 Found 457 hits with Last Name = 'root' and Initial = 'j'
Found 457 hits with Last Name = 'root' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50012544

(CHEMBL3260768)Show SMILES Cc1oc(cc1CNc1ccc(cc1)-c1ccc(OC(F)F)cc1)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C27H24F2N2O5S/c1-17-5-3-4-6-25(17)37(33,34)31-26(32)24-15-21(18(2)35-24)16-30-22-11-7-19(8-12-22)20-9-13-23(14-10-20)36-27(28)29/h3-15,27,30H,16H2,1-2H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting |
Bioorg Med Chem Lett 24: 2212-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.068
BindingDB Entry DOI: 10.7270/Q2125V6J |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50214370
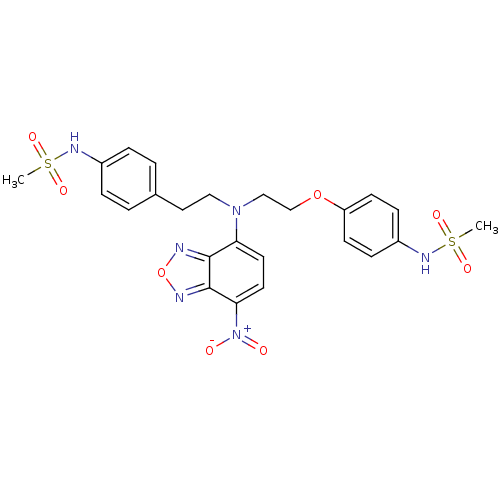
(CHEMBL227134 | N-(4-{2-[[2-(4-methanesulfonylamino...)Show SMILES CS(=O)(=O)Nc1ccc(CCN(CCOc2ccc(NS(C)(=O)=O)cc2)c2ccc([N+]([O-])=O)c3nonc23)cc1 Show InChI InChI=1S/C24H26N6O8S2/c1-39(33,34)27-18-5-3-17(4-6-18)13-14-29(21-11-12-22(30(31)32)24-23(21)25-38-26-24)15-16-37-20-9-7-19(8-10-20)28-40(2,35)36/h3-12,27-28H,13-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50012543

(CHEMBL3260767)Show SMILES Cc1noc(C)c1S(=O)(=O)NC(=O)c1cc(COc2ccc(cc2)-c2ccc(OC(F)F)cc2)c(C)o1 Show InChI InChI=1S/C25H22F2N2O7S/c1-14-23(16(3)36-28-14)37(31,32)29-24(30)22-12-19(15(2)34-22)13-33-20-8-4-17(5-9-20)18-6-10-21(11-7-18)35-25(26)27/h4-12,25H,13H2,1-3H3,(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting |
Bioorg Med Chem Lett 24: 2212-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.068
BindingDB Entry DOI: 10.7270/Q2125V6J |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50012502

(CHEMBL3260457)Show SMILES Cc1oc(cc1COc1ccc(cc1)-c1ccc(OC(F)F)cc1)C(=O)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C26H21F2NO6S/c1-17-20(15-24(34-17)25(30)29-36(31,32)23-5-3-2-4-6-23)16-33-21-11-7-18(8-12-21)19-9-13-22(14-10-19)35-26(27)28/h2-15,26H,16H2,1H3,(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting |
Bioorg Med Chem Lett 24: 2212-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.068
BindingDB Entry DOI: 10.7270/Q2125V6J |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50012532
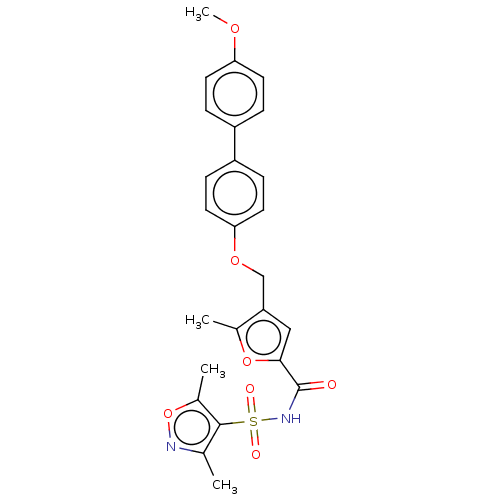
