 Found 363 hits with Last Name = 'roehr' and Initial = 'j'
Found 363 hits with Last Name = 'roehr' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50048803
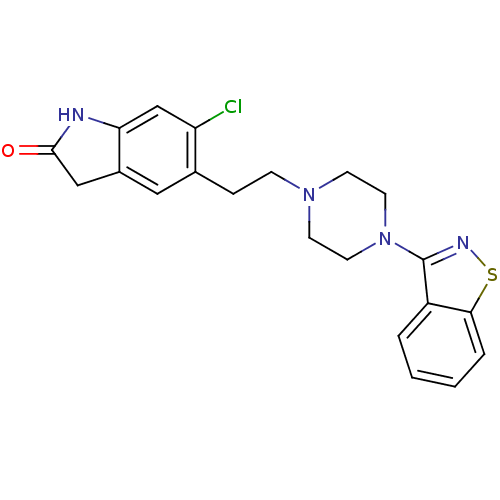
(5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 37-41 (2002)
Article DOI: 10.1016/s0014-2999(02)02074-5
BindingDB Entry DOI: 10.7270/Q2S75DX4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001786

(1-(2-{4-[5-chloro-1-(4-fluorophenyl)-1H-indol-3-yl...)Show SMILES Fc1ccc(cc1)-n1cc(C2CCN(CCN3CCNC3=O)CC2)c2cc(Cl)ccc12 Show InChI InChI=1S/C24H26ClFN4O/c25-18-1-6-23-21(15-18)22(16-30(23)20-4-2-19(26)3-5-20)17-7-10-28(11-8-17)13-14-29-12-9-27-24(29)31/h1-6,15-17H,7-14H2,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 37-41 (2002)
Article DOI: 10.1016/s0014-2999(02)02074-5
BindingDB Entry DOI: 10.7270/Q2S75DX4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards human Dopamine receptor D4.2 in CHO cells |
J Med Chem 39: 4044-57 (1996)
Article DOI: 10.1021/jm960268u
BindingDB Entry DOI: 10.7270/Q2ST7QH3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 37-41 (2002)
Article DOI: 10.1016/s0014-2999(02)02074-5
BindingDB Entry DOI: 10.7270/Q2S75DX4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50429069
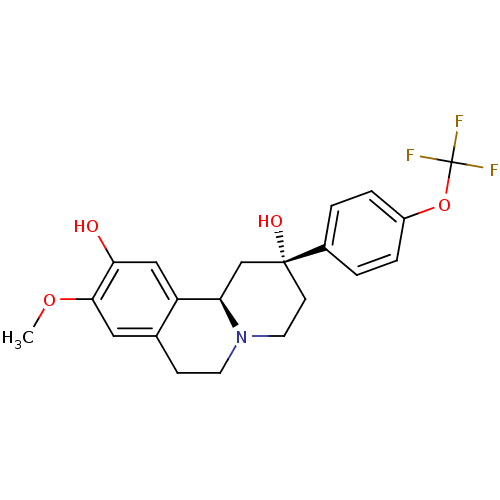
(CHEMBL2335736)Show SMILES COc1cc2CCN3CC[C@@](O)(C[C@@H]3c2cc1O)c1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C21H22F3NO4/c1-28-19-10-13-6-8-25-9-7-20(27,12-17(25)16(13)11-18(19)26)14-2-4-15(5-3-14)29-21(22,23)24/h2-5,10-11,17,26-27H,6-9,12H2,1H3/t17-,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH23390 from dopamine D1 receptor (unknown origin) expressed in CHO cell membranes after 60 mins |
Bioorg Med Chem Lett 23: 1498-501 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.046
BindingDB Entry DOI: 10.7270/Q290255G |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 37-41 (2002)
Article DOI: 10.1016/s0014-2999(02)02074-5
BindingDB Entry DOI: 10.7270/Q2S75DX4 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50001786

