

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3604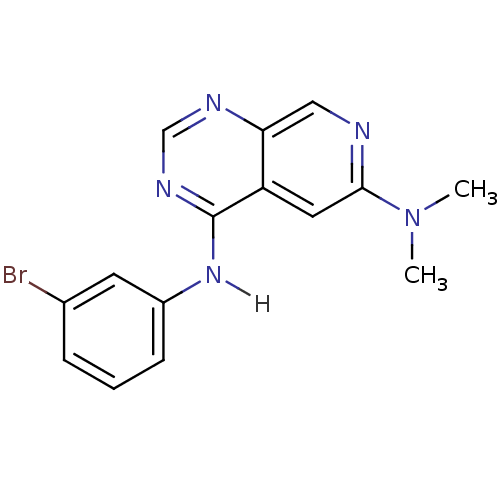 (4-N-(3-bromophenyl)-6-N,6-N-dimethylpyrido[3,4-d]p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3603 (4-N-(3-bromophenyl)-6-N-methylpyrido[3,4-d]pyrimid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3595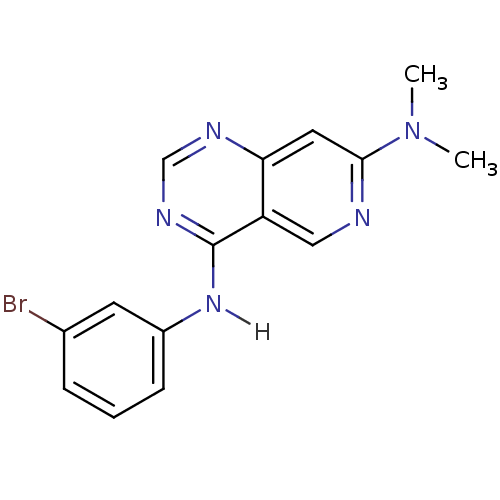 (4-N-(3-bromophenyl)-7-N,7-N-dimethylpyrido[4,3-d]p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3915-25 (1997) Article DOI: 10.1021/jm970366v BindingDB Entry DOI: 10.7270/Q2NS0S2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3594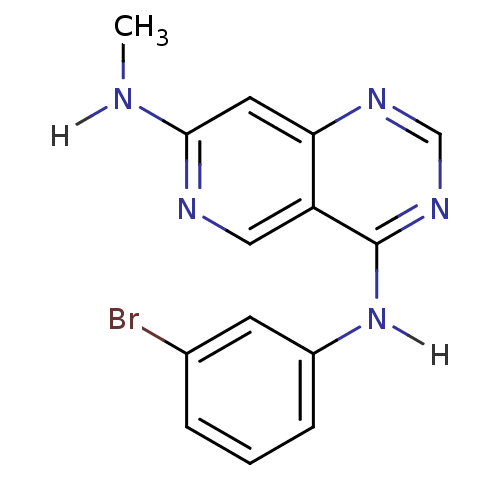 (4-N-(3-bromophenyl)-7-N-methylpyrido[4,3-d]pyrimid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3915-25 (1997) Article DOI: 10.1021/jm970366v BindingDB Entry DOI: 10.7270/Q2NS0S2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3600 (4-N-(3-bromophenyl)pyrido[3,4-d]pyrimidine-4,6-dia...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4780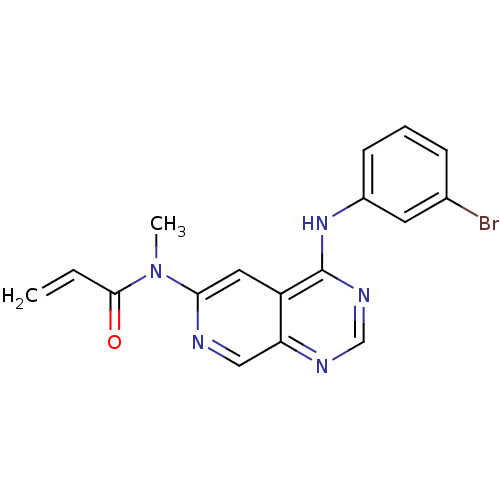 (4-Anilinopyrido[3,4-d]pyrimidine 7 | N-[4-(3-Bromo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description Enzyme assays for IC50 determinations were performed in 96-well filter plates. IC50 is the inhibitor concentration which inhibits 50% of kinase activ... | J Med Chem 44: 429-40 (2001) Article DOI: 10.1021/jm000372i BindingDB Entry DOI: 10.7270/Q2HX19WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3702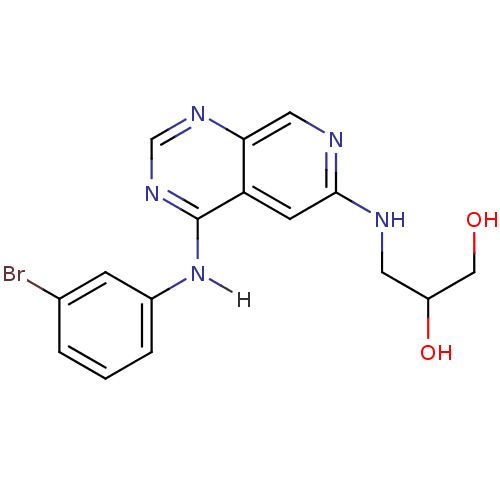 (3-({4-[(3-bromophenyl)amino]pyrido[3,4-d]pyrimidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3700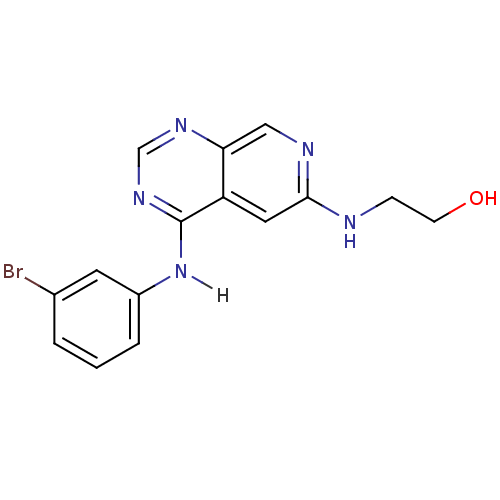 (2-({4-[(3-bromophenyl)amino]pyrido[3,4-d]pyrimidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3724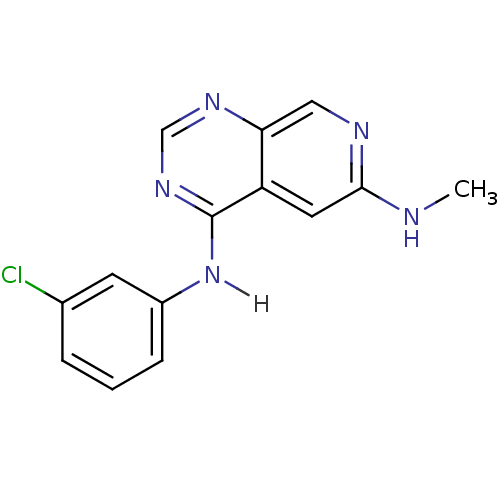 (4-N-(3-chlorophenyl)-6-N-methylpyrido[3,4-d]pyrimi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477513 (CHEMBL392246) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4566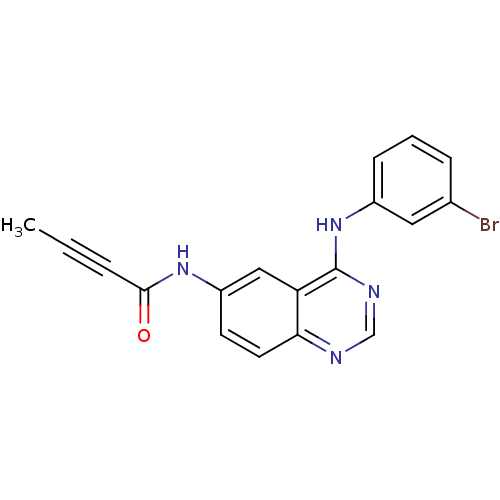 (4-anilinoquinazoline deriv. 1 | CHEMBL91867 | N-{4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB1 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3701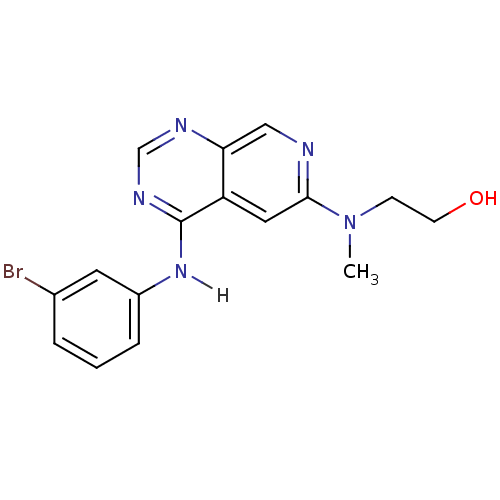 (2-({4-[(3-bromophenyl)amino]pyrido[3,4-d]pyrimidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3646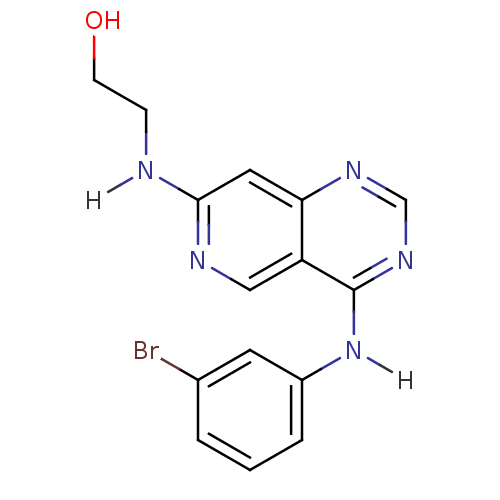 (2-({4-[(3-bromophenyl)amino]pyrido[4,3-d]pyrimidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3915-25 (1997) Article DOI: 10.1021/jm970366v BindingDB Entry DOI: 10.7270/Q2NS0S2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50077239 (CHEMBL52913 | N-[4-(3-Chloro-phenylamino)-quinazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of phosphorylation of glutamic acid/tyrosine random copolymer by isolated epidermal growth factor receptor (EGFR) | J Med Chem 42: 1803-15 (1999) Article DOI: 10.1021/jm9806603 BindingDB Entry DOI: 10.7270/Q2B56HZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3722 (3-({4-[(3-bromophenyl)amino]pyrido[3,4-d]pyrimidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3671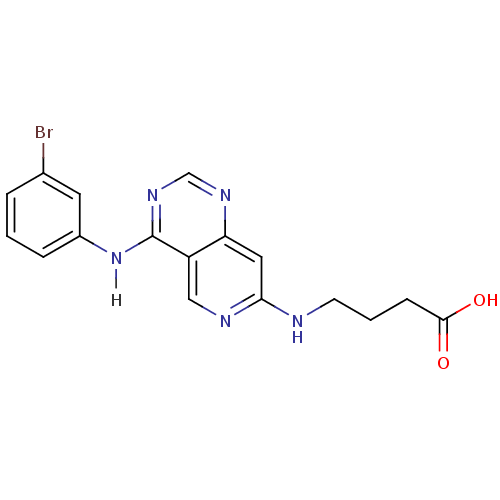 (4-({4-[(3-bromophenyl)amino]pyrido[4,3-d]pyrimidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3915-25 (1997) Article DOI: 10.1021/jm970366v BindingDB Entry DOI: 10.7270/Q2NS0S2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3720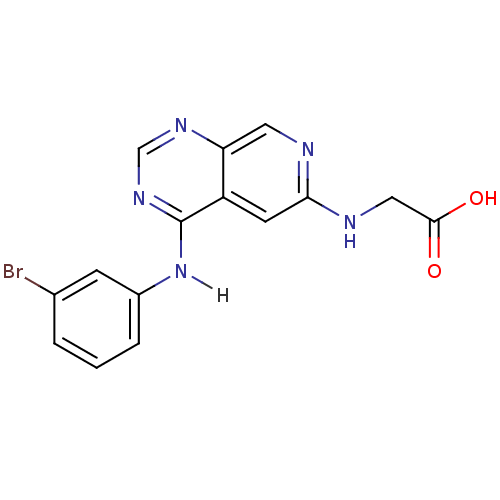 (2-({4-[(3-bromophenyl)amino]pyrido[3,4-d]pyrimidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477516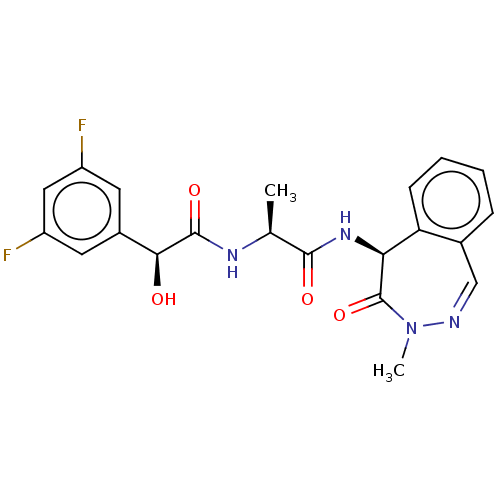 (BMS-433796 | CHEMBL247361) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50182693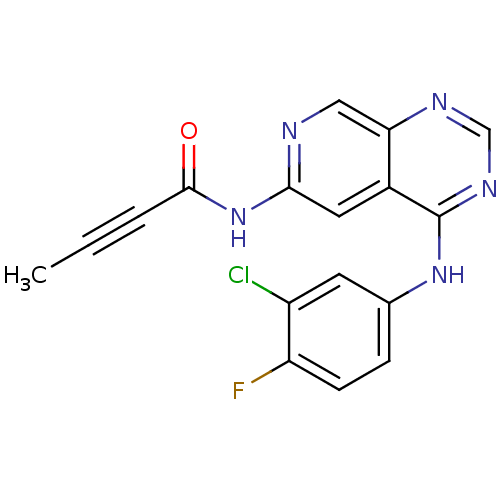 (CHEMBL203644 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB1 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3714 (2-{[3-({4-[(3-bromophenyl)amino]pyrido[3,4-d]pyrim...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4791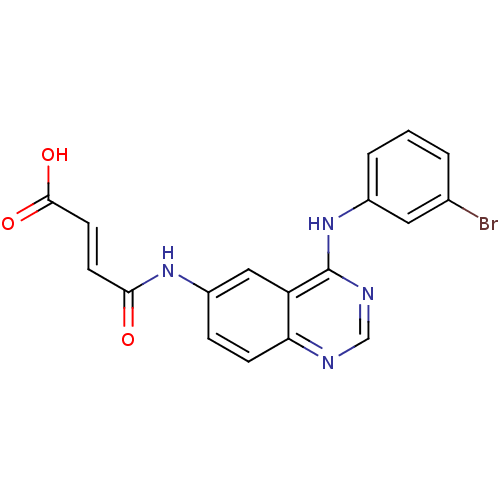 ((2E)-3-({4-[(3-bromophenyl)amino]quinazolin-6-yl}c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description Enzyme assays for IC50 determinations were performed in 96-well filter plates. IC50 is the inhibitor concentration which inhibits 50% of kinase activ... | J Med Chem 44: 429-40 (2001) Article DOI: 10.1021/jm000372i BindingDB Entry DOI: 10.7270/Q2HX19WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50077247 (CHEMBL51741 | N-[4-(6-Bromo-2,3-dihydro-indol-1-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of phosphorylation of glutamic acid/tyrosine random copolymer by isolated epidermal growth factor receptor (EGFR) | J Med Chem 42: 1803-15 (1999) Article DOI: 10.1021/jm9806603 BindingDB Entry DOI: 10.7270/Q2B56HZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477528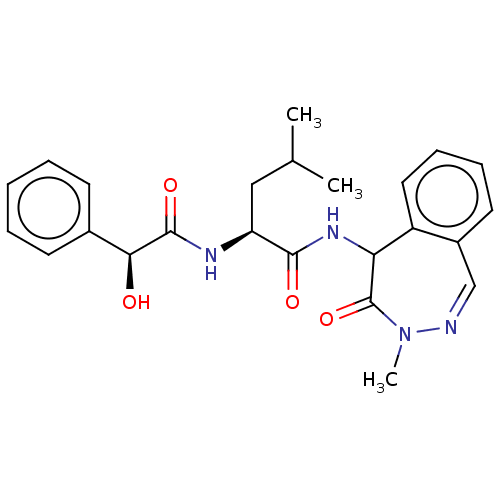 (CHEMBL397844) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50077244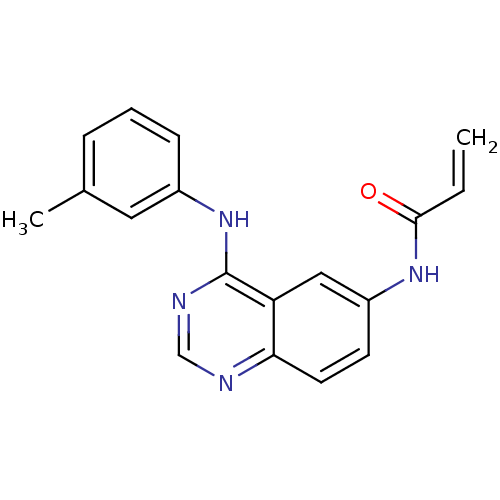 (CHEMBL31815 | N-(4-m-Tolylamino-quinazolin-6-yl)-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of phosphorylation of