

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19968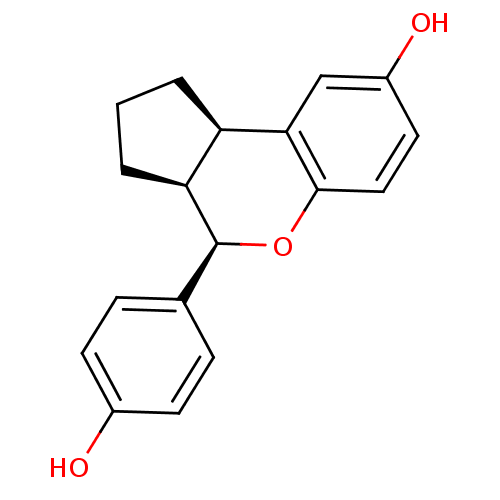 ((2R,6S,7R)-7-(4-hydroxyphenyl)-8-oxatricyclo[7.4.0...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.190 | -54.9 | n/a | n/a | 0.660 | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | J Med Chem 49: 6155-7 (2006) Article DOI: 10.1021/jm060491j BindingDB Entry DOI: 10.7270/Q2N014SV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50323737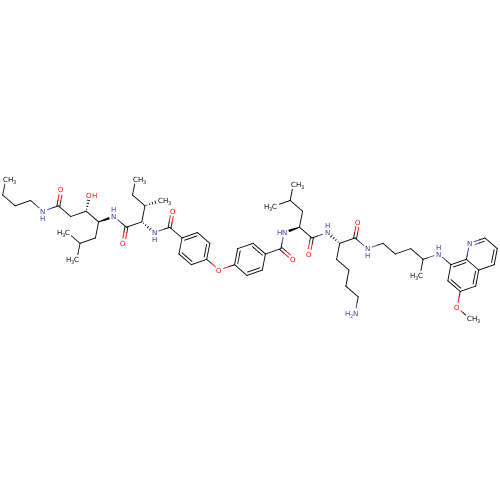 (CHEMBL1213687 | N-((2S)-1-((2S)-6-amino-1-(4-(6-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PLM2 using Lys-Glu-Phe-Val-Phe-NPhe-Ala-Leu-Lys as substrate by spectrophotometry | Bioorg Med Chem Lett 22: 5915-8 (2012) Article DOI: 10.1016/j.bmcl.2012.07.069 BindingDB Entry DOI: 10.7270/Q2M046HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50228078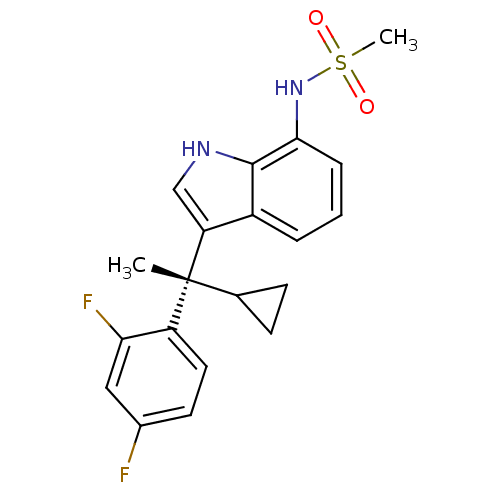 ((S)-N-(3-(1-cyclopropyl-1-(2,4-difluorophenyl)ethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.494 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human mineralocorticoid receptor | J Med Chem 50: 6443-5 (2007) Article DOI: 10.1021/jm701186z BindingDB Entry DOI: 10.7270/Q23B5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50200018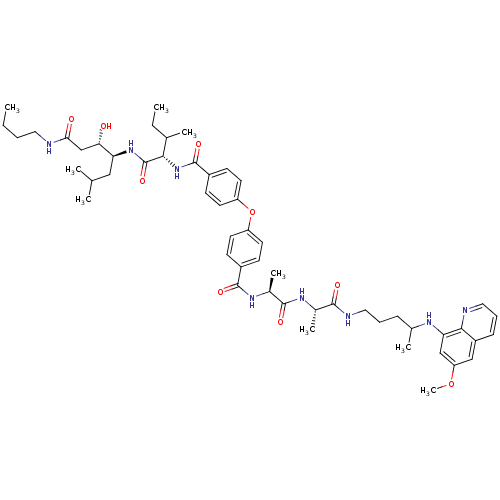 ((3S,4S)-4-((2S,3S)-2-(4-(4-((S)-((S)-1-(1-(4-(6-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum recombinant PLM1 | J Med Chem 49: 7440-9 (2006) Article DOI: 10.1021/jm061033d BindingDB Entry DOI: 10.7270/Q29P319W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50390636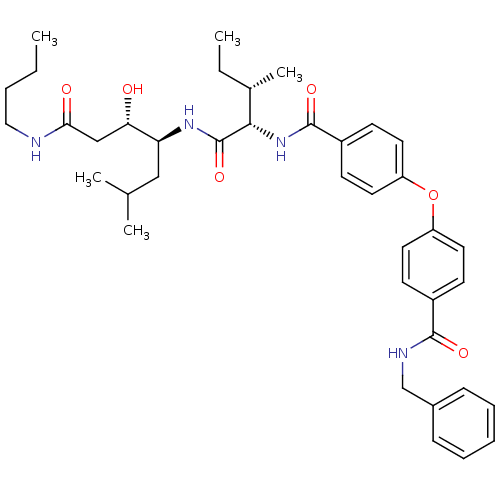 (CHEMBL2069615) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PLM2 using Lys-Glu-Phe-Val-Phe-NPhe-Ala-Leu-Lys as substrate by spectrophotometry | Bioorg Med Chem Lett 22: 5915-8 (2012) Article DOI: 10.1016/j.bmcl.2012.07.069 BindingDB Entry DOI: 10.7270/Q2M046HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM19969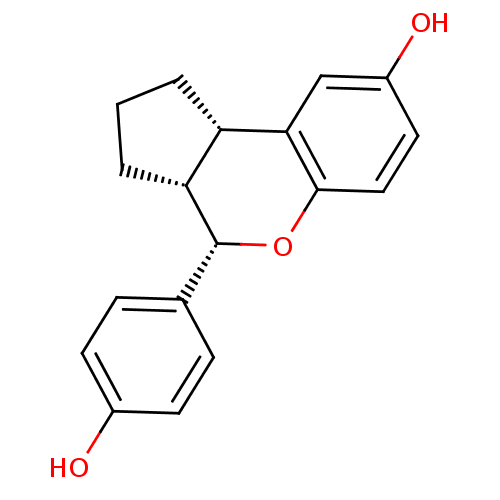 ((2S,6R,7S)-7-(4-hydroxyphenyl)-8-oxatricyclo[7.4.0...