(1-(2-{4-[5-chloro-1-(4-fluorophenyl)-1H-indol-3-yl...)Show SMILES Fc1ccc(cc1)-n1cc(C2CCN(CCN3CCNC3=O)CC2)c2cc(Cl)ccc12 Show InChI InChI=1S/C24H26ClFN4O/c25-18-1-6-23-21(15-18)22(16-30(23)20-4-2-19(26)3-5-20)17-7-10-28(11-8-17)13-14-29-12-9-27-24(29)31/h1-6,15-17H,7-14H2,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 37-41 (2002)
Article DOI: 10.1016/s0014-2999(02)02074-5
BindingDB Entry DOI: 10.7270/Q2S75DX4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50034043
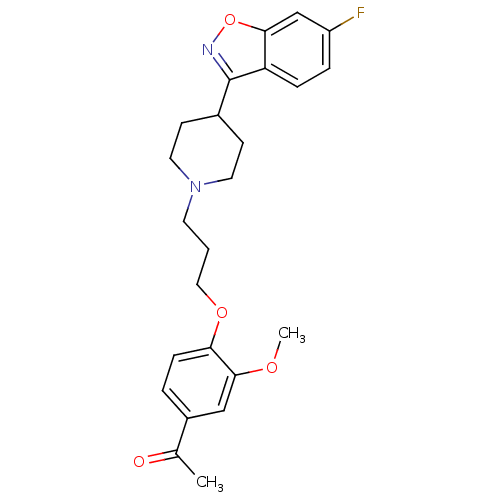
(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50429069