glutamic acid/tyrosine random copolymer by isolated epidermal growth factor receptor (EGFR) | J Med Chem 42: 1803-15 (1999) Article DOI: 10.1021/jm9806603 BindingDB Entry DOI: 10.7270/Q2B56HZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4808 (4-Anilinopyrido[3,4-d]pyrimidine 35 | N-(3-Bromoph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description Enzyme assays for IC50 determinations were performed in 96-well filter plates. IC50 is the inhibitor concentration which inhibits 50% of kinase activ... | J Med Chem 44: 429-40 (2001) Article DOI: 10.1021/jm000372i BindingDB Entry DOI: 10.7270/Q2HX19WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3721 (2-({4-[(3-bromophenyl)amino]pyrido[3,4-d]pyrimidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4798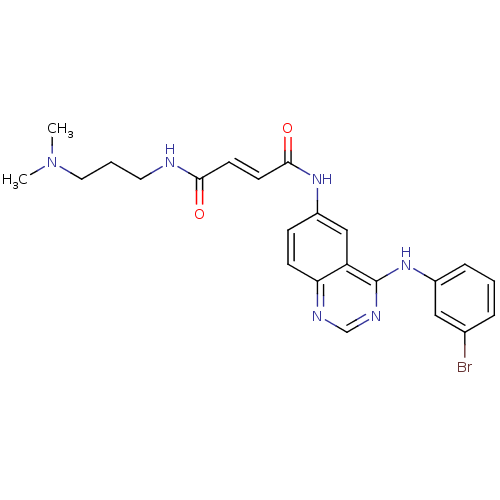 ((2E)-N-{4-[(3-bromophenyl)amino]quinazolin-6-yl}-N...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description Enzyme assays for IC50 determinations were performed in 96-well filter plates. IC50 is the inhibitor concentration which inhibits 50% of kinase activ... | J Med Chem 44: 429-40 (2001) Article DOI: 10.1021/jm000372i BindingDB Entry DOI: 10.7270/Q2HX19WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50077233 (CHEMBL443523 | N-(4-(3-bromophenylamino) quinazoli...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of phosphorylation of glutamic acid/tyrosine random copolymer by isolated epidermal growth factor receptor (EGFR) | J Med Chem 42: 1803-15 (1999) Article DOI: 10.1021/jm9806603 BindingDB Entry DOI: 10.7270/Q2B56HZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3727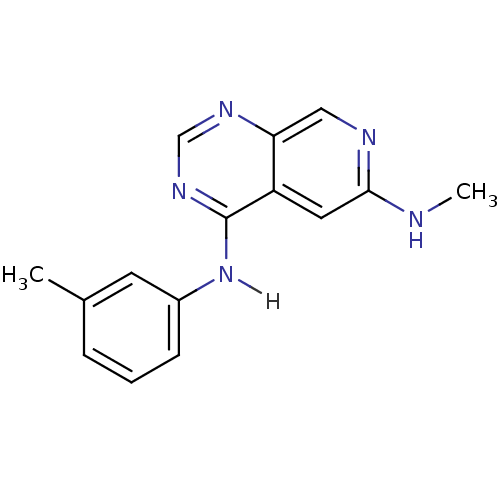 (6-(Methylamino)-4-[(3-methylphenyl)amino]pyrido[3,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50077236 (CHEMBL54088 | N-(4-m-Tolylamino-pyrido[3,4-d]pyrim...