

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Squalene synthase (Rattus norvegicus) | BDBM50038096 ((6R,7R)-1-((4S,5R)-4-Acetoxy-5-methyl-3-methylene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against squalene synthase in rat liver squalene synthase (RLSS) enzyme assay | Bioorg Med Chem Lett 4: 1591-1594 (1994) Article DOI: 10.1016/S0960-894X(01)80572-2 BindingDB Entry DOI: 10.7270/Q2TB16T4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125155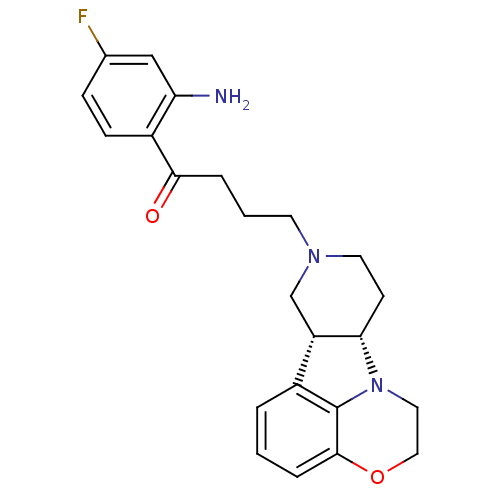 (1-(2-Amino-4-fluoro-phenyl)-4-(6bR,10aS)-1,2,6b,9,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50383163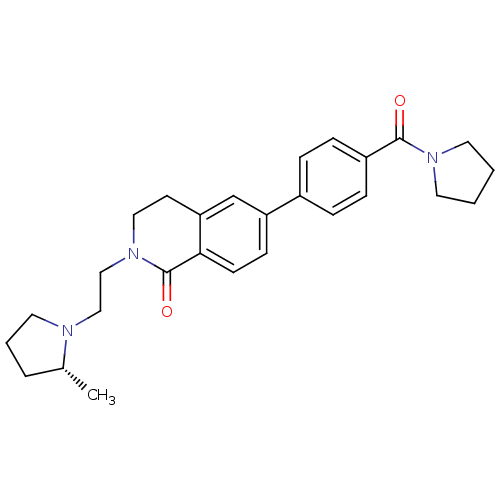 (CHEMBL2031885) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting | J Med Chem 55: 2452-68 (2012) Article DOI: 10.1021/jm300011d BindingDB Entry DOI: 10.7270/Q2736RZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50334730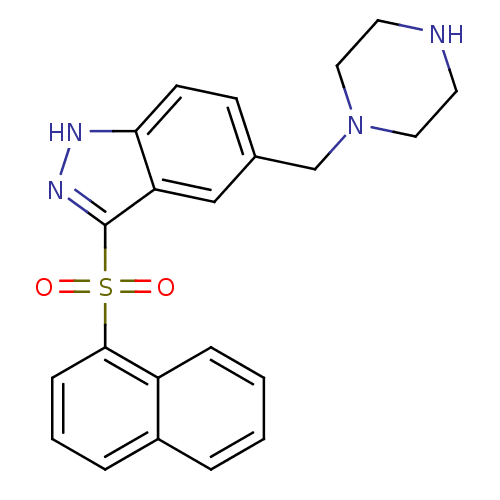 (3-(Naphthalen-1-ylsulfonyl)-5-(piperazin-1-ylmethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells | Bioorg Med Chem 19: 650-62 (2011) Article DOI: 10.1016/j.bmc.2010.10.033 BindingDB Entry DOI: 10.7270/Q29S1R9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50316949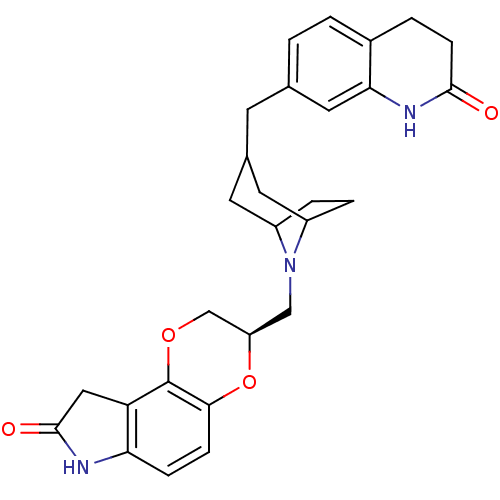 ((3R)-3-((3-((2-oxo-1,2,3,4-tetrahydroquinolin-7-yl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]citalopram form human SRET by liquid scintillation counting | Bioorg Med Chem Lett 20: 2983-6 (2010) Article DOI: 10.1016/j.bmcl.2010.02.105 BindingDB Entry DOI: 10.7270/Q2VH5P0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50383144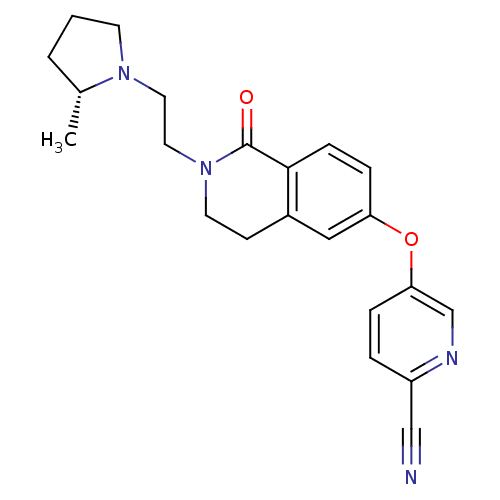 (CHEMBL2031864) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting | J Med Chem 55: 2452-68 (2012) Article DOI: 10.1021/jm300011d BindingDB Entry DOI: 10.7270/Q2736RZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM35226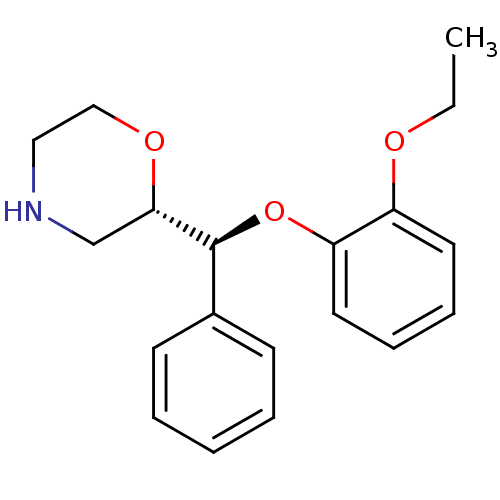 ((S,S)-reboxetine | Reboxetine | Vestra) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 0.300 | -56.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Wyeth Research | Assay Description Compounds were evaluated the inhibition of [3H] nisoxetine binding to MDCK-Net6 cells, stably transfected with the human norepinephrine transporter (... | Bioorg Med Chem 17: 7802-15 (2009) Article DOI: 10.1016/j.bmc.2009.09.023 BindingDB Entry DOI: 10.7270/Q26Q1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50300820 (CHEMBL565723 | N-(2-{3-[(3-Chlorophenyl)sulfonyl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in HeLa cells | Bioorg Med Chem 17: 5153-63 (2009) Article DOI: 10.1016/j.bmc.2009.05.055 BindingDB Entry DOI: 10.7270/Q25T3KJS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125161 (1-(2-Amino-4-fluoro-phenyl)-4-(6bR,10aS)-1,2,6b,9,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50354216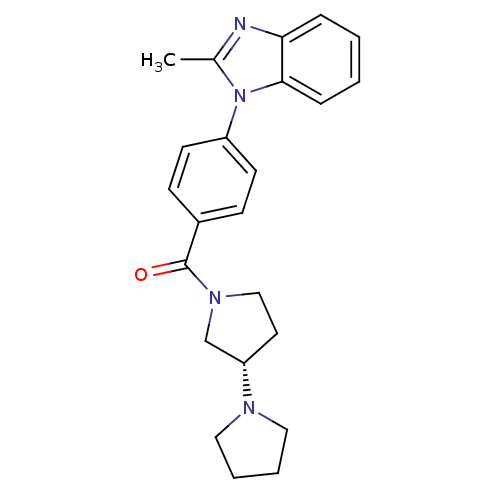 (CHEMBL1836004) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)alpha-methylhistamine from human histamine H3 receptor expressed in human HEK293T cells | Bioorg Med Chem Lett 21: 5957-60 (2011) Article DOI: 10.1016/j.bmcl.2011.07.061 BindingDB Entry DOI: 10.7270/Q2QN6753 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316673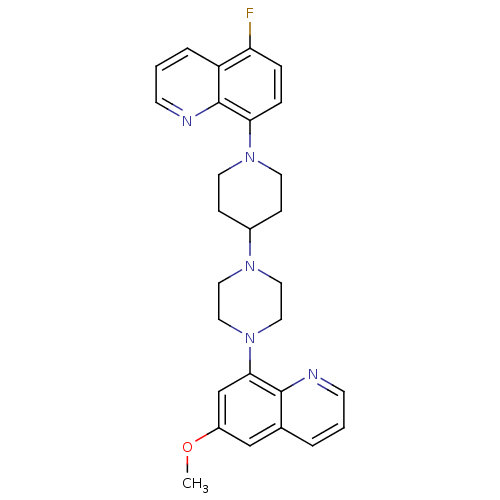 (5-Fluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50354216 (CHEMBL1836004) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting | J Med Chem 55: 2452-68 (2012) Article DOI: 10.1021/jm300011d BindingDB Entry DOI: 10.7270/Q2736RZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50302220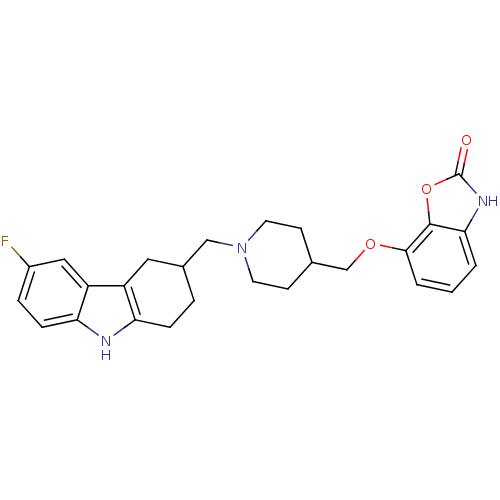 (7-((1-((6-fluoro-2,3,4,9-tetrahydro-1H-carbazol-3-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from human serotonin transporter expressed in HEK293 cells by scintillation proximity assay | Bioorg Med Chem Lett 19: 5552-5 (2009) Article DOI: 10.1016/j.bmcl.2009.08.050 BindingDB Entry DOI: 10.7270/Q2Z89DCC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125173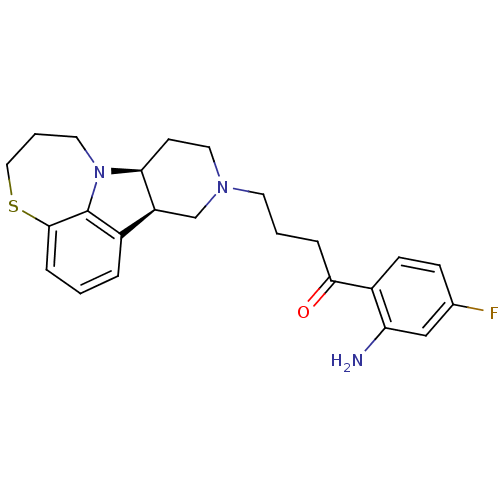 (1-(2-Amino-4-fluoro-phenyl)-4-(7bS,11aR)-6,7,8,9,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316677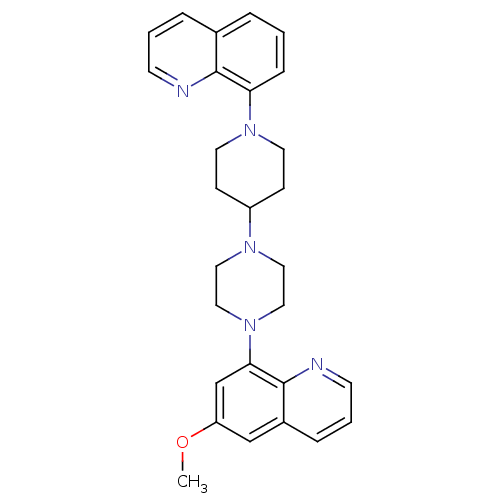 (6-Methoxy-8-{4-[1-(8-quinolinyl)-4-piperidinyl]-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316680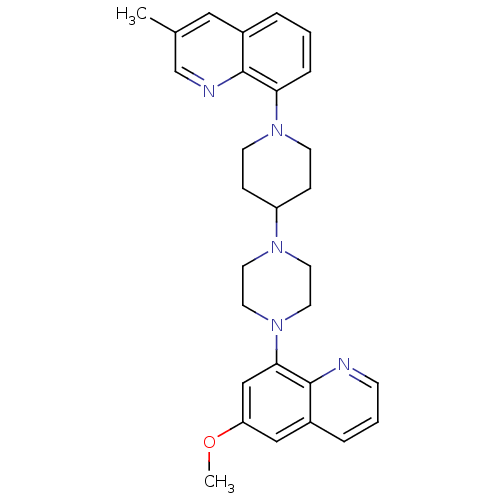 (8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316684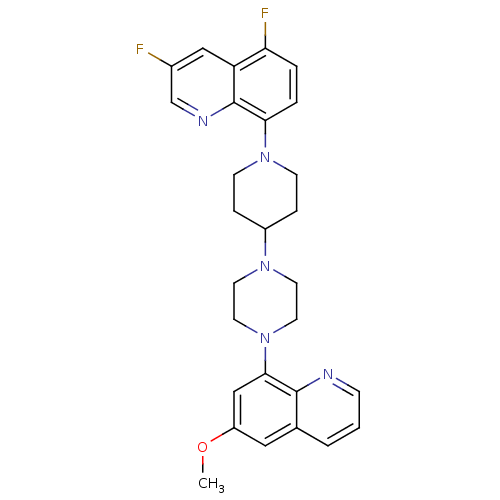 (3,5-Difluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piper...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125154 (1-(2-Amino-4-fluoro-phenyl)-4-(7aS,11aR)-5,6,8,9,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM35255 (2-Chlor-11-(2-dimethylaminoaethoxy)-dibenzo(b,f)-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Compounds were evaluated the inhibition of [3H] ketanserin binding to membranes from CHO cells, stably transfected with the human 5-HT2A receptor. Da... | Bioorg Med Chem 17: 7802-15 (2009) Article DOI: 10.1016/j.bmc.2009.09.023 BindingDB Entry DOI: 10.7270/Q26Q1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM28574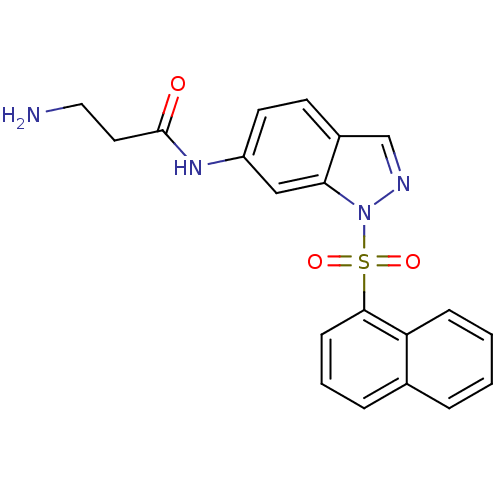 (1-sulfonylindazole, 6c | 3-amino-N-[1-(naphthalene...