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.54 | -49.8 | n/a | n/a | 3.61 | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | J Med Chem 49: 6155-7 (2006) Article DOI: 10.1021/jm060491j BindingDB Entry DOI: 10.7270/Q2N014SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50228079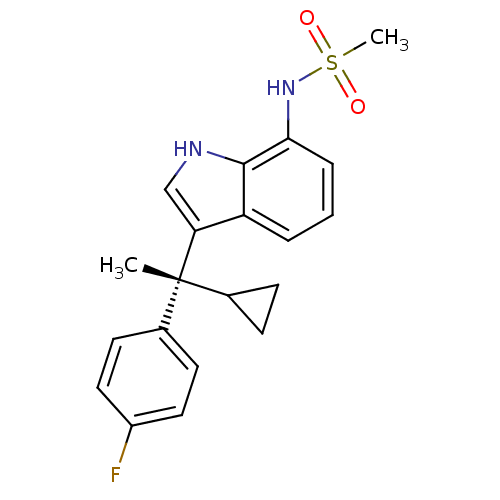 ((R)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human mineralocorticoid receptor | J Med Chem 50: 6443-5 (2007) Article DOI: 10.1021/jm701186z BindingDB Entry DOI: 10.7270/Q23B5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50390637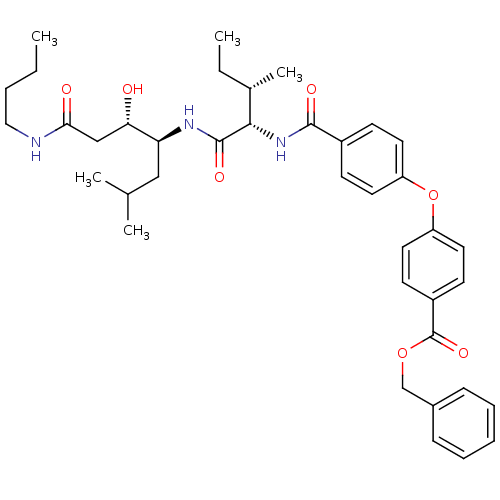 (CHEMBL2069616) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PLM2 using Lys-Glu-Phe-Val-Phe-NPhe-Ala-Leu-Lys as substrate by spectrophotometry | Bioorg Med Chem Lett 22: 5915-8 (2012) Article DOI: 10.1016/j.bmcl.2012.07.069 BindingDB Entry DOI: 10.7270/Q2M046HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50228076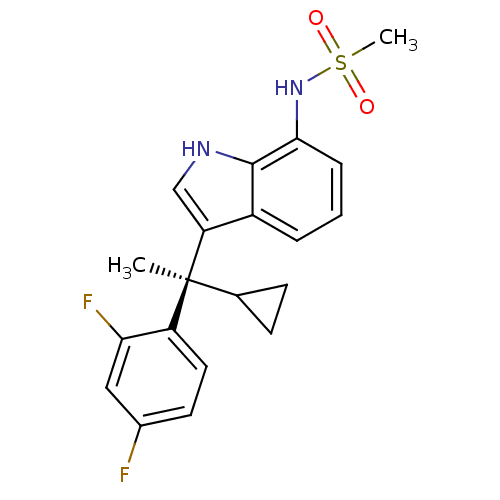 ((R)-N-(3-(1-cyclopropyl-1-(2,4-difluorophenyl)ethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human mineralocorticoid receptor | J Med Chem 50: 6443-5 (2007) Article DOI: 10.1021/jm701186z BindingDB Entry DOI: 10.7270/Q23B5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50390633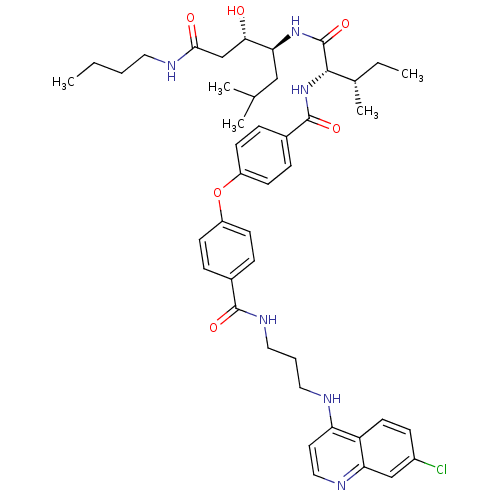 (CHEMBL2069612) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PLM2 using Lys-Glu-Phe-Val-Phe-NPhe-Ala-Leu-Lys as substrate by spectrophotometry | Bioorg Med Chem Lett 22: 5915-8 (2012) Article DOI: 10.1016/j.bmcl.2012.07.069 BindingDB Entry DOI: 10.7270/Q2M046HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50228080 (2',15'-dimethyl-5,5'-dioxo-(9'R)-spiro[tetrahydrof...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 2.32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human mineralocorticoid receptor | J Med Chem 50: 6443-5 (2007) Article DOI: 10.1021/jm701186z BindingDB Entry DOI: 10.7270/Q23B5ZVP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50390635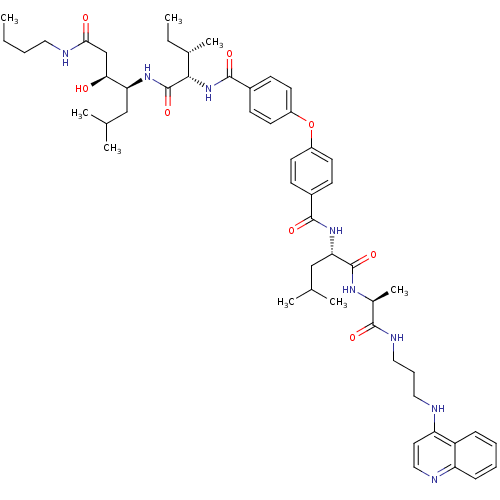 (CHEMBL2069614) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PLM2 using Lys-Glu-Phe-Val-Phe-NPhe-Ala-Leu-Lys as substrate by spectrophotometry | Bioorg Med Chem Lett 22: 5915-8 (2012) Article DOI: 10.1016/j.bmcl.2012.07.069 BindingDB Entry DOI: 10.7270/Q2M046HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19968 ((2R,6S,7R)-7-(4-hydroxyphenyl)-8-oxatricyclo[7.4.0...