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of phosphorylation of glutamic acid/tyrosine random copolymer by isolated epidermal growth factor receptor (EGFR) | J Med Chem 42: 1803-15 (1999) Article DOI: 10.1021/jm9806603 BindingDB Entry DOI: 10.7270/Q2B56HZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50182697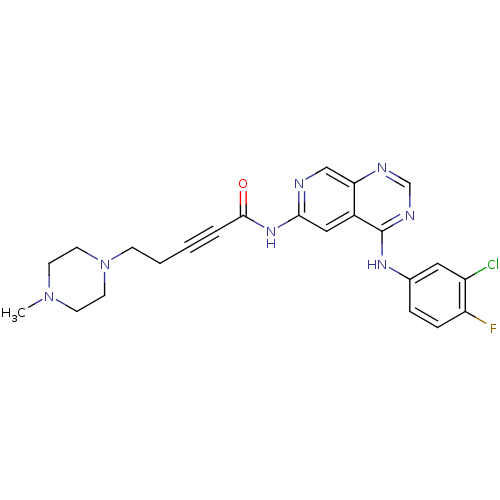 (CHEMBL203599 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB1 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50182684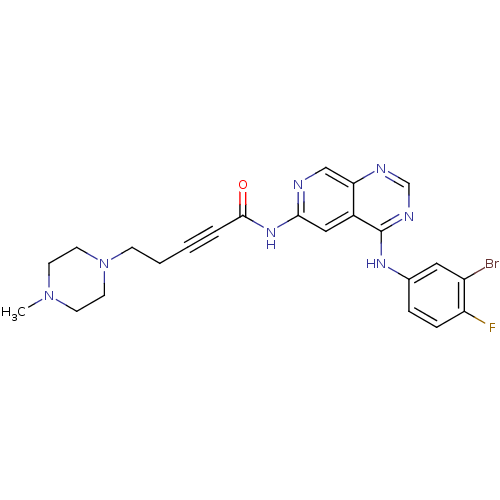 (CHEMBL437890 | N-[4-[(3-bromo-4-fluorophenyl)amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB2 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4783 ((2E)-N-[4-(3-Bromoanilino)pyrido[3,4-d]pyrimidin-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description Enzyme assays for IC50 determinations were performed in 96-well filter plates. IC50 is the inhibitor concentration which inhibits 50% of kinase activ... | J Med Chem 44: 429-40 (2001) Article DOI: 10.1021/jm000372i BindingDB Entry DOI: 10.7270/Q2HX19WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477531 (CHEMBL396808) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50182688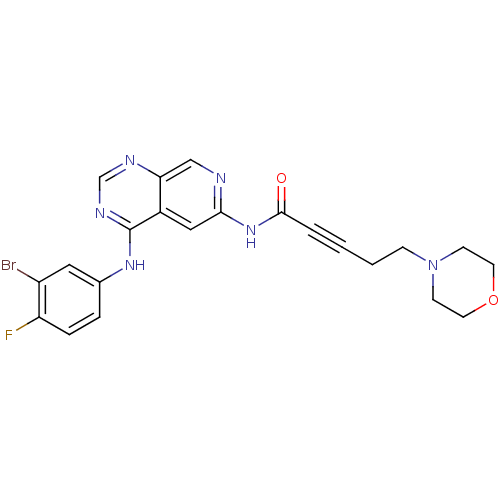 (CHEMBL204638 | N-[4-[(3-bromo-4-fluorophenyl)amino...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of HER stimulated human erbB autophosphorylation in MDA-MB-453 cells | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM50182693 (CHEMBL203644 | N-[4-[(3-chloro-4-fluorophenyl)amin...