(CHEMBL3260463)Show SMILES COc1ccc(cc1)-c1ccc(OCc2cc(oc2C)C(=O)NS(=O)(=O)c2c(C)noc2C)cc1 Show InChI InChI=1S/C25H24N2O7S/c1-15-24(17(3)34-26-15)35(29,30)27-25(28)23-13-20(16(2)33-23)14-32-22-11-7-19(8-12-22)18-5-9-21(31-4)10-6-18/h5-13H,14H2,1-4H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting |
Bioorg Med Chem Lett 24: 2212-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.068
BindingDB Entry DOI: 10.7270/Q2125V6J |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50012542

(CHEMBL3260766)Show SMILES Cc1oc(cc1CNc1ccc(cc1)-c1ccccc1)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C26H24N2O4S/c1-18-8-6-7-11-25(18)33(30,31)28-26(29)24-16-22(19(2)32-24)17-27-23-14-12-21(13-15-23)20-9-4-3-5-10-20/h3-16,27H,17H2,1-2H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting |
Bioorg Med Chem Lett 24: 2212-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.068
BindingDB Entry DOI: 10.7270/Q2125V6J |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM24226
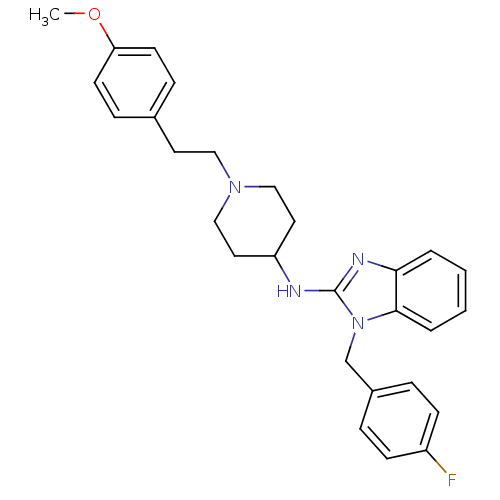
(1-[(4-fluorophenyl)methyl]-N-{1-[2-(4-methoxypheny...)Show SMILES COc1ccc(CCN2CCC(CC2)Nc2nc3ccccc3n2Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C28H31FN4O/c1-34-25-12-8-21(9-13-25)14-17-32-18-15-24(16-19-32)30-28-31-26-4-2-3-5-27(26)33(28)20-22-6-10-23(29)11-7-22/h2-13,24H,14-20H2,1H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at hERG expressed in HEK293 cells by fluorescence polarization assay |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50012545
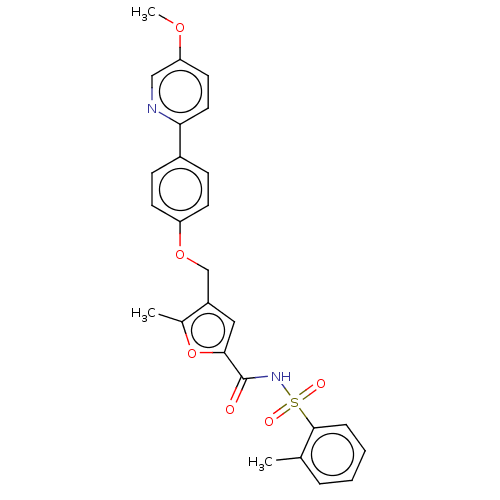
(BGC-201531 | CHEMBL1628698)Show SMILES COc1ccc(nc1)-c1ccc(OCc2cc(oc2C)C(=O)NS(=O)(=O)c2ccccc2C)cc1 Show InChI InChI=1S/C26H24N2O6S/c1-17-6-4-5-7-25(17)35(30,31)28-26(29)24-14-20(18(2)34-24)16-33-21-10-8-19(9-11-21)23-13-12-22(32-3)15-27-23/h4-15H,16H2,1-3H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting |
Bioorg Med Chem Lett 24: 2212-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.068
BindingDB Entry DOI: 10.7270/Q2125V6J |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50012534

(CHEMBL3260758)Show SMILES COc1ccc(cc1)-c1ccc(OCc2cc(oc2C)C(=O)NS(=O)(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C27H25NO6S/c1-19-23(16-26(34-19)27(29)28-35(30,31)18-20-6-4-3-5-7-20)17-33-25-14-10-22(11-15-25)21-8-12-24(32-2)13-9-21/h3-16H,17-18H2,1-2H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting |
Bioorg Med Chem Lett 24: 2212-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.068
BindingDB Entry DOI: 10.7270/Q2125V6J |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50012548
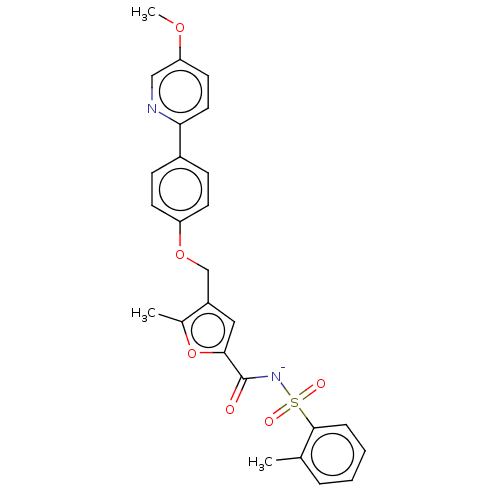
(CHEMBL3260771)Show SMILES [Na+].COc1ccc(nc1)-c1ccc(OCc2cc(oc2C)C(=O)[N-]S(=O)(=O)c2ccccc2C)cc1 Show InChI InChI=1S/C26H24N2O6S.Na/c1-17-6-4-5-7-25(17)35(30,31)28-26(29)24-14-20(18(2)34-24)16-33-21-10-8-19(9-11-21)23-13-12-22(32-3)15-27-23;/h4-15H,16H2,1-3H3,(H,28,29);/q;+1/p-1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting |
Bioorg Med Chem Lett 24: 2212-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.068
BindingDB Entry DOI: 10.7270/Q2125V6J |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50214364

(11-ethyl-1-(3-{[2-(4-methanesulfonylamino-phenoxy)...)Show SMILES CCN1CCCc2cc3NC4C=C5CCC[N+](CCCC(=O)N(CCOc6ccc(NS(C)(=O)=O)cc6)CCc6ccc(NS(C)(=O)=O)cc6)=C5C=C4Oc3cc12 |w:10.10,c:51,54,t:11| Show InChI InChI=1S/C42H53N6O7S2/c1-4-46-20-5-8-31-26-36-40(28-38(31)46)55-41-29-39-32(27-37(41)43-36)9-6-21-47(39)22-7-10-42(49)48(23-19-30-11-13-33(14-12-30)44-56(2,50)51)24-25-54-35-17-15-34(16-18-35)45-57(3,52)53/h11-18,26-29,37,43-45H,4-10,19-25H2,1-3H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50012533
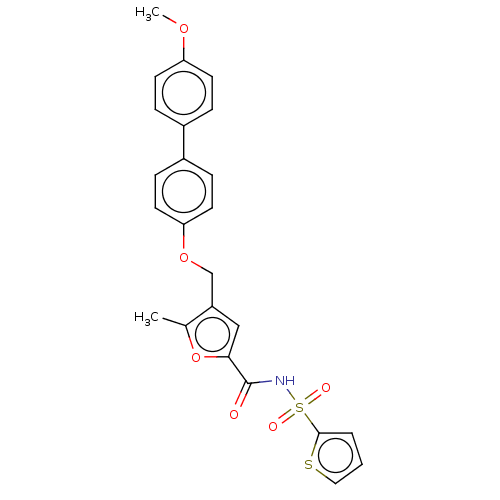
(CHEMBL3260757)Show SMILES COc1ccc(cc1)-c1ccc(OCc2cc(oc2C)C(=O)NS(=O)(=O)c2cccs2)cc1 Show InChI InChI=1S/C24H21NO6S2/c1-16-19(14-22(31-16)24(26)25-33(27,28)23-4-3-13-32-23)15-30-21-11-7-18(8-12-21)17-5-9-20(29-2)10-6-17/h3-14H,15H2,1-2H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting |
Bioorg Med Chem Lett 24: 2212-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.068
BindingDB Entry DOI: 10.7270/Q2125V6J |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50012501
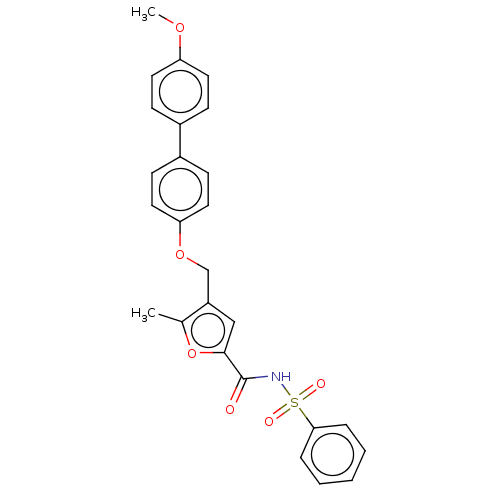
(CHEMBL3260456)Show SMILES COc1ccc(cc1)-c1ccc(OCc2cc(oc2C)C(=O)NS(=O)(=O)c2ccccc2)cc1 Show InChI InChI=1S/C26H23NO6S/c1-18-21(16-25(33-18)26(28)27-34(29,30)24-6-4-3-5-7-24)17-32-23-14-10-20(11-15-23)19-8-12-22(31-2)13-9-19/h3-16H,17H2,1-2H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting |
Bioorg Med Chem Lett 24: 2212-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.068
BindingDB Entry DOI: 10.7270/Q2125V6J |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50012530