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description Competition experiments were performed in the presence radioligand with membrane protein (obtained from cells expressing the receptor) and test compo... | Bioorg Med Chem Lett 19: 2413-5 (2009) Article DOI: 10.1016/j.bmcl.2009.03.071 BindingDB Entry DOI: 10.7270/Q28K77CF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50308167 ((3R)-N,N-Dimethyl-1-[3-(1-naphthylsulfonyl)-1H-ind...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5HT6 receptor by scintillation counting | J Med Chem 53: 2521-7 (2010) Article DOI: 10.1021/jm901674f BindingDB Entry DOI: 10.7270/Q22B8Z43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316688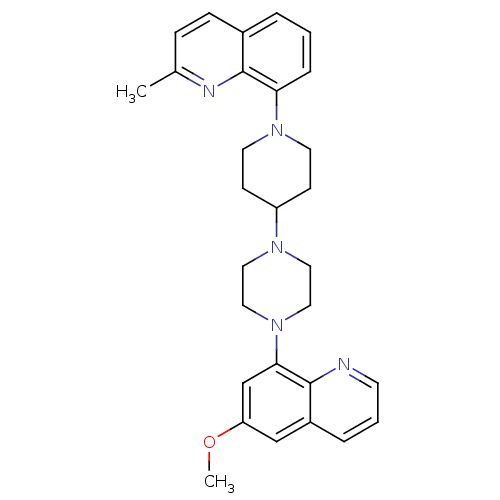 (8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50007659 (CHEMBL3233142) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc. Curated by ChEMBL | Assay Description Displacement of [125]DOI from human recombinant full length 5HT2A receptor expressed in HEK293E cells | J Med Chem 57: 2670-82 (2014) Article DOI: 10.1021/jm401958n BindingDB Entry DOI: 10.7270/Q27D2WNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50383142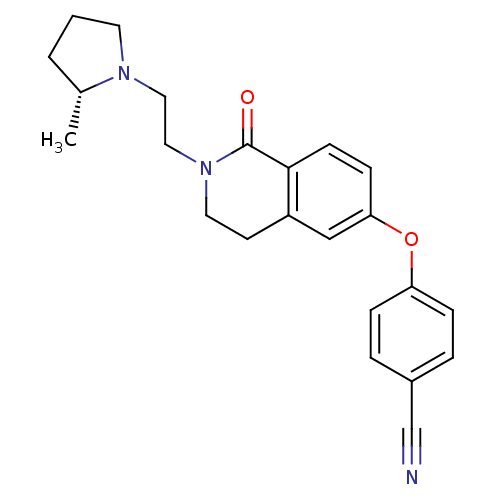 (CHEMBL2031862) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting | J Med Chem 55: 2452-68 (2012) Article DOI: 10.1021/jm300011d BindingDB Entry DOI: 10.7270/Q2736RZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125170 (1-(2-Amino-4-fluoro-phenyl)-4-(7bS,11aR)-4,5,6,7,8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent noradrenaline transporter (Homo sapiens (Human)) | BDBM35229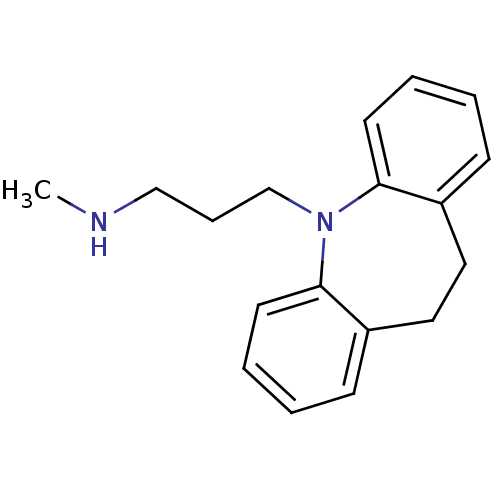 (3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.600 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Wyeth Research | Assay Description Compounds were evaluated the inhibition of [3H] nisoxetine binding to MDCK-Net6 cells, stably transfected with the human norepinephrine transporter (... | Bioorg Med Chem 17: 7802-15 (2009) Article DOI: 10.1016/j.bmc.2009.09.023 BindingDB Entry DOI: 10.7270/Q26Q1VK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50334734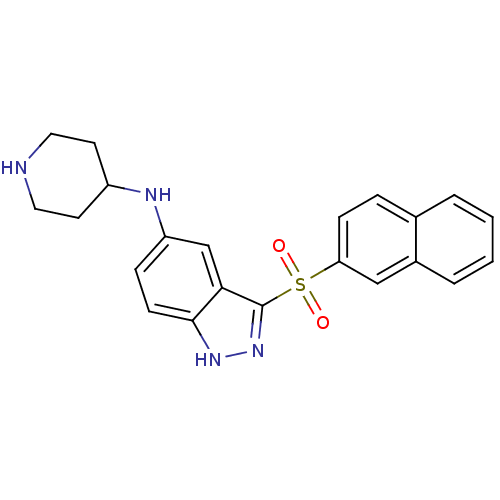 (3-(Naphthalen-2-ylsulfonyl)-N-(piperidin-4-yl)-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells | Bioorg Med Chem 19: 650-62 (2011) Article DOI: 10.1016/j.bmc.2010.10.033 BindingDB Entry DOI: 10.7270/Q29S1R9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125174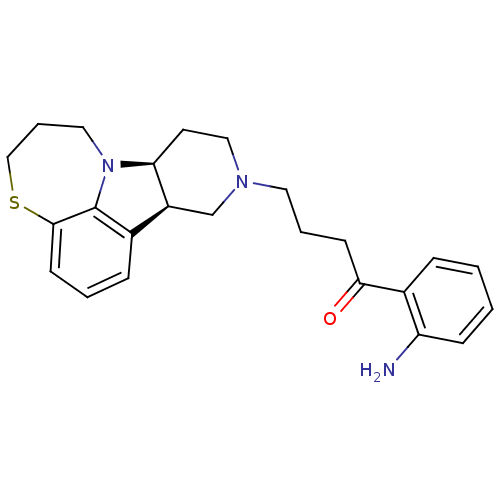 (1-(2-Amino-phenyl)-4-(7bS,11aR)-6,7,8,9,11,11a-hex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316690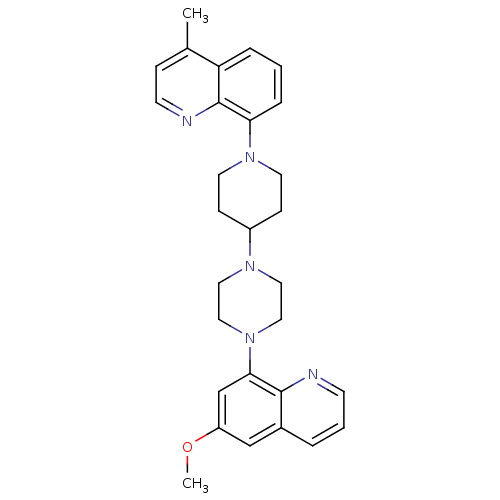 (8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316682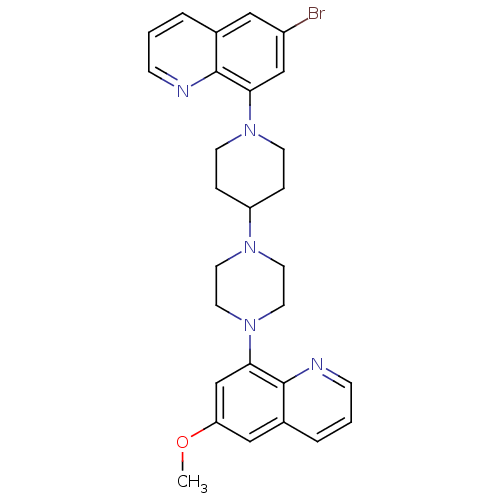 (6-Bromo-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316678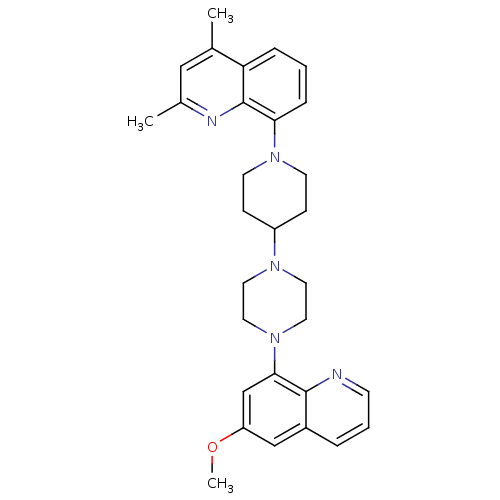 (8-{4-[4-(6-Methoxyquinolin-8-yl)piperazin-1-yl]pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50217902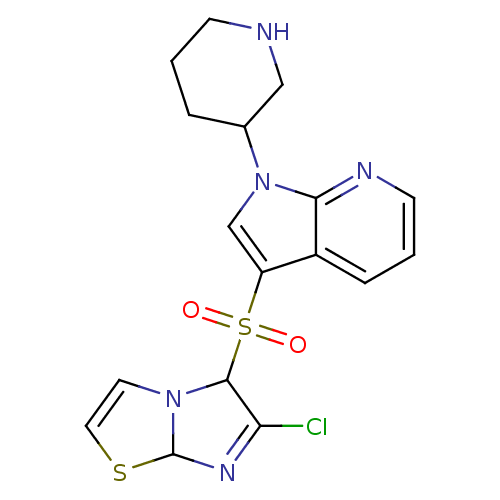 (3-[(6-chloroimidazo[2,1-b][1,3]thiazol-5-yl)sulfon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]LSD from cloned human 5HT6 expressed in HeLa cells | Bioorg Med Chem 15: 6208-26 (2007) Article DOI: 10.1016/j.bmc.2007.06.024 BindingDB Entry DOI: 10.7270/Q2HT2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50217922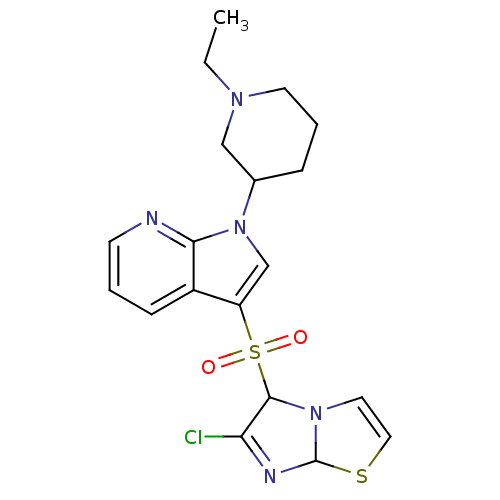 (3-[(6-chloroimidazo[2,1-b][1,3]thiazol-5-yl)sulfon...