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB4 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3667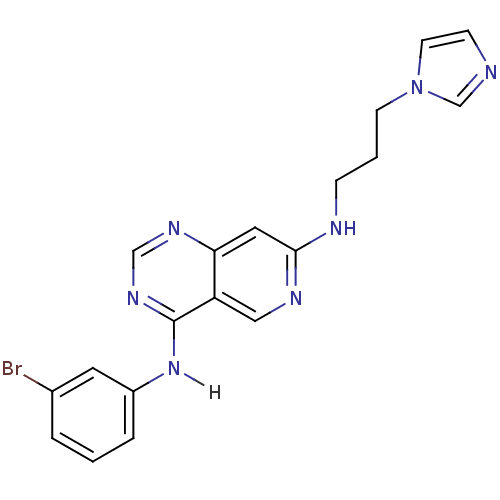 (4-N-(3-bromophenyl)-7-N-[3-(1H-imidazol-1-yl)propy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3915-25 (1997) Article DOI: 10.1021/jm970366v BindingDB Entry DOI: 10.7270/Q2NS0S2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50077246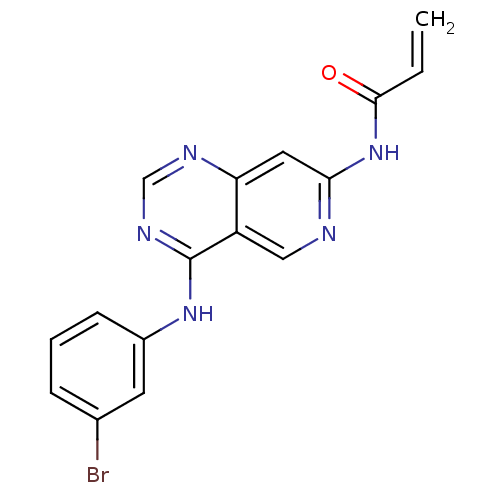 (CHEMBL49986 | N-[4-(3-Bromo-phenylamino)-pyrido[4,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of phosphorylation of glutamic acid/tyrosine random copolymer by isolated epidermal growth factor receptor (EGFR) | J Med Chem 42: 1803-15 (1999) Article DOI: 10.1021/jm9806603 BindingDB Entry DOI: 10.7270/Q2B56HZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4596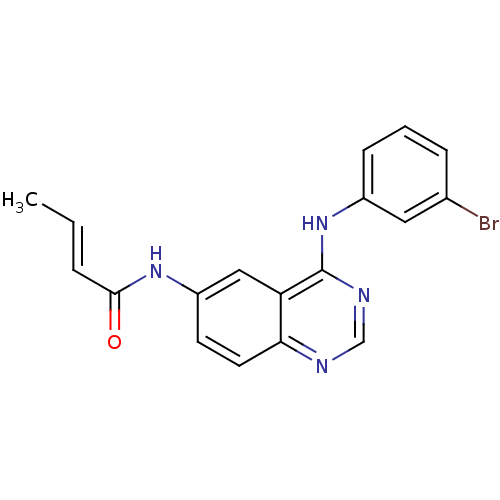 ((2E)-N-{4-[(3-bromophenyl)amino]quinazolin-6-yl}bu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description Enzyme assays for IC50 determinations were performed in 96-well filter plates. IC50 is the inhibitor concentration which inhibits 50% of kinase activ... | J Med Chem 44: 429-40 (2001) Article DOI: 10.1021/jm000372i BindingDB Entry DOI: 10.7270/Q2HX19WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3703 (3-({4-[(3-bromophenyl)amino]pyrido[3,4-d]pyrimidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4802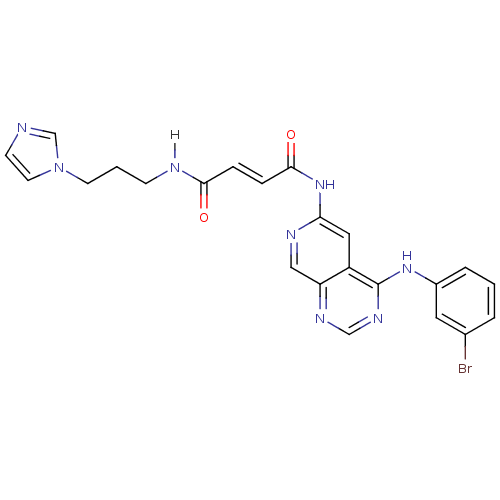 ((2E)-N-{4-[(3-bromophenyl)amino]pyrido[3,4-d]pyrim...