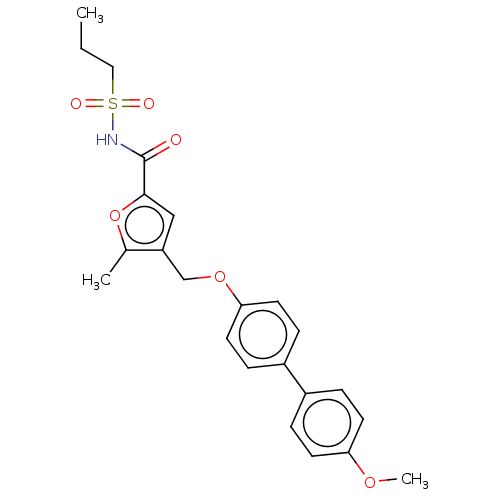
(CHEMBL3260461)Show SMILES CCCS(=O)(=O)NC(=O)c1cc(COc2ccc(cc2)-c2ccc(OC)cc2)c(C)o1 Show InChI InChI=1S/C23H25NO6S/c1-4-13-31(26,27)24-23(25)22-14-19(16(2)30-22)15-29-21-11-7-18(8-12-21)17-5-9-20(28-3)10-6-17/h5-12,14H,4,13,15H2,1-3H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting |
Bioorg Med Chem Lett 24: 2212-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.068
BindingDB Entry DOI: 10.7270/Q2125V6J |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50031720
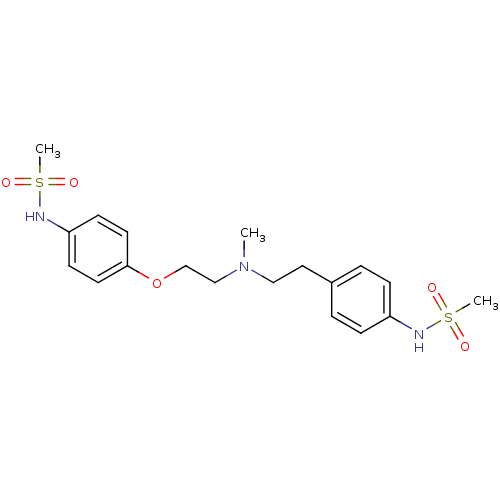
((Dofetilide) N-[4-(2-{[2-(4-Methanesulfonylamino-p...)Show SMILES CN(CCOc1ccc(NS(C)(=O)=O)cc1)CCc1ccc(NS(C)(=O)=O)cc1 Show InChI InChI=1S/C19H27N3O5S2/c1-22(13-12-16-4-6-17(7-5-16)20-28(2,23)24)14-15-27-19-10-8-18(9-11-19)21-29(3,25)26/h4-11,20-21H,12-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| DrugBank
Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50214372
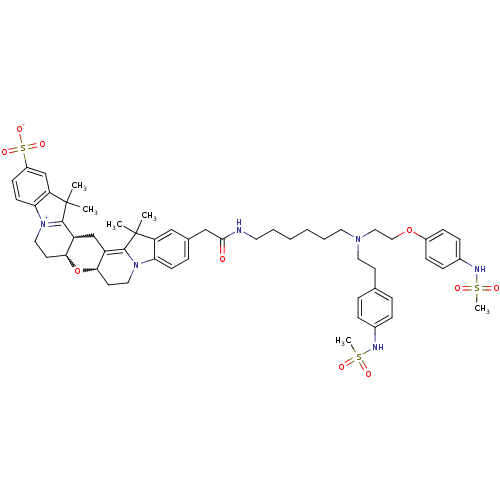
(24-{[(6-{[2-(4-methanesulfonamidophenoxy)ethyl][2-...)Show SMILES CC1(C)C2=C3CC4C(CC[N+]5=C4C(C)(C)c4cc(ccc54)S([O-])(=O)=O)OC3CCN2c2ccc(CC(=O)NCCCCCCN(CCOc3ccc(NS(C)(=O)=O)cc3)CCc3ccc(NS(C)(=O)=O)cc3)cc12 |w:26.28,6.5,7.27,c:3,10| Show InChI InChI=1S/C55H70N6O10S3/c1-54(2)45-33-38(13-21-47(45)60-29-24-49-43(52(54)60)36-44-50(71-49)25-30-61-48-22-20-42(74(67,68)69)35-46(48)55(3,4)53(44)61)34-51(62)56-26-9-7-8-10-27-59(28-23-37-11-14-39(15-12-37)57-72(5,63)64)31-32-70-41-18-16-40(17-19-41)58-73(6,65)66/h11-22,33,35,44,49-50,57-58H,7-10,23-32,34,36H2,1-6H3,(H-,56,62,67,68,69) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50012549
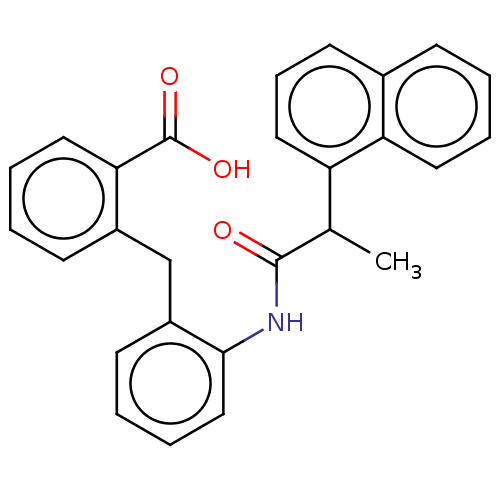
(CHEMBL3260772)Show SMILES CC(C(=O)Nc1ccccc1Cc1ccccc1C(O)=O)c1cccc2ccccc12 Show InChI InChI=1S/C27H23NO3/c1-18(22-15-8-12-19-9-2-5-13-23(19)22)26(29)28-25-16-7-4-11-21(25)17-20-10-3-6-14-24(20)27(30)31/h2-16,18H,17H2,1H3,(H,28,29)(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting |
Bioorg Med Chem Lett 24: 2212-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.068
BindingDB Entry DOI: 10.7270/Q2125V6J |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50031720