(CHEMBL2335736)Show SMILES COc1cc2CCN3CC[C@@](O)(C[C@@H]3c2cc1O)c1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C21H22F3NO4/c1-28-19-10-13-6-8-25-9-7-20(27,12-17(25)16(13)11-18(19)26)14-2-4-15(5-3-14)29-21(22,23)24/h2-5,10-11,17,26-27H,6-9,12H2,1H3/t17-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylspiperone from dopamine D2 receptor (unknown origin) expressed in CHO cell membranes after 60 mins |
Bioorg Med Chem Lett 23: 1498-501 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.046
BindingDB Entry DOI: 10.7270/Q290255G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM35254
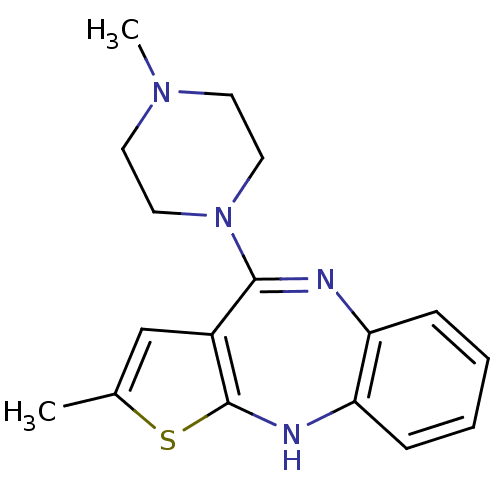
(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards human 5-HT2A receptor in BEK cells |
J Med Chem 39: 4044-57 (1996)
Article DOI: 10.1021/jm960268u
BindingDB Entry DOI: 10.7270/Q2ST7QH3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50053691
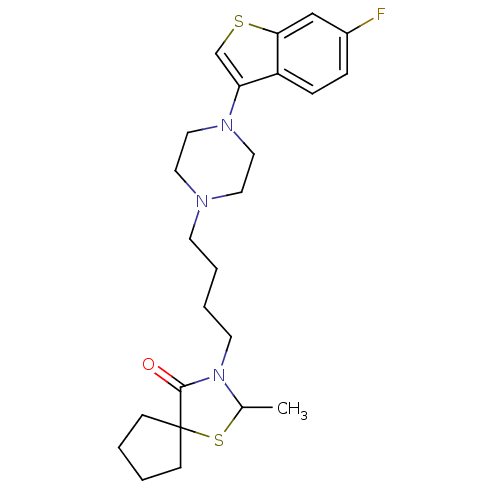
(3-{4-[4-(6-Fluoro-benzo[b]thiophen-3-yl)-piperazin...)Show SMILES CC1SC2(CCCC2)C(=O)N1CCCCN1CCN(CC1)c1csc2cc(F)ccc12 Show InChI InChI=1S/C24H32FN3OS2/c1-18-28(23(29)24(31-18)8-2-3-9-24)11-5-4-10-26-12-14-27(15-13-26)21-17-30-22-16-19(25)6-7-20(21)22/h6-7,16-18H,2-5,8-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards human 5-HT2A receptor in BEK cells |
J Med Chem 39: 4044-57 (1996)
Article DOI: 10.1021/jm960268u
BindingDB Entry DOI: 10.7270/Q2ST7QH3 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50429068
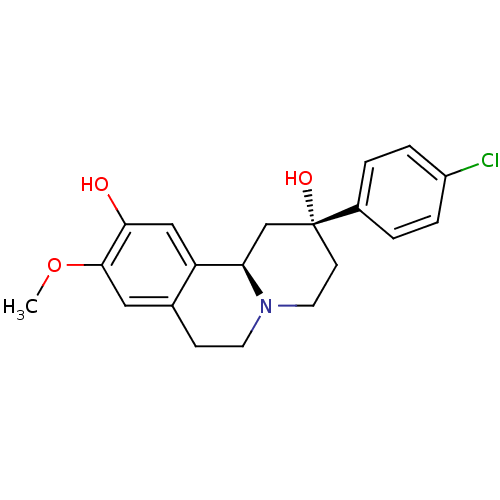
(CHEMBL2335740)Show SMILES COc1cc2CCN3CC[C@@](O)(C[C@@H]3c2cc1O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C20H22ClNO3/c1-25-19-10-13-6-8-22-9-7-20(24,14-2-4-15(21)5-3-14)12-17(22)16(13)11-18(19)23/h2-5,10-11,17,23-24H,6-9,12H2,1H3/t17-,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH23390 from dopamine D1 receptor (unknown origin) expressed in CHO cell membranes after 60 mins |
Bioorg Med Chem Lett 23: 1498-501 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.046
BindingDB Entry DOI: 10.7270/Q290255G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards human 5-HT2A receptor in BEK cells |
J Med Chem 39: 4044-57 (1996)
Article DOI: 10.1021/jm960268u
BindingDB Entry DOI: 10.7270/Q2ST7QH3 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50002107
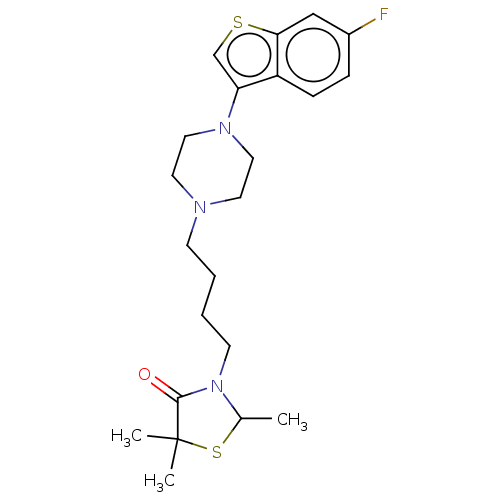
(3-{4-[4-(6-Fluoro-benzo[b]thiophen-3-yl)-piperazin...)Show SMILES CC1SC(C)(C)C(=O)N1CCCCN1CCN(CC1)c1csc2cc(F)ccc12 Show InChI InChI=1S/C22H30FN3OS2/c1-16-26(21(27)22(2,3)29-16)9-5-4-8-24-10-12-25(13-11-24)19-15-28-20-14-17(23)6-7-18(19)20/h6-7,14-16H,4-5,8-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against human Dopamine receptor D4.2 in CHO cells |
J Med Chem 39: 4044-57 (1996)
Article DOI: 10.1021/jm960268u
BindingDB Entry DOI: 10.7270/Q2ST7QH3 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50429067
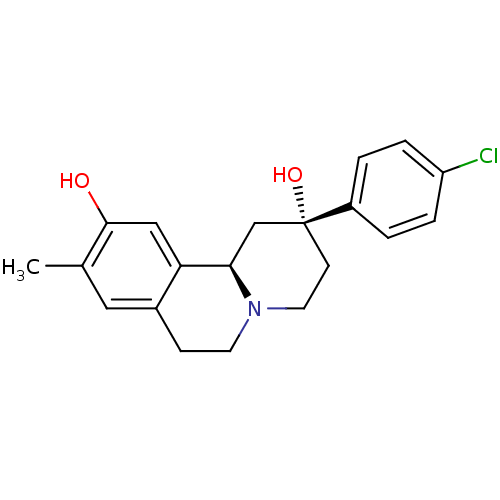
(CHEMBL2335737)Show SMILES Cc1cc2CCN3CC[C@@](O)(C[C@@H]3c2cc1O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C20H22ClNO2/c1-13-10-14-6-8-22-9-7-20(24,15-2-4-16(21)5-3-15)12-18(22)17(14)11-19(13)23/h2-5,10-11,18,23-24H,6-9,12H2,1H3/t18-,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]SCH23390 from dopamine D1 receptor (unknown origin) expressed in CHO cell membranes after 60 mins |
Bioorg Med Chem Lett 23: 1498-501 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.046
BindingDB Entry DOI: 10.7270/Q290255G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM82479
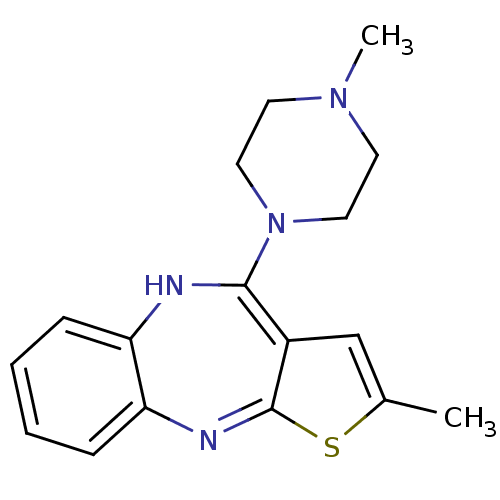
(CAS_132539-06-1 | NSC_4585 | OLANZAPINE | USRE4934...)Show SMILES CN1CCN(CC1)C1=c2cc(C)sc2=Nc2ccccc2N1 |c:8,15| Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,18H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50001786

