

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4696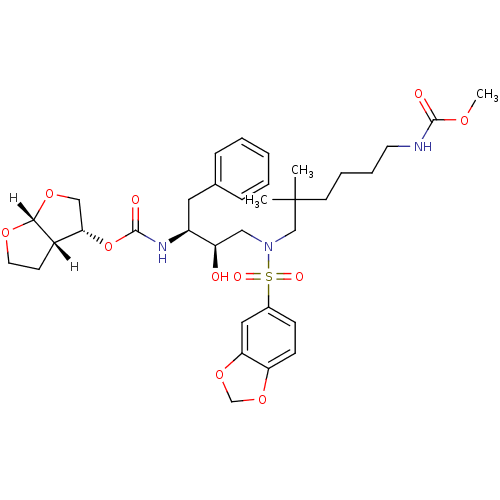 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0000140 | -80.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Bioorg Med Chem Lett 14: 959-63 (2004) Article DOI: 10.1016/j.bmcl.2003.12.008 BindingDB Entry DOI: 10.7270/Q21V5C57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4699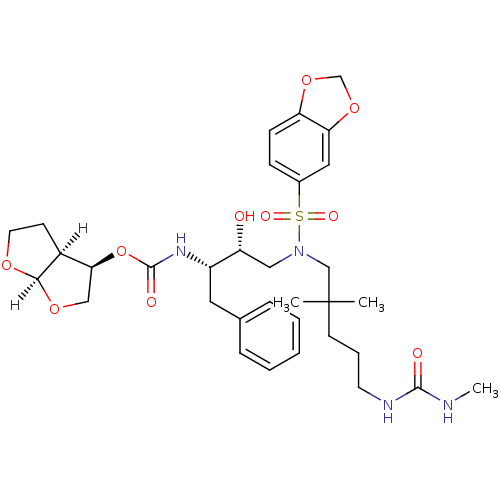 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0000480 | -77.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Bioorg Med Chem Lett 14: 959-63 (2004) Article DOI: 10.1016/j.bmcl.2003.12.008 BindingDB Entry DOI: 10.7270/Q21V5C57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4698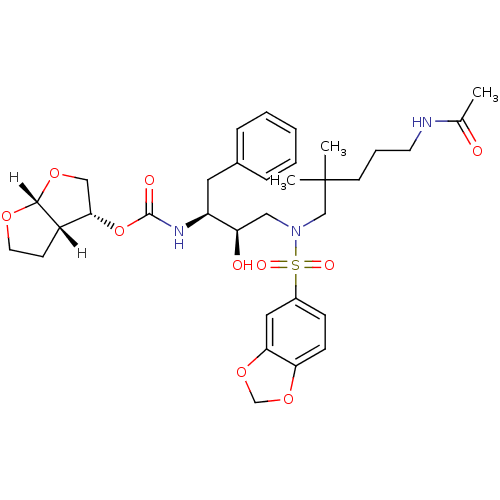 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0000540 | -77.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Bioorg Med Chem Lett 14: 959-63 (2004) Article DOI: 10.1016/j.bmcl.2003.12.008 BindingDB Entry DOI: 10.7270/Q21V5C57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4700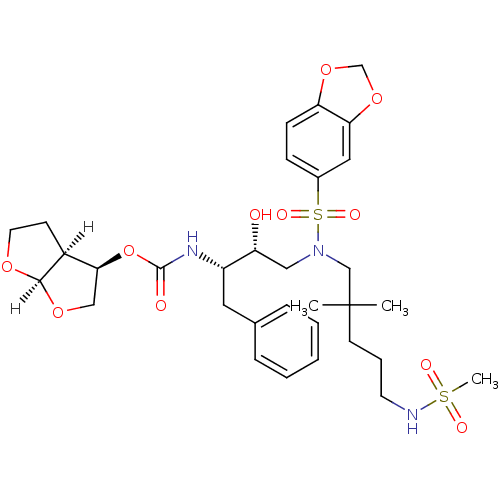 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0000580 | -76.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Bioorg Med Chem Lett 14: 959-63 (2004) Article DOI: 10.1016/j.bmcl.2003.12.008 BindingDB Entry DOI: 10.7270/Q21V5C57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4691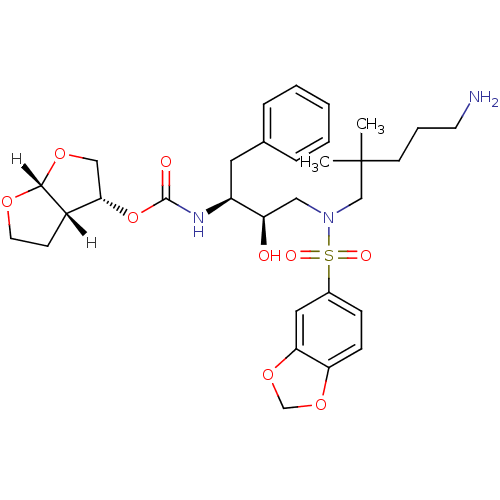 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0000610 | -76.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Bioorg Med Chem Lett 14: 959-63 (2004) Article DOI: 10.1016/j.bmcl.2003.12.008 BindingDB Entry DOI: 10.7270/Q21V5C57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4697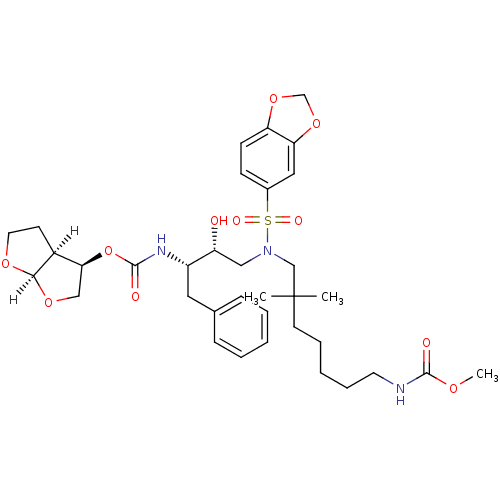 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.000120 | -75.