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.68 | -48.4 | n/a | n/a | 19.4 | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | J Med Chem 49: 6155-7 (2006) Article DOI: 10.1021/jm060491j BindingDB Entry DOI: 10.7270/Q2N014SV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50390634 (CHEMBL2069613) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum PLM2 using Lys-Glu-Phe-Val-Phe-NPhe-Ala-Leu-Lys as substrate by spectrophotometry | Bioorg Med Chem Lett 22: 5915-8 (2012) Article DOI: 10.1016/j.bmcl.2012.07.069 BindingDB Entry DOI: 10.7270/Q2M046HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50200018 ((3S,4S)-4-((2S,3S)-2-(4-(4-((S)-((S)-1-(1-(4-(6-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum recombinant PLM2 | J Med Chem 49: 7440-9 (2006) Article DOI: 10.1021/jm061033d BindingDB Entry DOI: 10.7270/Q29P319W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029462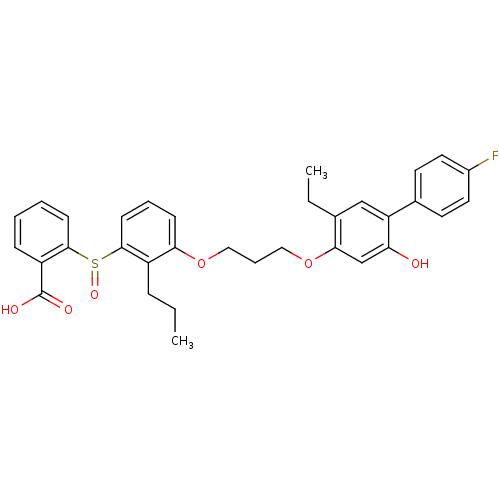 (2-{3-[3-(5-Ethyl-4'-fluoro-2-hydroxy-biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards LTB4 receptor guinea pig spleen cells using [3H]-LTB4 as radioligand | J Med Chem 38: 4411-32 (1995) BindingDB Entry DOI: 10.7270/Q20V8BSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50228081 ((S)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human mineralocorticoid receptor | J Med Chem 50: 6443-5 (2007) Article DOI: 10.1021/jm701186z BindingDB Entry DOI: 10.7270/Q23B5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50228078 ((S)-N-(3-(1-cyclopropyl-1-(2,4-difluorophenyl)ethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human glucocorticoid receptor | J Med Chem 50: 6443-5 (2007) Article DOI: 10.1021/jm701186z BindingDB Entry DOI: 10.7270/Q23B5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50200015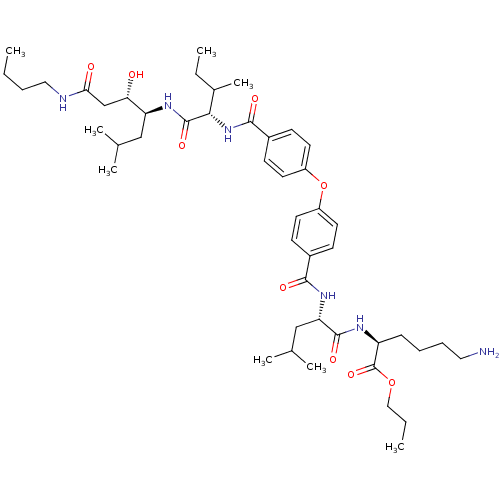 ((S)-2-(2S-(4-(4-(((2S,3S)-1-((3S,4S)-1-(butylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum recombinant PLM2 | J Med Chem 49: 7440-9 (2006) Article DOI: 10.1021/jm061033d BindingDB Entry DOI: 10.7270/Q29P319W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50200019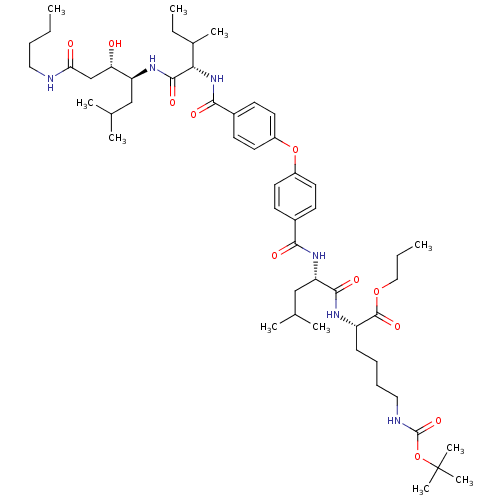 ((S)-2-(2S-(4-(4-(((2S,3S)-1-((3S,4S)-1-(butylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum recombinant PLM2 | J Med Chem 49: 7440-9 (2006) Article DOI: 10.1021/jm061033d BindingDB Entry DOI: 10.7270/Q29P319W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50200015 ((S)-2-(2S-(4-(4-(((2S,3S)-1-((3S,4S)-1-(butylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum recombinant PLM1 | J Med Chem 49: 7440-9 (2006) Article DOI: 10.1021/jm061033d BindingDB Entry DOI: 10.7270/Q29P319W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50200017 ((3S,4S)-4-((2S,3S)-2-(4-(4-((S)-((S)-1-(6-amino-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum recombinant PLM2 | J Med Chem 49: 7440-9 (2006) Article DOI: 10.1021/jm061033d BindingDB Entry DOI: 10.7270/Q29P319W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50200020 ((3S,4S)-4-((2S,3S)-2-(4-(4-((4-(6-methoxyquinolin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum recombinant PLM2 | J Med Chem 49: 7440-9 (2006) Article DOI: 10.1021/jm061033d BindingDB Entry DOI: 10.7270/Q29P319W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50200021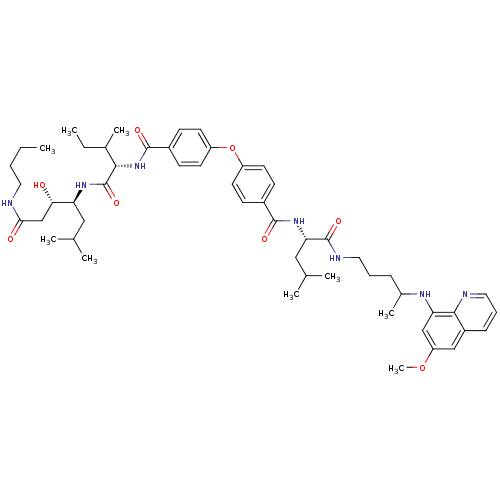 ((3S,4S)-4-((2S,3S)-2-(4-(4-((S)-(1-(4-(6-methoxyqu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum recombinant PLM2 | J Med Chem 49: 7440-9 (2006) Article DOI: 10.1021/jm061033d BindingDB Entry DOI: 10.7270/Q29P319W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM19969 ((2S,6R,7S)-7-(4-hydroxyphenyl)-8-oxatricyclo[7.4.0...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 14.5 | -44.3 | n/a | n/a | 32.5 | n/a | n/a | 7.5 | 22 |