(1-(2-{4-[5-chloro-1-(4-fluorophenyl)-1H-indol-3-yl...)Show SMILES Fc1ccc(cc1)-n1cc(C2CCN(CCN3CCNC3=O)CC2)c2cc(Cl)ccc12 Show InChI InChI=1S/C24H26ClFN4O/c25-18-1-6-23-21(15-18)22(16-30(23)20-4-2-19(26)3-5-20)17-7-10-28(11-8-17)13-14-29-12-9-27-24(29)31/h1-6,15-17H,7-14H2,(H,27,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 6.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 37-41 (2002)
Article DOI: 10.1016/s0014-2999(02)02074-5
BindingDB Entry DOI: 10.7270/Q2S75DX4 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(RAT) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50002107

(3-{4-[4-(6-Fluoro-benzo[b]thiophen-3-yl)-piperazin...)Show SMILES CC1SC(C)(C)C(=O)N1CCCCN1CCN(CC1)c1csc2cc(F)ccc12 Show InChI InChI=1S/C22H30FN3OS2/c1-16-26(21(27)22(2,3)29-16)9-5-4-8-24-10-12-25(13-11-24)19-15-28-20-14-17(23)6-7-18(19)20/h6-7,14-16H,4-5,8-13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards human 5-HT2A receptor in BEK cells |
J Med Chem 39: 4044-57 (1996)
Article DOI: 10.1021/jm960268u
BindingDB Entry DOI: 10.7270/Q2ST7QH3 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50048803