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Bioorg Med Chem Lett 14: 959-63 (2004) Article DOI: 10.1016/j.bmcl.2003.12.008 BindingDB Entry DOI: 10.7270/Q21V5C57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4695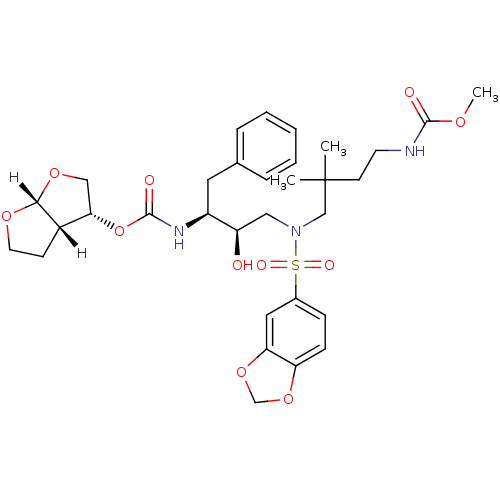 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.000160 | -74.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Bioorg Med Chem Lett 14: 959-63 (2004) Article DOI: 10.1016/j.bmcl.2003.12.008 BindingDB Entry DOI: 10.7270/Q21V5C57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4693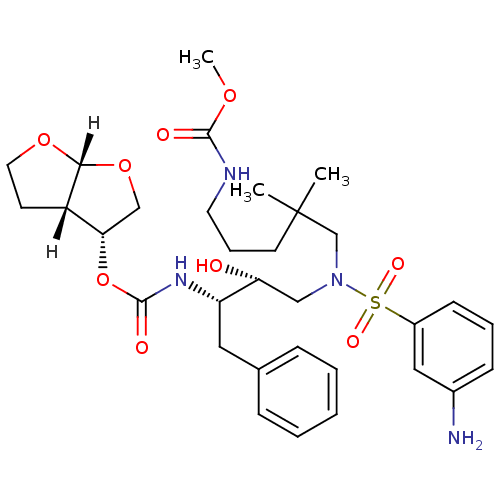 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.000210 | -73.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Bioorg Med Chem Lett 14: 959-63 (2004) Article DOI: 10.1016/j.bmcl.2003.12.008 BindingDB Entry DOI: 10.7270/Q21V5C57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4692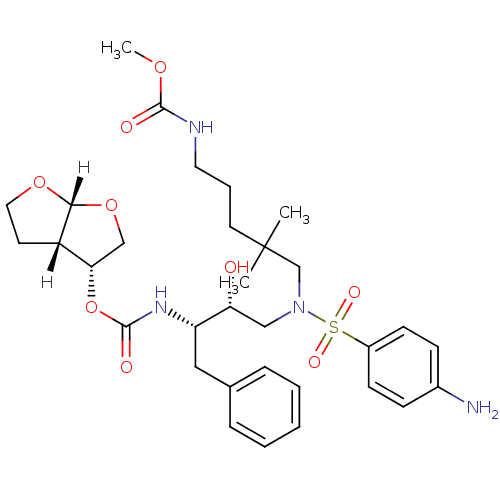 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.000260 | -73.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Bioorg Med Chem Lett 14: 959-63 (2004) Article DOI: 10.1016/j.bmcl.2003.12.008 BindingDB Entry DOI: 10.7270/Q21V5C57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4694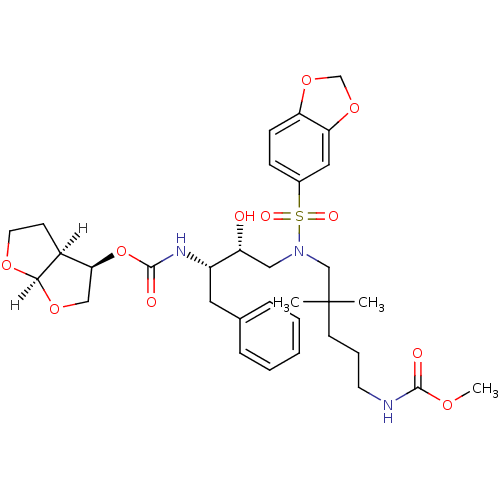 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.000520 | -71.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
GlaxoSmithKline | Assay Description The method was a competitive displacement assay used to determine binding affinities of other inhibitors relative to that of GW0385. The inhibitor of... | Bioorg Med Chem Lett 14: 959-63 (2004) Article DOI: 10.1016/j.bmcl.2003.12.008 BindingDB Entry DOI: 10.7270/Q21V5C57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50366495 ((+)butaclamol | CHEMBL1255588) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]- spiperone radioligand binding at the dopamine binding site of rat caudate | J Med Chem 26: 974-80 (1983) BindingDB Entry DOI: 10.7270/Q2DR2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50437440 (CHEMBL2409219) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human coagulation factor 7A | Bioorg Med Chem Lett 23: 2432-5 (2013) Article DOI: 10.1016/j.bmcl.2013.02.013 BindingDB Entry DOI: 10.7270/Q2R78GN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50437438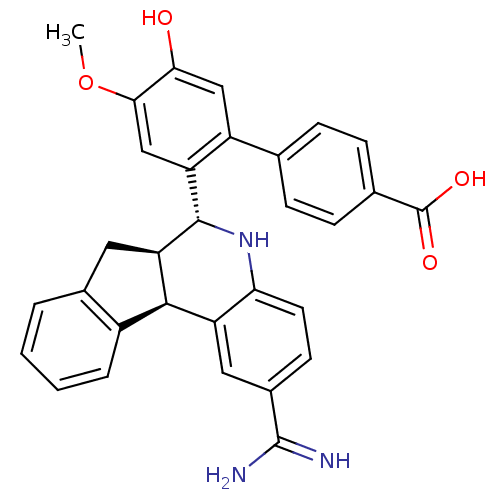 (CHEMBL2409314) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human coagulation factor 7A | Bioorg Med Chem Lett 23: 2432-5 (2013) Article DOI: 10.1016/j.bmcl.2013.02.013 BindingDB Entry DOI: 10.7270/Q2R78GN6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]- spiperone radioligand binding at the dopamine binding site of rat caudate | J Med Chem 26: 974-80 (1983) BindingDB Entry DOI: 10.7270/Q2DR2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007692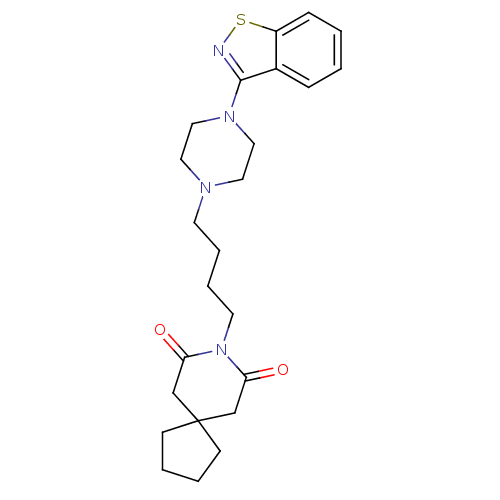 (8-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50048806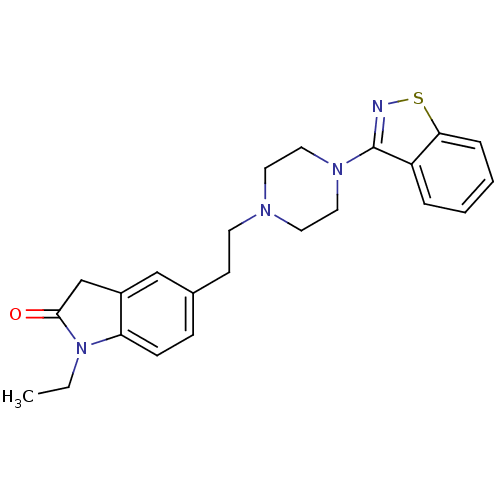 (5-[2-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50048807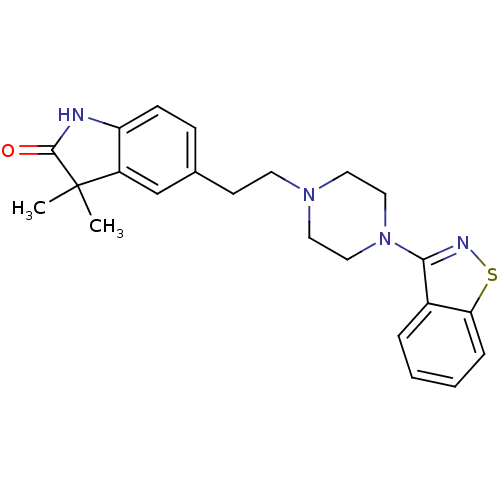 (5-[2-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50048804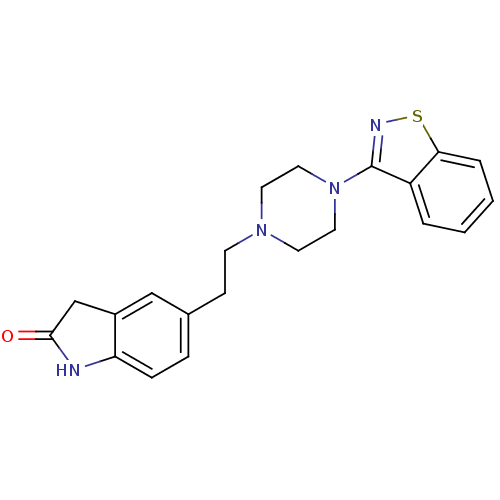 (5-[2-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50017721 (1-Methyl-4-(5H-dibenzo(a,d)cycloheptenylidene)pipe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]- QNB binding at the muscarinic-cholinergic binding site of rat brain S1 | J Med Chem 26: 974-80 (1983) BindingDB Entry DOI: 10.7270/Q2DR2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50048802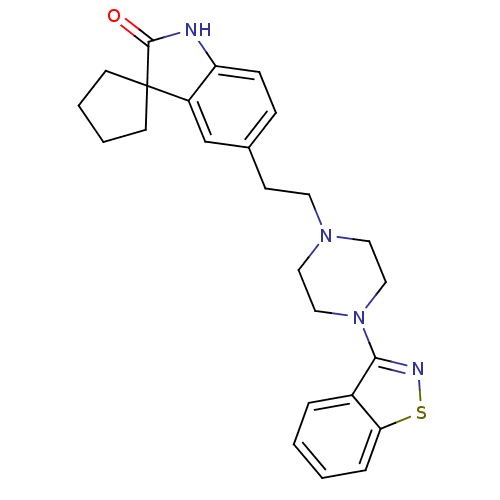 (5'-[2-(4-benzo[d]isothiazol-3-ylhexahydro-1-pyrazi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50048805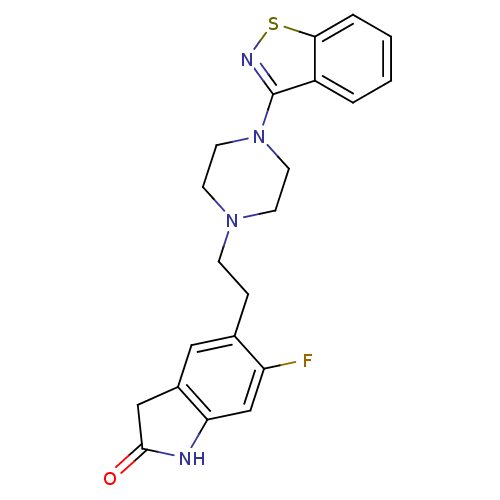 (5-[2-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50048803 (5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50048800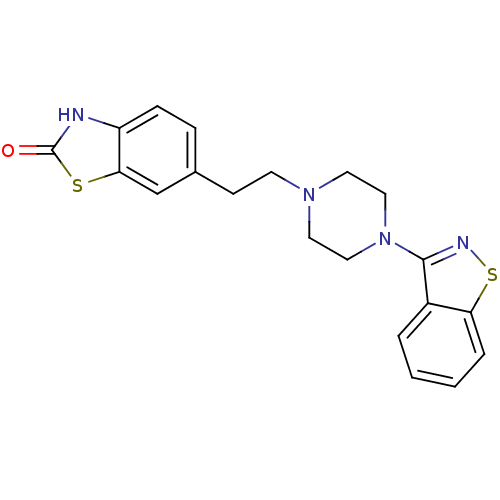 (6-[2-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50130099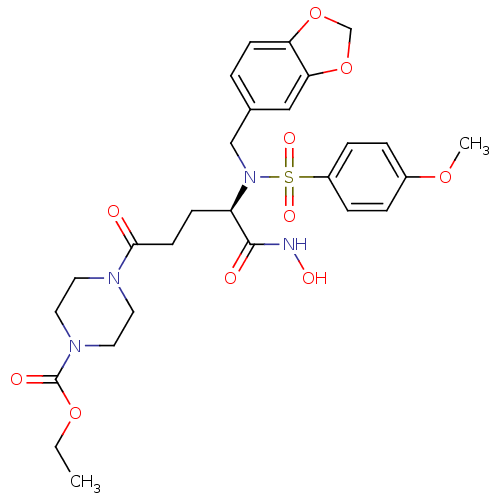 (4-{(R)-4-[Benzo[1,3]dioxol-5-ylmethyl-(4-methoxy-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem Inc. Curated by ChEMBL | Assay Description Inhibition of MMP-8 | Bioorg Med Chem Lett 13: 2381-4 (2003) BindingDB Entry DOI: 10.7270/Q26H4GS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50048801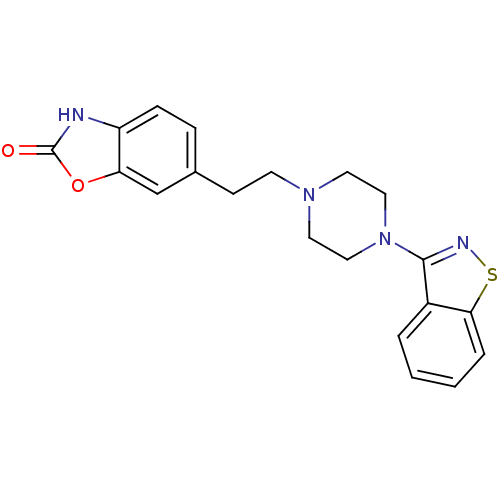 (6-[2-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-NPA from rat brain Dopamine receptor D2 | J Med Chem 39: 143-8 (1996) Article DOI: 10.1021/jm950625l BindingDB Entry DOI: 10.7270/Q2ZG6RB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50026964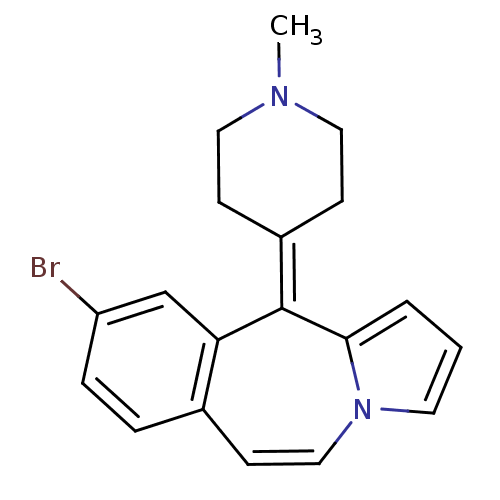 (9-Bromo-11-(1-methyl-piperidin-4-ylidene)-11H-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]- apomorphine radioligand binding at the dopamine binding site of rat caudate | J Med Chem 26: 974-80 (1983) BindingDB Entry DOI: 10.7270/Q2DR2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50026968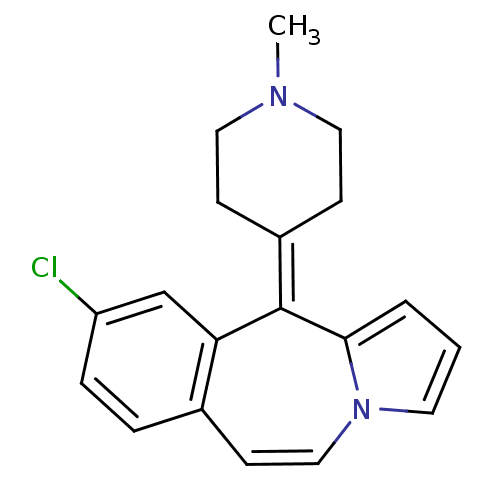 (9-Chloro-11-(1-methyl-piperidin-4-ylidene)-11H-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]- apomorphine radioligand binding at the dopamine binding site of rat caudate | J Med Chem 26: 974-80 (1983) BindingDB Entry DOI: 10.7270/Q2DR2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50129204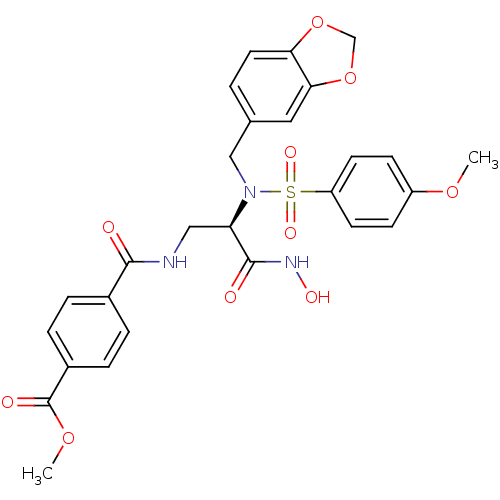 (CHEMBL65208 | N-{2-[Benzo[1,3]dioxol-5-ylmethyl-((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against procollagen C-terminal proteinase (PCP) in HT-1080 cells using synthetic peptide as substrate | Bioorg Med Chem Lett 13: 2101-4 (2003) BindingDB Entry DOI: 10.7270/Q2TD9WQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50026964 (9-Bromo-11-(1-methyl-piperidin-4-ylidene)-11H-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]- spiperone radioligand binding at the dopamine binding site of rat caudate | J Med Chem 26: 974-80 (1983) BindingDB Entry DOI: 10.7270/Q2DR2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50026974 (9-Bromo-11-(1-methyl-piperidin-4-ylidene)-6,11-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]- apomorphine radioligand binding at the dopamine binding site of rat caudate | J Med Chem 26: 974-80 (1983) BindingDB Entry DOI: 10.7270/Q2DR2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50026968 (9-Chloro-11-(1-methyl-piperidin-4-ylidene)-11H-ben...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]LSD radioligand binding at the serotonin-1 binding site of rat cortex | J Med Chem 26: 974-80 (1983) BindingDB Entry DOI: 10.7270/Q2DR2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50026974 (9-Bromo-11-(1-methyl-piperidin-4-ylidene)-6,11-dih...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]LSD radioligand binding at the serotonin-1 binding site of rat cortex | J Med Chem 26: 974-80 (1983) BindingDB Entry DOI: 10.7270/Q2DR2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50129206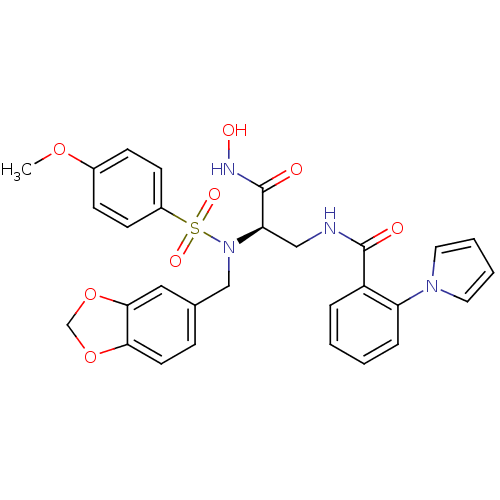 (CHEMBL292073 | N-{2-[Benzo[1,3]dioxol-5-ylmethyl-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against procollagen C-terminal proteinase (PCP) in