((Dofetilide) N-[4-(2-{[2-(4-Methanesulfonylamino-p...)Show SMILES CN(CCOc1ccc(NS(C)(=O)=O)cc1)CCc1ccc(NS(C)(=O)=O)cc1 Show InChI InChI=1S/C19H27N3O5S2/c1-22(13-12-16-4-6-17(7-5-16)20-28(2,23)24)14-15-27-19-10-8-18(9-11-19)21-29(3,25)26/h4-11,20-21H,12-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| DrugBank
Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at hERG expressed in HEK293 cells by fluorescence polarization assay |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50019696
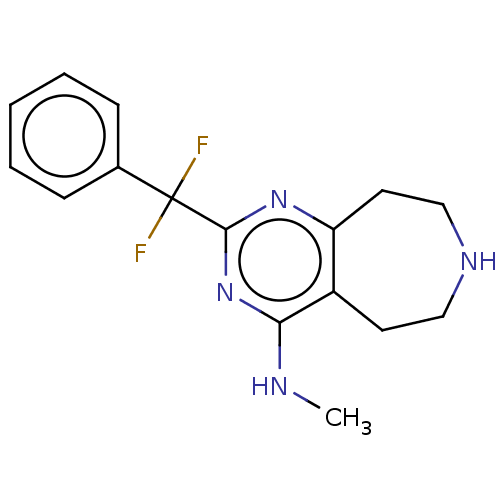
(CHEMBL3286557)Show InChI InChI=1S/C16H18F2N4/c1-19-14-12-7-9-20-10-8-13(12)21-15(22-14)16(17,18)11-5-3-2-4-6-11/h2-6,20H,7-10H2,1H3,(H,19,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
J Med Chem 57: 5258-69 (2014)
Article DOI: 10.1021/jm5003292
BindingDB Entry DOI: 10.7270/Q2J104RF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50214373
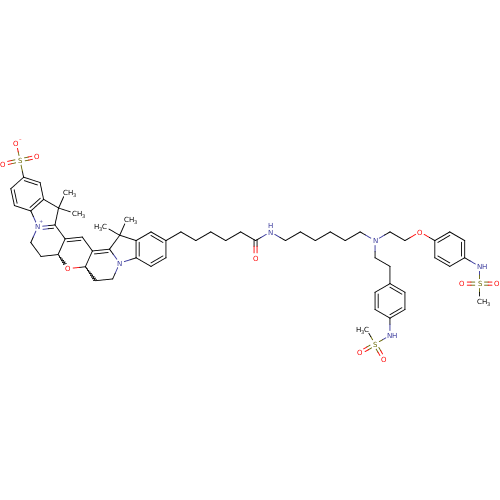
(24-{5-[(6-{[2-(4-methanesulfonamidophenoxy)ethyl][...)Show SMILES CC1(C)C2=C3C=C4C(CC[N+]5=C4C(C)(C)c4cc(ccc54)S([O-])(=O)=O)OC3CCN2c2ccc(CCCCCC(=O)NCCCCCCN(CCOc3ccc(NS(C)(=O)=O)cc3)CCc3ccc(NS(C)(=O)=O)cc3)cc12 |w:7.27,26.30,c:3,5,10| Show InChI InChI=1S/C59H76N6O10S3/c1-58(2)49-38-42(18-26-51(49)64-34-29-53-47(56(58)64)40-48-54(75-53)30-35-65-52-27-25-46(78(71,72)73)39-50(52)59(3,4)57(48)65)14-10-9-11-15-55(66)60-31-12-7-8-13-32-63(33-28-41-16-19-43(20-17-41)61-76(5,67)68)36-37-74-45-23-21-44(22-24-45)62-77(6,69)70/h16-27,38-40,53-54,61-62H,7-15,28-37H2,1-6H3,(H-,60,66,71,72,73) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50012531

(CHEMBL3260462)Show SMILES COc1ccc(cc1)-c1ccc(OCc2cc(oc2C)C(=O)NS(=O)(=O)c2ccc(O)cc2)cc1 Show InChI InChI=1S/C26H23NO7S/c1-17-20(15-25(34-17)26(29)27-35(30,31)24-13-7-21(28)8-14-24)16-33-23-11-5-19(6-12-23)18-3-9-22(32-2)10-4-18/h3-15,28H,16H2,1-2H3,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting |
Bioorg Med Chem Lett 24: 2212-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.068
BindingDB Entry DOI: 10.7270/Q2125V6J |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50214369