(5-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)et...)Show SMILES Clc1cc2NC(=O)Cc2cc1CCN1CCN(CC1)c1nsc2ccccc12 Show InChI InChI=1S/C21H21ClN4OS/c22-17-13-18-15(12-20(27)23-18)11-14(17)5-6-25-7-9-26(10-8-25)21-16-3-1-2-4-19(16)28-24-21/h1-4,11,13H,5-10,12H2,(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 37-41 (2002)
Article DOI: 10.1016/s0014-2999(02)02074-5
BindingDB Entry DOI: 10.7270/Q2S75DX4 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity towards human Dopamine receptor D4.2 in CHO cells |
J Med Chem 39: 4044-57 (1996)
Article DOI: 10.1021/jm960268u
BindingDB Entry DOI: 10.7270/Q2ST7QH3 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for in vitro binding affinity towards Dopamine receptor D2 in rat striatum using [3H]- spiperone as radioligand |
J Med Chem 35: 2712-5 (1992)
BindingDB Entry DOI: 10.7270/Q2TH8KMC |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50334150
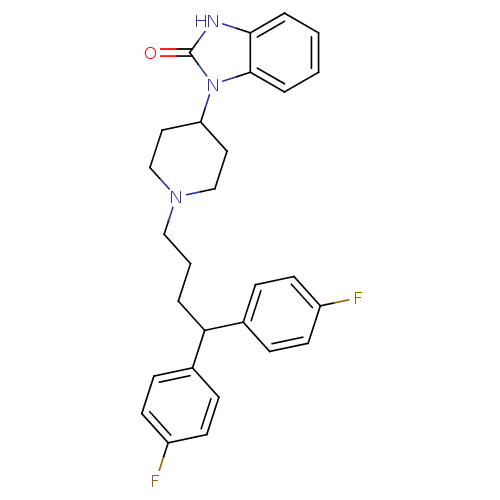
(1-(1-(4,4-bis(4-fluorophenyl)butyl)piperidin-4-yl)...)Show SMILES Fc1ccc(cc1)C(CCCN1CCC(CC1)n1c2ccccc2[nH]c1=O)c1ccc(F)cc1 Show InChI InChI=1S/C28H29F2N3O/c29-22-11-7-20(8-12-22)25(21-9-13-23(30)14-10-21)4-3-17-32-18-15-24(16-19-32)33-27-6-2-1-5-26(27)31-28(33)34/h1-2,5-14,24-25H,3-4,15-19H2,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 11.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 37-41 (2002)
Article DOI: 10.1016/s0014-2999(02)02074-5
BindingDB Entry DOI: 10.7270/Q2S75DX4 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50429067

(CHEMBL2335737)Show SMILES Cc1cc2CCN3CC[C@@](O)(C[C@@H]3c2cc1O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C20H22ClNO2/c1-13-10-14-6-8-22-9-7-20(24,15-2-4-16(21)5-3-15)12-18(22)17(14)11-19(13)23/h2-5,10-11,18,23-24H,6-9,12H2,1H3/t18-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylspiperone from dopamine D2 receptor (unknown origin) expressed in CHO cell membranes after 60 mins |
Bioorg Med Chem Lett 23: 1498-501 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.046
BindingDB Entry DOI: 10.7270/Q290255G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 13.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM82479

(CAS_132539-06-1 | NSC_4585 | OLANZAPINE | USRE4934...)Show SMILES CN1CCN(CC1)C1=c2cc(C)sc2=Nc2ccccc2N1 |c:8,15| Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,18H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 13.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 14.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50429068

