc5)ncc4c3)c2cc1NC(=O)\C=C\CN1CCCC1 Show InChI InChI=1S/C32H33N7O2/c1-2-41-30-19-27-26(18-28(30)37-31(40)11-8-16-38-14-6-7-15-38)32(34-22-33-27)36-25-12-13-29-24(17-25)20-35-39(29)21-23-9-4-3-5-10-23/h3-5,8-13,17-20,22H,2,6-7,14-16,21H2,1H3,(H,37,40)(H,33,34,36)/b11-8+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Irreversible inhibition of full length human ERBB1 autophosphorylation transfected in EGF-stimulated mouse NIH/3T3 cells incubated for 2 hrs followed... |
J Med Chem 59: 8103-24 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00883
BindingDB Entry DOI: 10.7270/Q2FF3VB3 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM50191502

(CHEMBL3981551)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(OCc4cccc(F)c4)c(Cl)c3)ncnc2cn1 Show InChI InChI=1S/C26H24ClFN6O2/c1-34(2)10-4-7-25(35)33-24-13-20-22(14-29-24)30-16-31-26(20)32-19-8-9-23(21(27)12-19)36-15-17-5-3-6-18(28)11-17/h3-9,11-14,16H,10,15H2,1-2H3,(H,29,33,35)(H,30,31,32)/b7-4+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Irreversible inhibition of GST-tagged ERBB4 (unknown origin) (Gly-259 to Gly-690 residues) expressed in baculovirus infected Sf9 insect cells assesse... |
J Med Chem 59: 8103-24 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00883
BindingDB Entry DOI: 10.7270/Q2FF3VB3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50191641
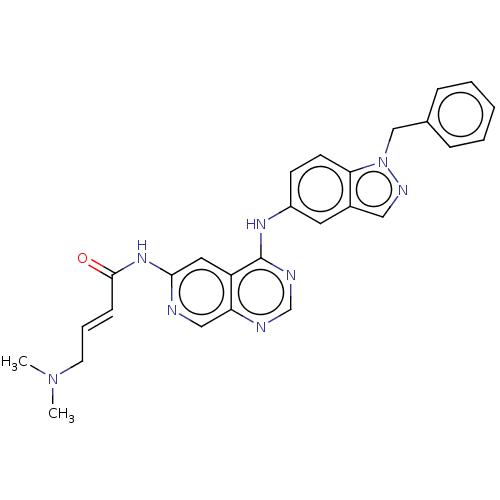
(CHEMBL3949347)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc4n(Cc5ccccc5)ncc4c3)ncnc2cn1 Show InChI InChI=1S/C27H26N8O/c1-34(2)12-6-9-26(36)33-25-14-22-23(16-28-25)29-18-30-27(22)32-21-10-11-24-20(13-21)15-31-35(24)17-19-7-4-3-5-8-19/h3-11,13-16,18H,12,17H2,1-2H3,(H,28,33,36)(H,29,30,32)/b9-6+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Irreversible inhibition of full length human ERBB1 autophosphorylation transfected in EGF-stimulated mouse NIH/3T3 cells incubated for 2 hrs followed... |
J Med Chem 59: 8103-24 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00883
BindingDB Entry DOI: 10.7270/Q2FF3VB3 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50191481
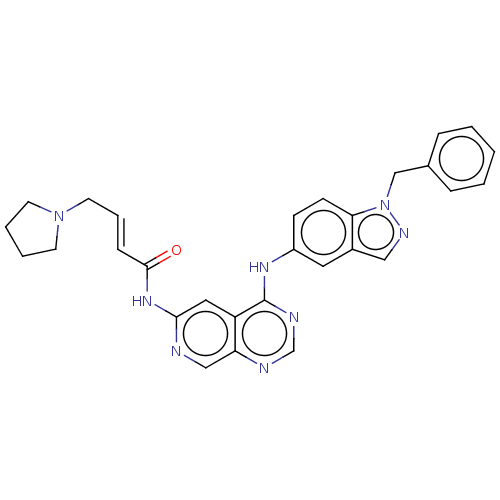
(CHEMBL3948267)Show SMILES O=C(Nc1cc2c(Nc3ccc4n(Cc5ccccc5)ncc4c3)ncnc2cn1)\C=C\CN1CCCC1 Show InChI InChI=1S/C29H28N8O/c38-28(9-6-14-36-12-4-5-13-36)35-27-16-24-25(18-30-27)31-20-32-29(24)34-23-10-11-26-22(15-23)17-33-37(26)19-21-7-2-1-3-8-21/h1-3,6-11,15-18,20H,4-5,12-14,19H2,(H,30,35,38)(H,31,32,34)/b9-6+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Irreversible inhibition of full length human ERBB1 autophosphorylation transfected in EGF-stimulated mouse NIH/3T3 cells incubated for 2 hrs followed... |
J Med Chem 59: 8103-24 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00883
BindingDB Entry DOI: 10.7270/Q2FF3VB3 |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-4
(Homo sapiens (Human)) | BDBM50191586
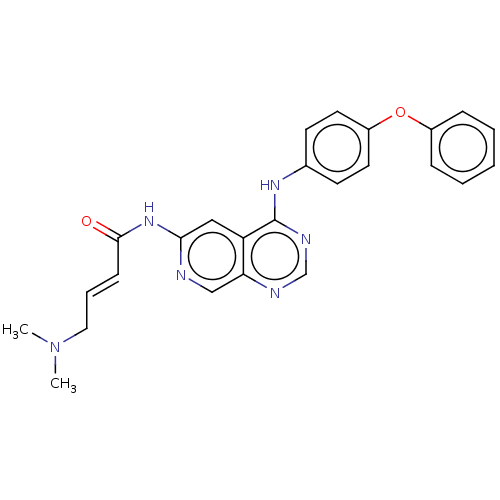
(CHEMBL3977504)Show SMILES CN(C)C\C=C\C(=O)Nc1cc2c(Nc3ccc(Oc4ccccc4)cc3)ncnc2cn1 Show InChI InChI=1S/C25H24N6O2/c1-31(2)14-6-9-24(32)30-23-15-21-22(16-26-23)27-17-28-25(21)29-18-10-12-20(13-11-18)33-19-7-4-3-5-8-19/h3-13,15-17H,14H2,1-2H3,(H,26,30,32)(H,27,28,29)/b9-6+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Irreversible inhibition of GST-tagged ERBB4 (unknown origin) (Gly-259 to Gly-690 residues) expressed in baculovirus infected Sf9 insect cells assesse... |
J Med Chem 59: 8103-24 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00883
BindingDB Entry DOI: 10.7270/Q2FF3VB3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data