(24-{[(5-{[2-(4-methanesulfonamidophenoxy)ethyl][2-...)Show SMILES CC1(C)C2=C3C=C4C(CC[N+]5=C4C(C)(C)c4cc(ccc54)S([O-])(=O)=O)OC3CCN2c2ccc(CC(=O)NCCCCCN(CCOc3ccc(NS(C)(=O)=O)cc3)CCc3ccc(NS(C)(=O)=O)cc3)cc12 |w:26.28,7.27,c:3,5,10| Show InChI InChI=1S/C54H66N6O10S3/c1-53(2)44-32-37(12-20-46(44)59-28-23-48-42(51(53)59)35-43-49(70-48)24-29-60-47-21-19-41(73(66,67)68)34-45(47)54(3,4)52(43)60)33-50(61)55-25-8-7-9-26-58(27-22-36-10-13-38(14-11-36)56-71(5,62)63)30-31-69-40-17-15-39(16-18-40)57-72(6,64)65/h10-21,32,34-35,48-49,56-57H,7-9,22-31,33H2,1-6H3,(H-,55,61,66,67,68) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50012546

(CHEMBL3260769)Show SMILES COc1ccc(nc1)-c1ccc(OCc2cc(oc2C)C(=O)NS(=O)(=O)c2c(C)noc2C)cc1 Show InChI InChI=1S/C24H23N3O7S/c1-14-23(16(3)34-26-14)35(29,30)27-24(28)22-11-18(15(2)33-22)13-32-19-7-5-17(6-8-19)21-10-9-20(31-4)12-25-21/h5-12H,13H2,1-4H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting |
Bioorg Med Chem Lett 24: 2212-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.068
BindingDB Entry DOI: 10.7270/Q2125V6J |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM24226

(1-[(4-fluorophenyl)methyl]-N-{1-[2-(4-methoxypheny...)Show SMILES COc1ccc(CCN2CCC(CC2)Nc2nc3ccccc3n2Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C28H31FN4O/c1-34-25-12-8-21(9-13-25)14-17-32-18-15-24(16-19-32)30-28-31-26-4-2-3-5-27(26)33(28)20-22-6-10-23(29)11-7-22/h2-13,24H,14-20H2,1H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50012541
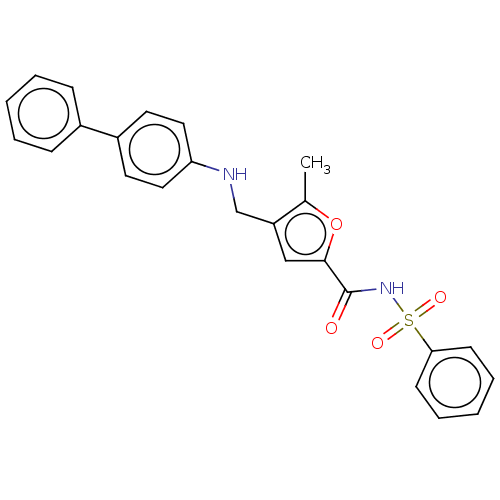
(CHEMBL3260765)Show SMILES Cc1oc(cc1CNc1ccc(cc1)-c1ccccc1)C(=O)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C25H22N2O4S/c1-18-21(17-26-22-14-12-20(13-15-22)19-8-4-2-5-9-19)16-24(31-18)25(28)27-32(29,30)23-10-6-3-7-11-23/h2-16,26H,17H2,1H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting |
Bioorg Med Chem Lett 24: 2212-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.068
BindingDB Entry DOI: 10.7270/Q2125V6J |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50012539
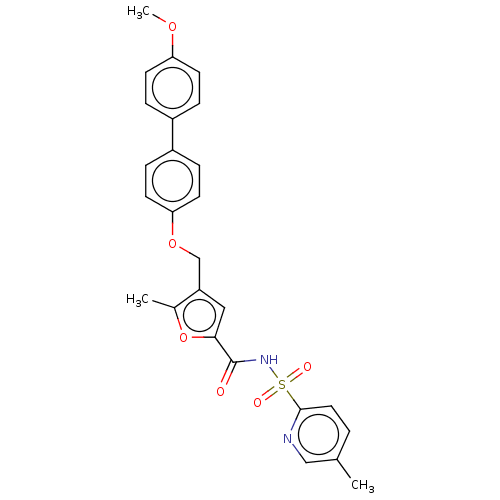
(CHEMBL3260763)Show SMILES COc1ccc(cc1)-c1ccc(OCc2cc(oc2C)C(=O)NS(=O)(=O)c2ccc(C)cn2)cc1 Show InChI InChI=1S/C26H24N2O6S/c1-17-4-13-25(27-15-17)35(30,31)28-26(29)24-14-21(18(2)34-24)16-33-23-11-7-20(8-12-23)19-5-9-22(32-3)10-6-19/h4-15H,16H2,1-3H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting |
Bioorg Med Chem Lett 24: 2212-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.068
BindingDB Entry DOI: 10.7270/Q2125V6J |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50214376
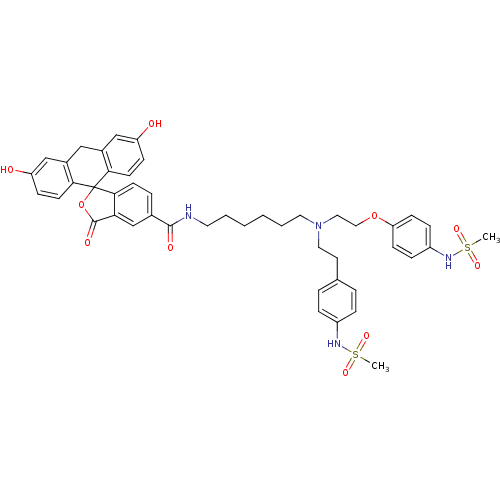