(CHEMBL2335740)Show SMILES COc1cc2CCN3CC[C@@](O)(C[C@@H]3c2cc1O)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C20H22ClNO3/c1-25-19-10-13-6-8-22-9-7-20(24,14-2-4-15(21)5-3-14)12-17(22)16(13)11-18(19)23/h2-5,10-11,17,23-24H,6-9,12H2,1H3/t17-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi US
Curated by ChEMBL
| Assay Description
Displacement of [3H]N-methylspiperone from dopamine D2 receptor (unknown origin) expressed in CHO cell membranes after 60 mins |
Bioorg Med Chem Lett 23: 1498-501 (2013)
Article DOI: 10.1016/j.bmcl.2012.12.046
BindingDB Entry DOI: 10.7270/Q290255G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50334150

(1-(1-(4,4-bis(4-fluorophenyl)butyl)piperidin-4-yl)...)Show SMILES Fc1ccc(cc1)C(CCCN1CCC(CC1)n1c2ccccc2[nH]c1=O)c1ccc(F)cc1 Show InChI InChI=1S/C28H29F2N3O/c29-22-11-7-20(8-12-22)25(21-9-13-23(30)14-10-21)4-3-17-32-18-15-24(16-19-32)33-27-6-2-1-5-26(27)31-28(33)34/h1-2,5-14,24-25H,3-4,15-19H2,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharmaceuticals, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 450: 37-41 (2002)
Article DOI: 10.1016/s0014-2999(02)02074-5
BindingDB Entry DOI: 10.7270/Q2S75DX4 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM22869

(6-chloro-10-(4-methylpiperazin-1-yl)-2,9-diazatric...)Show SMILES CN1CCN(CC1)C1=c2ccccc2=Nc2ccc(Cl)cc2N1 |c:8,15| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,21H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
Serotonin 2 (5-HT2) receptor
(RAT-Rattus norvegicus (Rat)-Rattus norvegicus (rat...) | BDBM50002107

(3-{4-[4-(6-Fluoro-benzo[b]thiophen-3-yl)-piperazin...)Show SMILES CC1SC(C)(C)C(=O)N1CCCCN1CCN(CC1)c1csc2cc(F)ccc12 Show InChI InChI=1S/C22H30FN3OS2/c1-16-26(21(27)22(2,3)29-16)9-5-4-8-24-10-12-25(13-11-24)19-15-28-20-14-17(23)6-7-18(19)20/h6-7,14-16H,4-5,8-13H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst-Roussel Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Compound was evaluated for in vitro binding affinity towards serotonin 5-hydroxytryptamine 2 receptor in rat cortex using [3H]- spiperone as radiolig... |
J Med Chem 35: 2712-5 (1992)
BindingDB Entry DOI: 10.7270/Q2TH8KMC |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50001885

((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...)Show SMILES Cc1nc2CCCCn2c(=O)c1CCN1CCC(CC1)c1noc2cc(F)ccc12 Show InChI InChI=1S/C23H27FN4O2/c1-15-18(23(29)28-10-3-2-4-21(28)25-15)9-13-27-11-7-16(8-12-27)22-19-6-5-17(24)14-20(19)30-26-22/h5-6,14,16H,2-4,7-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM50034043

(1-(4-{3-[4-(6-Fluoro-benzo[d]isoxazol-3-yl)-piperi...)Show SMILES COc1cc(ccc1OCCCN1CCC(CC1)c1noc2cc(F)ccc12)C(C)=O Show InChI InChI=1S/C24H27FN2O4/c1-16(28)18-4-7-21(23(14-18)29-2)30-13-3-10-27-11-8-17(9-12-27)24-20-6-5-19(25)15-22(20)31-26-24/h4-7,14-15,17H,3,8-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM82479

(CAS_132539-06-1 | NSC_4585 | OLANZAPINE | USRE4934...)Show SMILES CN1CCN(CC1)C1=c2cc(C)sc2=Nc2ccccc2N1 |c:8,15| Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,18H,7-10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 24.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel, Inc.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 317: 417-23 (1996)
Article DOI: 10.1016/s0014-2999(96)00840-0
BindingDB Entry DOI: 10.7270/Q2DF6PR7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data