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]LSD from cloned human 5HT6 expressed in HeLa cells | Bioorg Med Chem 15: 6208-26 (2007) Article DOI: 10.1016/j.bmc.2007.06.024 BindingDB Entry DOI: 10.7270/Q2HT2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125173 (1-(2-Amino-4-fluoro-phenyl)-4-(7bS,11aR)-6,7,8,9,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards DA D2 receptor using [3H]-N-methyl-spiperone as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125163 (1-(2-Amino-phenyl)-4-(7bS,11aR)-4,5,6,7,8,9,11,11a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50334731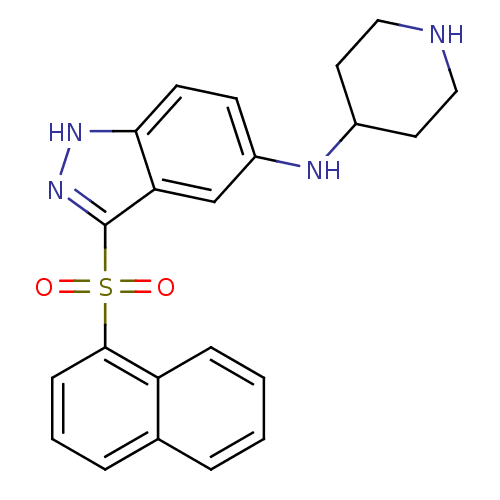 (CHEMBL1642851 | [3-(Naphthalen-1-sulfonyl)-1H-inda...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells | Bioorg Med Chem 19: 650-62 (2011) Article DOI: 10.1016/j.bmc.2010.10.033 BindingDB Entry DOI: 10.7270/Q29S1R9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316679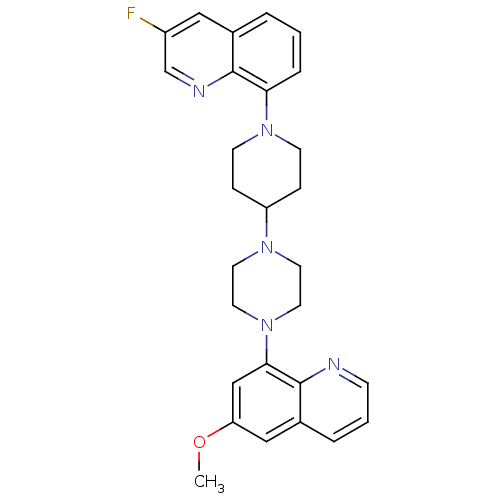 (3-Fluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125169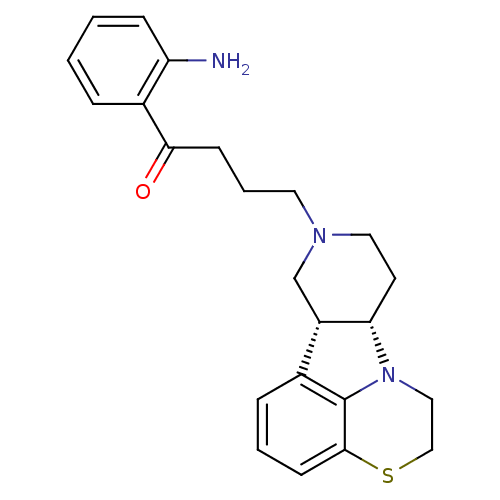 (1-(2-Amino-phenyl)-4-(6bR,10aS)-1,2,6b,9,10,10a-he...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50217909 (3-(6-chloro-imidazo[2,1-b]thiazole-5-sulfonyl)-1-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]LSD from cloned human 5HT6 expressed in HeLa cells | Bioorg Med Chem 15: 6208-26 (2007) Article DOI: 10.1016/j.bmc.2007.06.024 BindingDB Entry DOI: 10.7270/Q2HT2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125177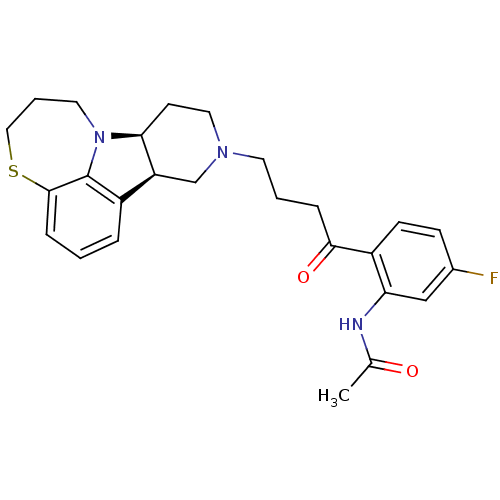 (CHEMBL162768 | N-[5-Fluoro-2-((7bS,11aR)-4-6,7,8,9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50383138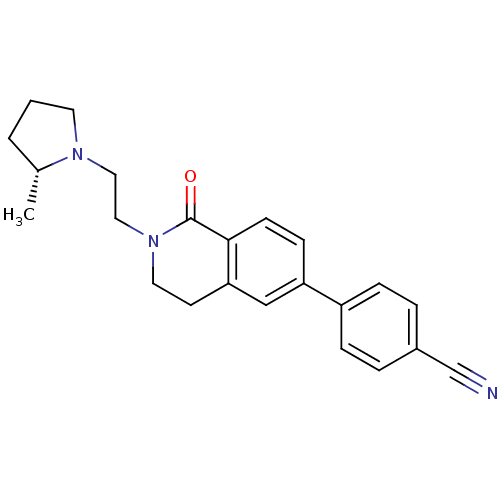 (CHEMBL2031762) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting | J Med Chem 55: 2452-68 (2012) Article DOI: 10.1021/jm300011d BindingDB Entry DOI: 10.7270/Q2736RZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50334754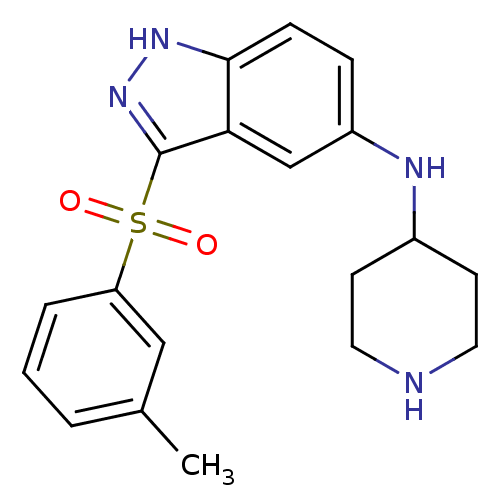 (CHEMBL1642883 | N-(Piperidin-4-yl)-3-(m-tolylsulfo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from cloned human 5-HT6 receptor expressed in human HeLa cells | Bioorg Med Chem 19: 650-62 (2011) Article DOI: 10.1016/j.bmc.2010.10.033 BindingDB Entry DOI: 10.7270/Q29S1R9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125151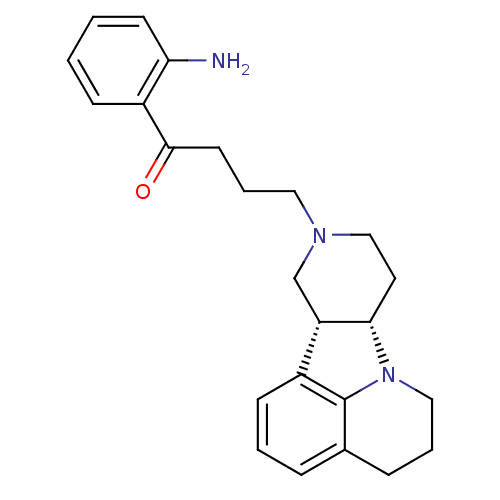 (1-(2-Amino-phenyl)-4-(7aS,11aR)-5,6,8,9,11,11a-hex...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50383167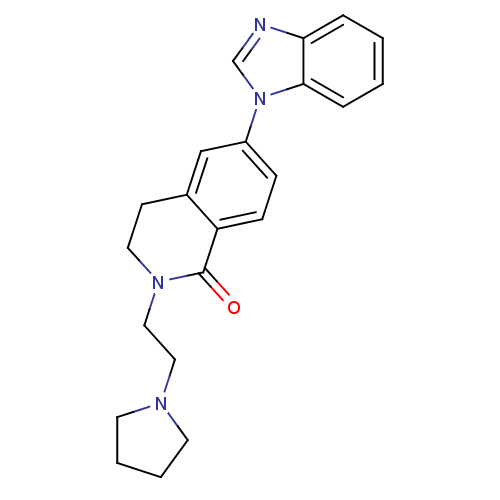 (CHEMBL2031741) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting | J Med Chem 55: 2452-68 (2012) Article DOI: 10.1021/jm300011d BindingDB Entry DOI: 10.7270/Q2736RZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50316683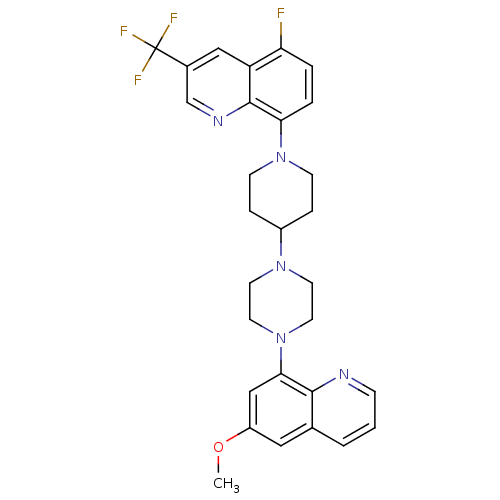 (5-Fluoro-8-{4-[4-(6-methoxyquinolin-8-yl)piperazin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5HT1A receptor expressed in CHO cells | J Med Chem 53: 4066-84 (2010) Article DOI: 10.1021/jm1000908 BindingDB Entry DOI: 10.7270/Q28P60P7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50007666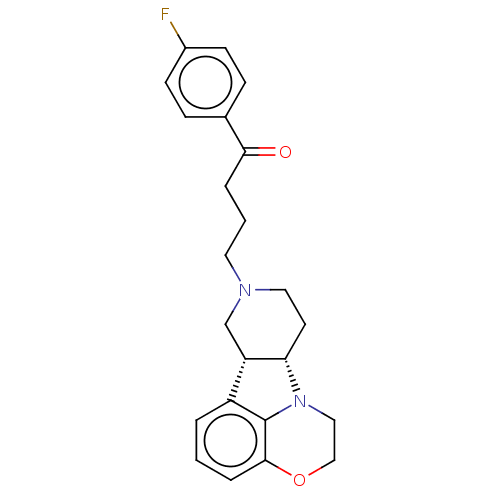 (CHEMBL3233432) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Intra-Cellular Therapies, Inc. Curated by ChEMBL | Assay Description Displacement