(3,6-dihydroxy-N-(6-{[2-(4-methanesulfonamidophenox...)Show SMILES CS(=O)(=O)Nc1ccc(CCN(CCCCCCNC(=O)c2ccc3c(c2)C(=O)OC32c3ccc(O)cc3Cc3cc(O)ccc23)CCOc2ccc(NS(C)(=O)=O)cc2)cc1 Show InChI InChI=1S/C46H50N4O10S2/c1-61(55,56)48-35-10-7-31(8-11-35)21-24-50(25-26-59-39-16-12-36(13-17-39)49-62(2,57)58)23-6-4-3-5-22-47-44(53)32-9-18-43-40(30-32)45(54)60-46(43)41-19-14-37(51)28-33(41)27-34-29-38(52)15-20-42(34)46/h7-20,28-30,48-49,51-52H,3-6,21-27H2,1-2H3,(H,47,53) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50019685
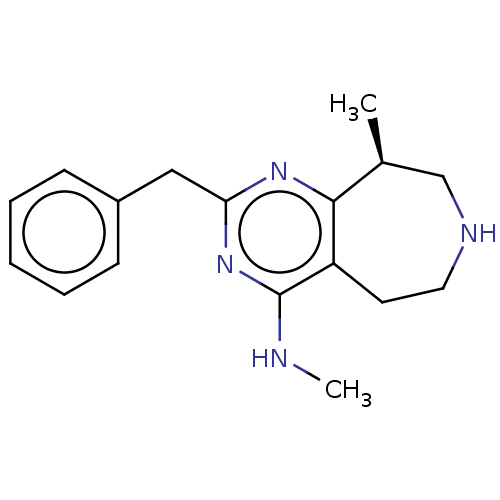
(CHEMBL3286556)Show InChI InChI=1S/C17H22N4/c1-12-11-19-9-8-14-16(12)20-15(21-17(14)18-2)10-13-6-4-3-5-7-13/h3-7,12,19H,8-11H2,1-2H3,(H,18,20,21)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
J Med Chem 57: 5258-69 (2014)
Article DOI: 10.1021/jm5003292
BindingDB Entry DOI: 10.7270/Q2J104RF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50161646
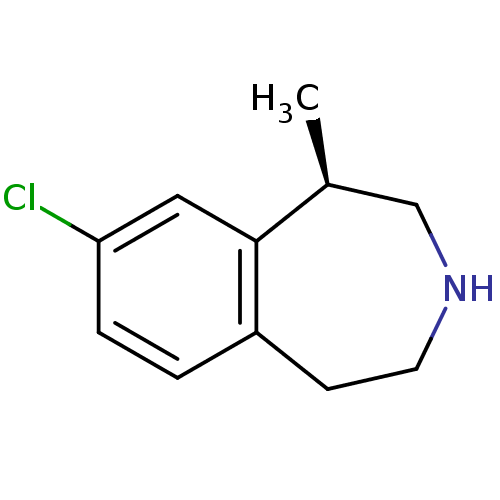
((1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-ben...)Show InChI InChI=1S/C11H14ClN/c1-8-7-13-5-4-9-2-3-10(12)6-11(8)9/h2-3,6,8,13H,4-5,7H2,1H3/t8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2C receptor expressed in HEK293 cells by scintillation counting |
Bioorg Med Chem Lett 21: 2715-20 (2011)
Article DOI: 10.1016/j.bmcl.2010.11.120
BindingDB Entry DOI: 10.7270/Q2HD7VZZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50214371
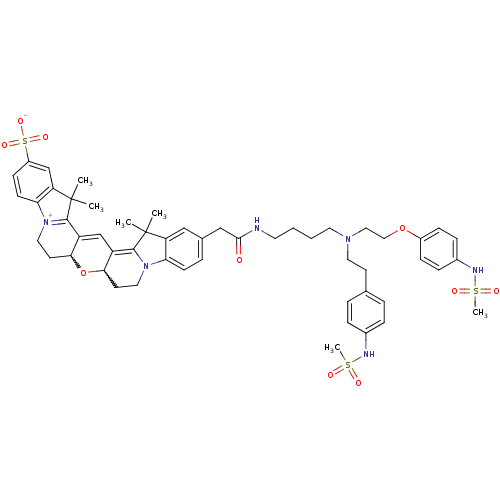
(24-{[(4-{[2-(4-methanesulfonamidophenoxy)ethyl][2-...)Show SMILES CC1(C)C2=C3C=C4C(CC[N+]5=C4C(C)(C)c4cc(ccc54)S([O-])(=O)=O)OC3CCN2c2ccc(CC(=O)NCCCCN(CCOc3ccc(NS(C)(=O)=O)cc3)CCc3ccc(NS(C)(=O)=O)cc3)cc12 |w:26.30,7.27,c:3,5,10| Show InChI InChI=1S/C53H64N6O10S3/c1-52(2)43-31-36(11-19-45(43)58-27-22-47-41(50(52)58)34-42-48(69-47)23-28-59-46-20-18-40(72(65,66)67)33-44(46)53(3,4)51(42)59)32-49(60)54-24-7-8-25-57(26-21-35-9-12-37(13-10-35)55-70(5,61)62)29-30-68-39-16-14-38(15-17-39)56-71(6,63)64/h9-20,31,33-34,47-48,55-56H,7-8,21-30,32H2,1-6H3,(H-,54,60,65,66,67) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50117930
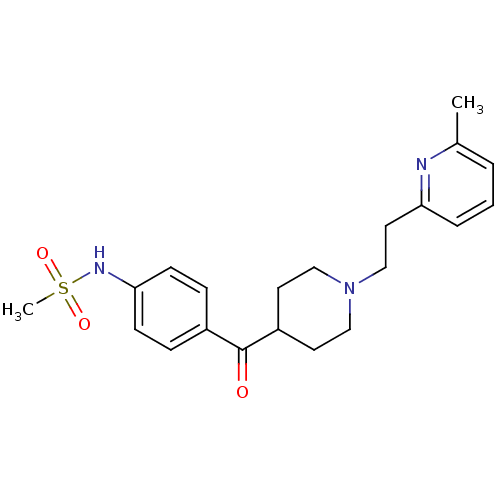