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description Enzyme assays for IC50 determinations were performed in 96-well filter plates. IC50 is the inhibitor concentration which inhibits 50% of kinase activ... | J Med Chem 44: 429-40 (2001) Article DOI: 10.1021/jm000372i BindingDB Entry DOI: 10.7270/Q2HX19WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM5446 (CHEMBL553 | ERLOTINIB HYDROCHLORIDE | Erlotinib | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB1 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50182689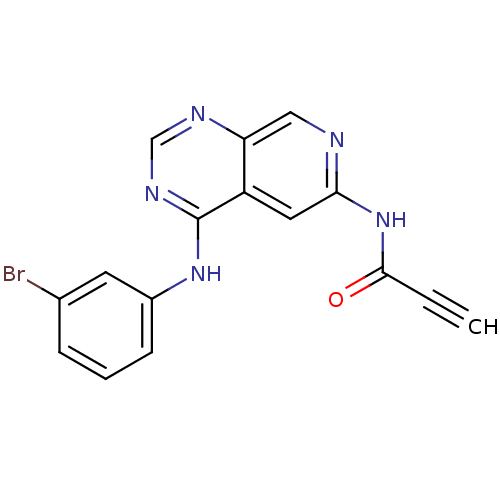 (CHEMBL378144 | N-[4-[(3-bromophenyl)amino]pyrido[3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of erbB1 fusion protein expressed in baculovirus by ELISA | J Med Chem 49: 1475-85 (2006) Article DOI: 10.1021/jm050936o BindingDB Entry DOI: 10.7270/Q2ZS2X96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3670 (3-({4-[(3-bromophenyl)amino]pyrido[4,3-d]pyrimidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 40: 3915-25 (1997) Article DOI: 10.1021/jm970366v BindingDB Entry DOI: 10.7270/Q2NS0S2V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4804 ((2E)-N-{4-[(3-chloro-4-fluorophenyl)amino]pyrido[3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland | Assay Description Enzyme assays for IC50 determinations were performed in 96-well filter plates. IC50 is the inhibitor concentration which inhibits 50% of kinase activ... | J Med Chem 44: 429-40 (2001) Article DOI: 10.1021/jm000372i BindingDB Entry DOI: 10.7270/Q2HX19WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3710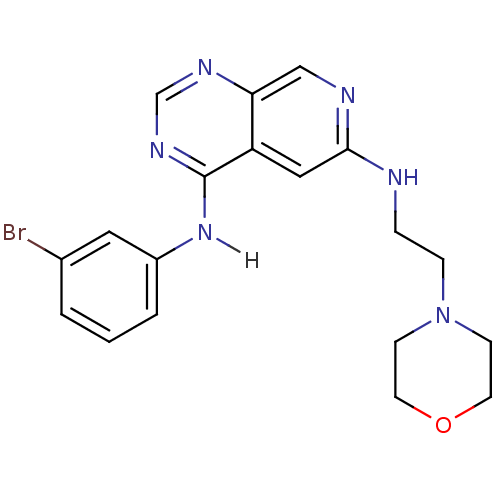 (4-N-(3-bromophenyl)-6-N-[2-(morpholin-4-yl)ethyl]p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of Auckland | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | J Med Chem 41: 742-51 (1998) Article DOI: 10.1021/jm970641d BindingDB Entry DOI: 