Lilly Research Laboratories | Assay Description The binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer. The Kd for 3H-estradiol was determin... | J Med Chem 49: 6155-7 (2006) Article DOI: 10.1021/jm060491j BindingDB Entry DOI: 10.7270/Q2N014SV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50200019 ((S)-2-(2S-(4-(4-(((2S,3S)-1-((3S,4S)-1-(butylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum recombinant PLM1 | J Med Chem 49: 7440-9 (2006) Article DOI: 10.1021/jm061033d BindingDB Entry DOI: 10.7270/Q29P319W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029452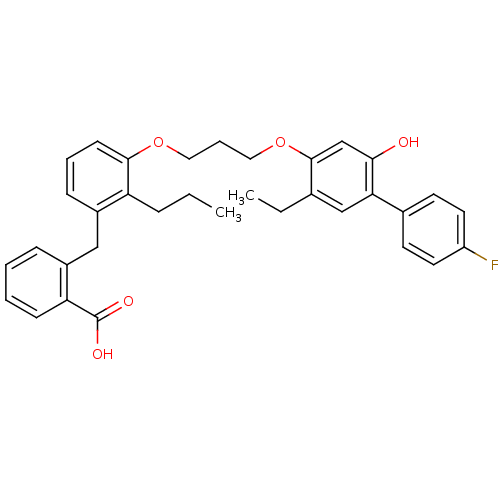 (2-{3-[3-(5-Ethyl-4'-fluoro-2-hydroxy-biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards LTB4 receptor guinea pig spleen cells using [3H]-LTB4 as radioligand | J Med Chem 38: 4411-32 (1995) BindingDB Entry DOI: 10.7270/Q20V8BSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50200014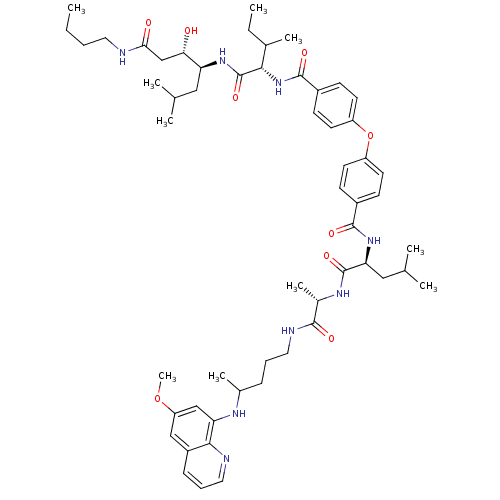 ((3S,4S)-4-((2S,3S)-2-(4-(4-((S)-((S)-1-(1-(4-(6-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum recombinant PLM2 | J Med Chem 49: 7440-9 (2006) Article DOI: 10.1021/jm061033d BindingDB Entry DOI: 10.7270/Q29P319W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50228080 (2',15'-dimethyl-5,5'-dioxo-(9'R)-spiro[tetrahydrof...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 32.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human glucocorticoid receptor | J Med Chem 50: 6443-5 (2007) Article DOI: 10.1021/jm701186z BindingDB Entry DOI: 10.7270/Q23B5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029468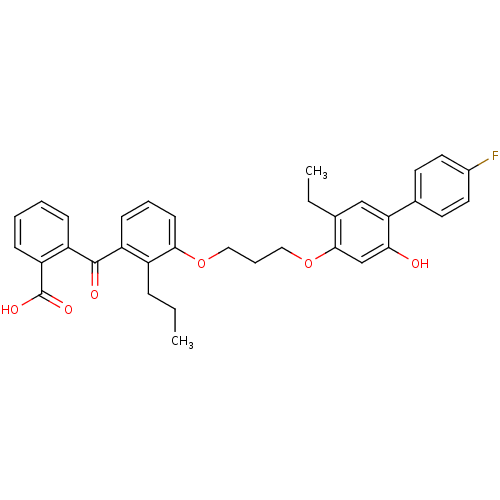 (2-{3-[3-(5-Ethyl-4'-fluoro-2-hydroxy-biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards LTB4 receptor guinea pig spleen cells using [3H]-LTB4 as radioligand | J Med Chem 38: 4411-32 (1995) BindingDB Entry DOI: 10.7270/Q20V8BSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50200017 ((3S,4S)-4-((2S,3S)-2-(4-(4-((S)-((S)-1-(6-amino-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum recombinant PLM1 | J Med Chem 49: 7440-9 (2006) Article DOI: 10.1021/jm061033d BindingDB Entry DOI: 