HT-1080 cells using synthetic peptide as substrate | Bioorg Med Chem Lett 13: 2101-4 (2003) BindingDB Entry DOI: 10.7270/Q2TD9WQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50437439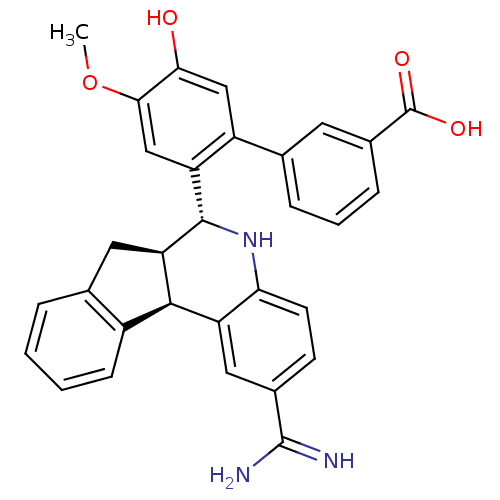 (CHEMBL2409220) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant human coagulation factor 7A | Bioorg Med Chem Lett 23: 2432-5 (2013) Article DOI: 10.1016/j.bmcl.2013.02.013 BindingDB Entry DOI: 10.7270/Q2R78GN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50026970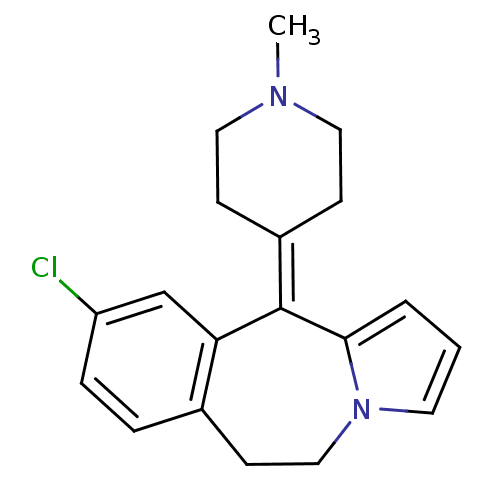 (9-Chloro-11-(1-methyl-piperidin-4-ylidene)-6,11-di...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]LSD radioligand binding at the serotonin-1 binding site of rat cortex | J Med Chem 26: 974-80 (1983) BindingDB Entry DOI: 10.7270/Q2DR2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50026964 (9-Bromo-11-(1-methyl-piperidin-4-ylidene)-11H-benz...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]LSD radioligand binding at the serotonin-1 binding site of rat cortex | J Med Chem 26: 974-80 (1983) BindingDB Entry DOI: 10.7270/Q2DR2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50130108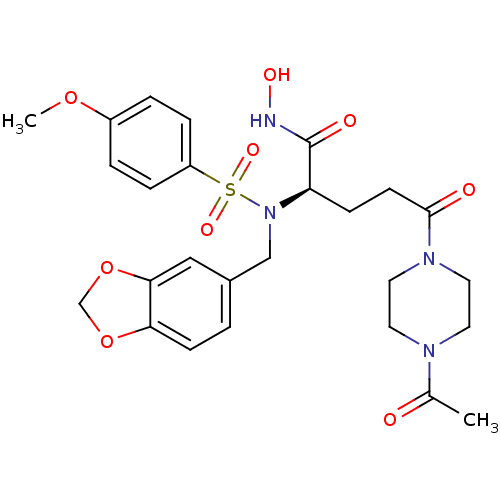 ((R)-5-(4-Acetyl-piperazin-1-yl)-2-[benzo[1,3]dioxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem Inc. Curated by ChEMBL | Assay Description Inhibition of MMP-8 | Bioorg Med Chem Lett 13: 2381-4 (2003) BindingDB Entry DOI: 10.7270/Q26H4GS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50130114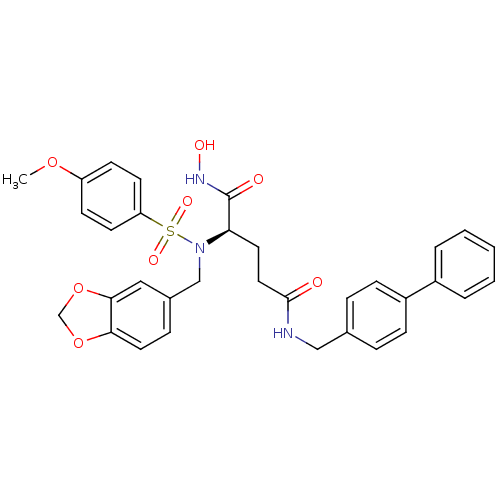 ((R)-2-[Benzo[1,3]dioxol-5-ylmethyl-(4-methoxy-benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem Inc. Curated by ChEMBL | Assay Description Ability to inhibit procollagen C-terminal proteinase (PCP) tested in vitro | Bioorg Med Chem Lett 13: 