10.7270/Q2DB800T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50077238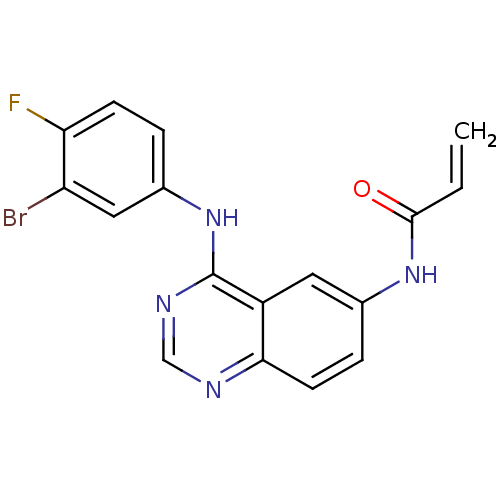 (CHEMBL280757 | N-[4-(3-Bromo-4-fluoro-phenylamino)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of phosphorylation of glutamic acid/tyrosine random copolymer by isolated epidermal growth factor receptor (EGFR) | J Med Chem 42: 1803-15 (1999) Article DOI: 10.1021/jm9806603 BindingDB Entry DOI: 10.7270/Q2B56HZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4785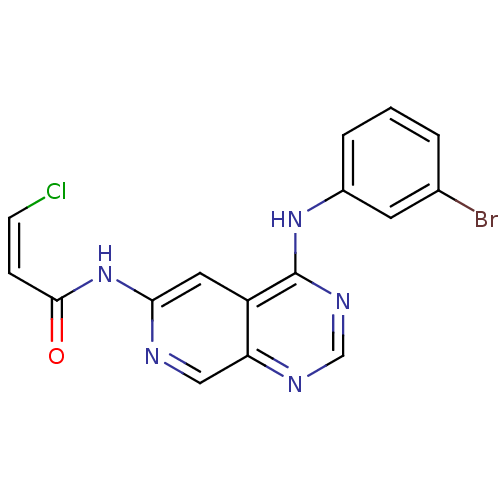 ((2Z)-N-[4-(3-Bromoanilino)-6-pyrido[3,4-d]pyrimidi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Auckland | Assay Description Enzyme assays for IC50 determinations were performed in 96-well filter plates. IC50 is the inhibitor concentration which inhibits 50% of kinase activ... | J Med Chem 44: 429-40 (2001) Article DOI: 10.1021/jm000372i BindingDB Entry DOI: 10.7270/Q2HX19WB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50477536 (CHEMBL247155) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of gamma secretase assessed as reduction of amyloid beta level in H4 cells | Bioorg Med Chem Lett 17: 4006-11 (2007) Article DOI: 10.1016/j.bmcl.2007.04.082 BindingDB Entry DOI: 10.7270/Q2XD14GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4567 (4-anilinoquinazoline deriv. 2 | BMC163482 Compound...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of phosphorylation of glutamic acid/tyrosine random copolymer by isolated epidermal growth factor receptor (EGFR) | J Med Chem 42: 1803-15 (1999) Article DOI: 10.1021/jm9806603 BindingDB Entry DOI: 10.7270/Q2B56HZG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 738 total ) | Next | Last >> |