10.7270/Q29P319W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029467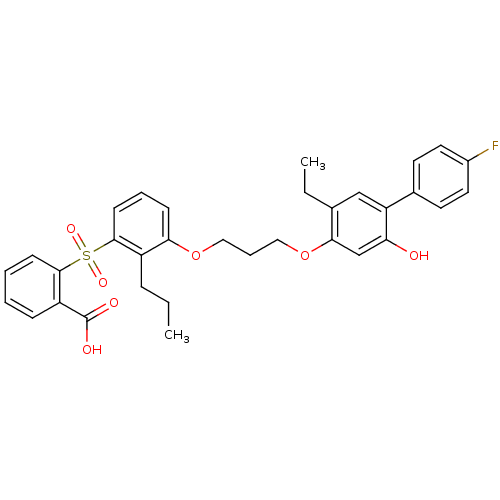 (2-{3-[3-(5-Ethyl-4'-fluoro-2-hydroxy-biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards LTB4 receptor guinea pig spleen cells using [3H]-LTB4 as radioligand | J Med Chem 38: 4411-32 (1995) BindingDB Entry DOI: 10.7270/Q20V8BSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50228080 (2',15'-dimethyl-5,5'-dioxo-(9'R)-spiro[tetrahydrof...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 39.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human androgen receptor | J Med Chem 50: 6443-5 (2007) Article DOI: 10.1021/jm701186z BindingDB Entry DOI: 10.7270/Q23B5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029484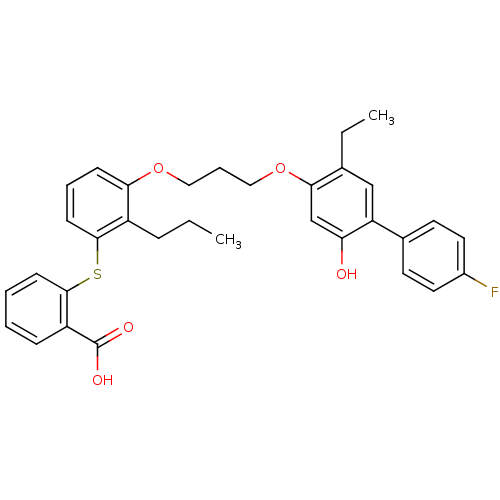 (2-{3-[3-(5-Ethyl-4'-fluoro-2-hydroxy-biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards LTB4 receptor guinea pig spleen cells using [3H]-LTB4 as radioligand | J Med Chem 38: 4411-32 (1995) BindingDB Entry DOI: 10.7270/Q20V8BSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50200023 ((3S,4S)-4-((2S,3S)-2-(4-(4-((S)-((S)-1-(1-(4-(6-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum recombinant PLM1 | J Med Chem 49: 7440-9 (2006) Article DOI: 10.1021/jm061033d BindingDB Entry DOI: 10.7270/Q29P319W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50146527 ((3S,4S)-N-butyl-3-hydroxy-6-methyl-4-((2S,3S)-3-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum recombinant PLM2 | J Med Chem 49: 7440-9 (2006) Article DOI: 10.1021/jm061033d BindingDB Entry DOI: 10.7270/Q29P319W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50200014 ((3S,4S)-4-((2S,3S)-2-(4-(4-((S)-((S)-1-(1-(4-(6-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum recombinant PLM1 | J Med Chem 49: 7440-9 (2006) Article DOI: 10.1021/jm061033d BindingDB Entry DOI: 10.7270/Q29P319W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50200020 ((3S,4S)-4-((2S,3S)-2-(4-(4-((4-(6-methoxyquinolin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum recombinant PLM1 | J Med Chem 49: 7440-9 (2006) Article DOI: 10.1021/jm061033d