2381-4 (2003) BindingDB Entry DOI: 10.7270/Q26H4GS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50026974 (9-Bromo-11-(1-methyl-piperidin-4-ylidene)-6,11-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]- spiperone radioligand binding at the dopamine binding site of rat caudate | J Med Chem 26: 974-80 (1983) BindingDB Entry DOI: 10.7270/Q2DR2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50026970 (9-Chloro-11-(1-methyl-piperidin-4-ylidene)-6,11-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]- apomorphine radioligand binding at the dopamine binding site of rat caudate | J Med Chem 26: 974-80 (1983) BindingDB Entry DOI: 10.7270/Q2DR2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50026968 (9-Chloro-11-(1-methyl-piperidin-4-ylidene)-11H-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]- spiperone radioligand binding at the dopamine binding site of rat caudate | J Med Chem 26: 974-80 (1983) BindingDB Entry DOI: 10.7270/Q2DR2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50026971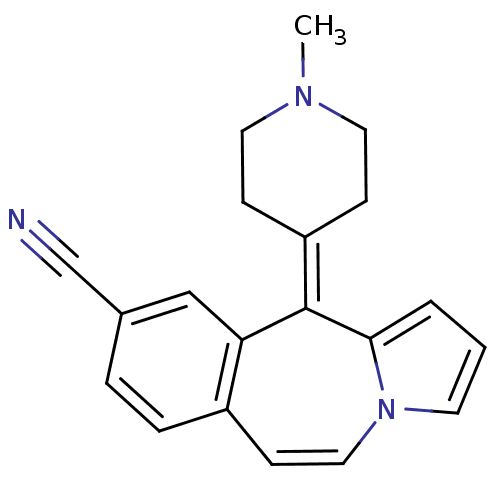 (11-(1-Methyl-piperidin-4-ylidene)-11H-benzo[d]pyrr...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]LSD radioligand binding at the serotonin-1 binding site of rat cortex | J Med Chem 26: 974-80 (1983) BindingDB Entry DOI: 10.7270/Q2DR2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50026973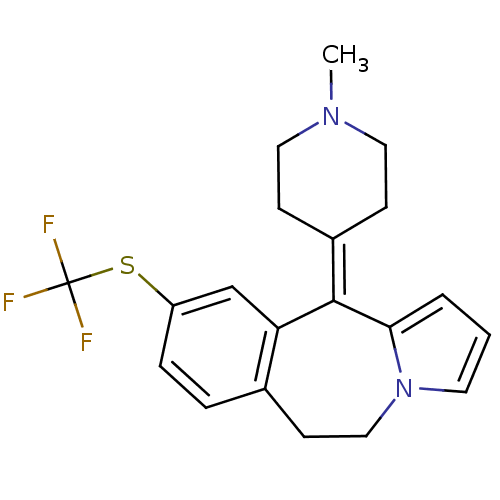 (11-(1-Methyl-piperidin-4-ylidene)-9-trifluoromethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]- spiperone radioligand binding at the dopamine binding site of rat caudate | J Med Chem 26: 974-80 (1983) BindingDB Entry DOI: 10.7270/Q2DR2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (CALF) | BDBM50026967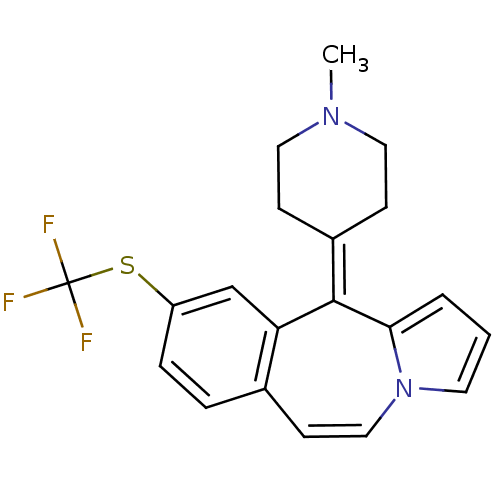 (11-(1-Methyl-piperidin-4-ylidene)-9-trifluoromethy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]- prazosin radioligand binding at the alpha 1-adrenergic binding site of calf cerebral cortex | J Med Chem 26: 974-80 (1983) BindingDB Entry DOI: 10.7270/Q2DR2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50026972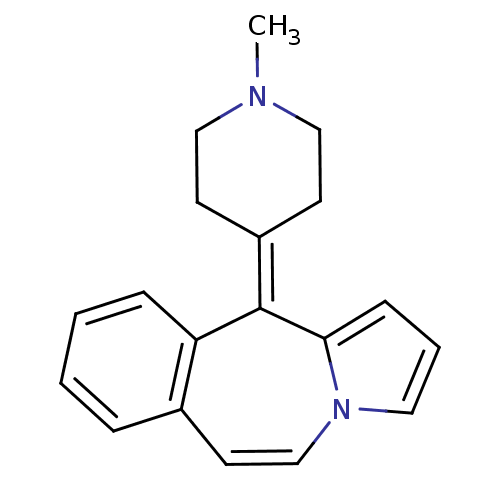 (11-(1-Methyl-piperidin-4-ylidene)-11H-benzo[d]pyrr...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]LSD radioligand binding at the serotonin-1 binding site of rat cortex | J Med Chem 26: 974-80 (1983) BindingDB Entry DOI: 10.7270/Q2DR2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50129212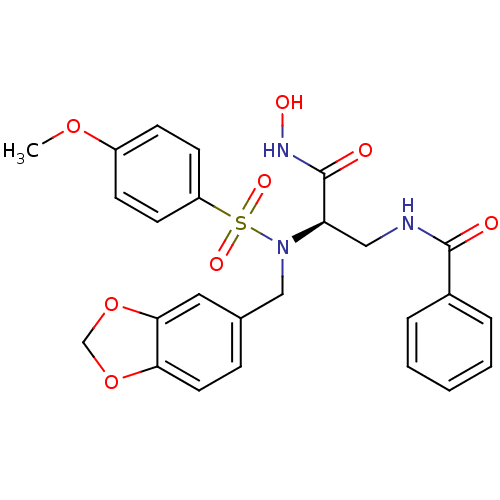 (CHEMBL294742 | N-{2-[Benzo[1,3]dioxol-5-ylmethyl-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against procollagen C-terminal proteinase (PCP) in HT-1080 cells using synthetic peptide as substrate | Bioorg Med Chem Lett 13: 2101-4 (2003) BindingDB Entry DOI: 10.7270/Q2TD9WQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A/1B/1D/1F (RAT-Rattus norvegicus (rat)-Rattus norvegicus (Rat...) | BDBM50026963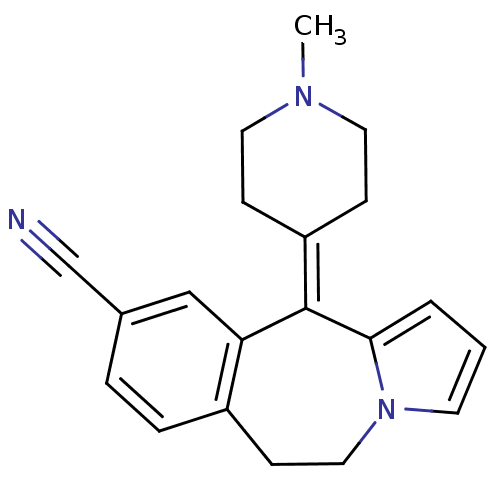 (11-(1-Methyl-piperidin-4-ylidene)-6,11-dihydro-5H-...) | PDB MMDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]LSD radioligand binding at the serotonin-1 binding site of rat cortex | J Med Chem 26: 974-80 (1983) BindingDB Entry DOI: 10.7270/Q2DR2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50026972 (11-(1-Methyl-piperidin-4-ylidene)-11H-benzo[d]pyrr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]- QNB binding at the muscarinic-cholinergic binding site of rat brain S1 | J Med Chem 26: 974-80 (1983) BindingDB Entry DOI: 10.7270/Q2DR2W2B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 409 total ) | Next | Last >> |