of [125]DOI from human recombinant full length 5HT2A receptor expressed in HEK293E cells | J Med Chem 57: 2670-82 (2014) Article DOI: 10.1021/jm401958n BindingDB Entry DOI: 10.7270/Q27D2WNZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50383153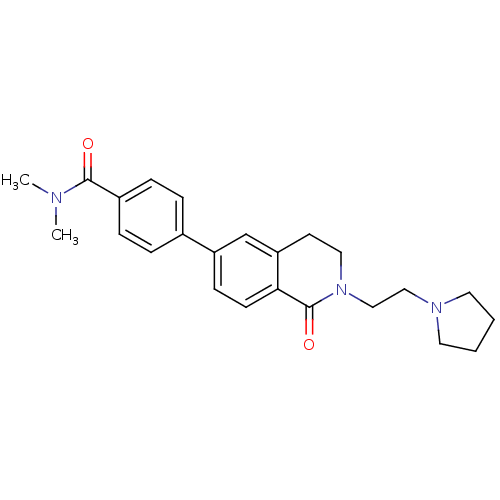 (CHEMBL2031874) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-(R)-alpha-methylhistamine from human H3 receptor expressed in HEK293T cells after 120 mins by scintillation counting | J Med Chem 55: 2452-68 (2012) Article DOI: 10.1021/jm300011d BindingDB Entry DOI: 10.7270/Q2736RZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50330507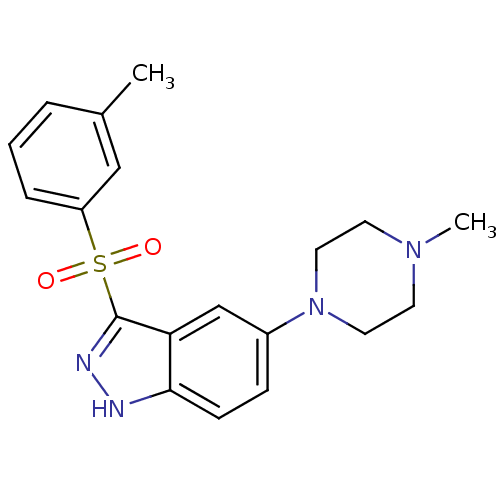 (5-(4-Methylpiperazin-1-yl)-3-(m-tolylsulfonyl)-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human cloned 5HT6 receptor expressed in human HeLa cells | J Med Chem 53: 7639-46 (2010) Article DOI: 10.1021/jm1007825 BindingDB Entry DOI: 10.7270/Q2GQ6XZ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50125150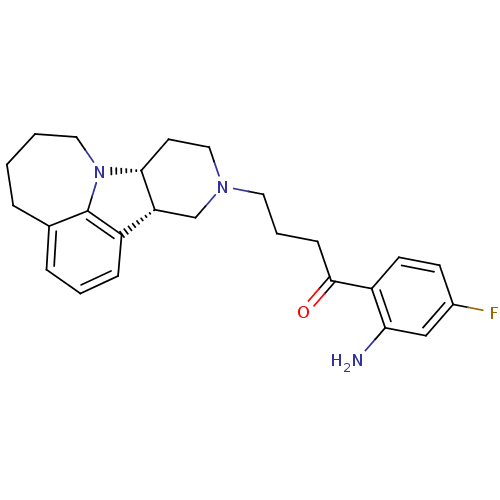 (1-(2-Amino-4-fluoro-phenyl)-4-(7bR,11aS)-4,5,6,7,8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 2A receptor using [125I]-DOI as radioligand. | Bioorg Med Chem Lett 13: 767-70 (2003) BindingDB Entry DOI: 10.7270/Q25H7FNW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50217891 (3-(3-bromo-benzenesulfonyl)-1-(1-methyl-pyrrolidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Displacement of [3H]LSD from cloned human 5HT6 expressed in HeLa cells | Bioorg Med Chem 15: 6208-26 (2007) Article DOI: 10.1016/j.bmc.2007.06.024 BindingDB Entry DOI: 10.7270/Q2HT2P2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3676 total ) | Next | Last >> |