BindingDB Entry DOI: 10.7270/Q29P319W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50200023 ((3S,4S)-4-((2S,3S)-2-(4-(4-((S)-((S)-1-(1-(4-(6-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum recombinant PLM2 | J Med Chem 49: 7440-9 (2006) Article DOI: 10.1021/jm061033d BindingDB Entry DOI: 10.7270/Q29P319W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50146524 ((S)-3-(S)-Hydroxy-4-{(S)-3-methyl-2-[2-(naphthalen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan Curated by ChEMBL | Assay Description In vitro binding affinity towards Plasmodium falciparum plasmepsin-2 | Bioorg Med Chem Lett 14: 2931-4 (2004) Article DOI: 10.1016/j.bmcl.2004.03.030 BindingDB Entry DOI: 10.7270/Q2G44QVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50228077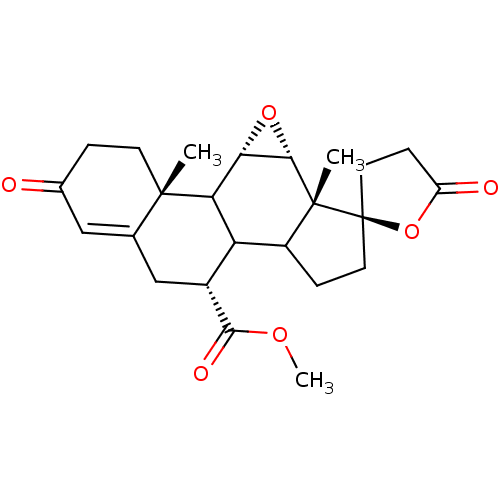 (CHEMBL237122 | epierenone) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human mineralocorticoid receptor | J Med Chem 50: 6443-5 (2007) Article DOI: 10.1021/jm701186z BindingDB Entry DOI: 10.7270/Q23B5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50228079 ((R)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 137 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human progesterone receptor | J Med Chem 50: 6443-5 (2007) Article DOI: 10.1021/jm701186z BindingDB Entry DOI: 10.7270/Q23B5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50228078 ((S)-N-(3-(1-cyclopropyl-1-(2,4-difluorophenyl)ethy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 163 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human progesterone receptor | J Med Chem 50: 6443-5 (2007) Article DOI: 10.1021/jm701186z BindingDB Entry DOI: 10.7270/Q23B5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50228079 ((R)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 167 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human glucocorticoid receptor | J Med Chem 50: 6443-5 (2007) Article DOI: 10.1021/jm701186z BindingDB Entry DOI: 10.7270/Q23B5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50223627 (CHEMBL3215350) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan Curated by ChEMBL | Assay Description In vitro binding affinity towards Plasmodium falciparum plasmepsin-2 | Bioorg Med Chem Lett 14: 2931-4 (2004) Article DOI: 10.1016/j.bmcl.2004.03.030 BindingDB Entry DOI: 10.7270/Q2G44QVR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin I (Plasmodium falciparum) | BDBM50200016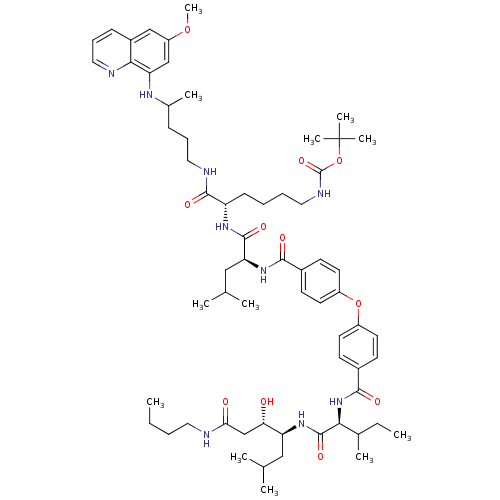 ((3S,4S)-4-((2S,3S)-2-(4-(4-((S)-((S)-1-(6-(tert-bu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum recombinant PLM1 | J Med Chem 49: 7440-9 (2006) Article DOI: 10.1021/jm061033d BindingDB Entry DOI: 10.7270/Q29P319W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50228081 ((S)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 313 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human progesterone receptor | J Med Chem 50: 6443-5 (2007) Article DOI: 10.1021/jm701186z BindingDB Entry DOI: 10.7270/Q23B5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50228081 ((S)-N-(3-(1-cyclopropyl-1-(4-fluorophenyl)ethyl)-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 387 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human glucocorticoid receptor | J Med Chem 50: 6443-5 (2007) Article DOI: 10.1021/jm701186z BindingDB Entry DOI: 10.7270/Q23B5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50228080 (2',15'-dimethyl-5,5'-dioxo-(9'R)-spiro[tetrahydrof...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human progesterone receptor | J Med Chem 50: 6443-5 (2007) Article DOI: 10.1021/jm701186z BindingDB Entry DOI: 10.7270/Q23B5ZVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasmepsin II (Plasmodium falciparum) | BDBM50200016 ((3S,4S)-4-((2S,3S)-2-(4-(4-((S)-((S)-1-(6-(tert-bu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 403 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Milan Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum recombinant PLM2 | J Med Chem 49: 7440-9 (2006) Article DOI: 10.1021/jm061033d BindingDB Entry DOI: 10.7270/Q29P319W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 73 total ) | Next | Last >> |