((4-{1-[2-(6-Methyl-pyridin-2-yl)-ethyl]-piperidine...)Show SMILES Cc1cccc(CCN2CCC(CC2)C(=O)c2ccc(NS(C)(=O)=O)cc2)n1 Show InChI InChI=1S/C21H27N3O3S/c1-16-4-3-5-19(22-16)12-15-24-13-10-18(11-14-24)21(25)17-6-8-20(9-7-17)23-28(2,26)27/h3-9,18,23H,10-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity at hERG expressed in HEK293 cells by fluorescence polarization assay |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50214374
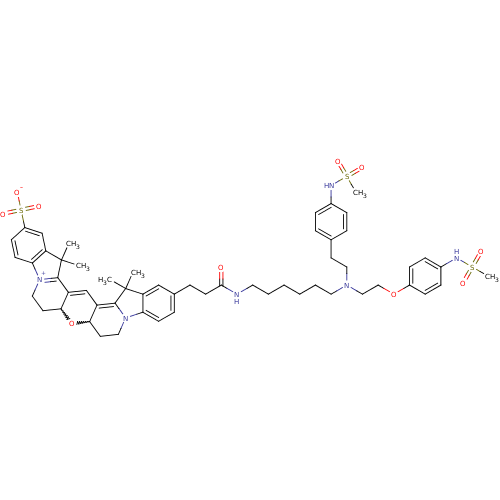
(24-{2-[(6-{[2-(4-methanesulfonamidophenoxy)ethyl][...)Show SMILES CC1(C)C2=C3C=C4C(CC[N+]5=C4C(C)(C)c4cc(ccc54)S([O-])(=O)=O)OC3CCN2c2ccc(CCC(=O)NCCCCCCN(CCOc3ccc(NS(C)(=O)=O)cc3)CCc3ccc(NS(C)(=O)=O)cc3)cc12 |w:26.28,7.27,c:3,5,10| Show InChI InChI=1S/C56H70N6O10S3/c1-55(2)46-35-39(13-22-48(46)61-31-26-50-44(53(55)61)37-45-51(72-50)27-32-62-49-23-21-43(75(68,69)70)36-47(49)56(3,4)54(45)62)14-24-52(63)57-28-9-7-8-10-29-60(30-25-38-11-15-40(16-12-38)58-73(5,64)65)33-34-71-42-19-17-41(18-20-42)59-74(6,66)67/h11-13,15-23,35-37,50-51,58-59H,7-10,14,24-34H2,1-6H3,(H-,57,63,68,69,70) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50117930

((4-{1-[2-(6-Methyl-pyridin-2-yl)-ethyl]-piperidine...)Show SMILES Cc1cccc(CCN2CCC(CC2)C(=O)c2ccc(NS(C)(=O)=O)cc2)n1 Show InChI InChI=1S/C21H27N3O3S/c1-16-4-3-5-19(22-16)12-15-24-13-10-18(11-14-24)21(25)17-6-8-20(9-7-17)23-28(2,26)27/h3-9,18,23H,10-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from hERG expressed in HEK293 cells by SPA |
J Med Chem 50: 2931-41 (2007)
Article DOI: 10.1021/jm0700565
BindingDB Entry DOI: 10.7270/Q2736RR2 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50012486

(CHEMBL3260443)Show SMILES Cc1oc(cc1COc1ccc(cc1)-c1ccc(OC(F)F)cc1)C(O)=O Show InChI InChI=1S/C20H16F2O5/c1-12-15(10-18(26-12)19(23)24)11-25-16-6-2-13(3-7-16)14-4-8-17(9-5-14)27-20(21)22/h2-10,20H,11H2,1H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting |
Bioorg Med Chem Lett 24: 2212-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.068
BindingDB Entry DOI: 10.7270/Q2125V6J |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50012511
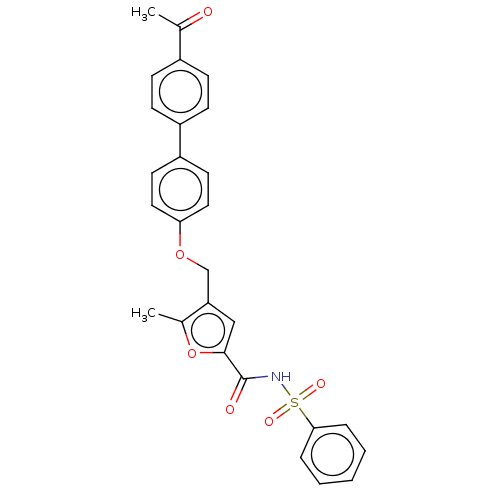
(CHEMBL3260459)Show SMILES CC(=O)c1ccc(cc1)-c1ccc(OCc2cc(oc2C)C(=O)NS(=O)(=O)c2ccccc2)cc1 Show InChI InChI=1S/C27H23NO6S/c1-18(29)20-8-10-21(11-9-20)22-12-14-24(15-13-22)33-17-23-16-26(34-19(23)2)27(30)28-35(31,32)25-6-4-3-5-7-25/h3-16H,17H2,1-2H3,(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting |
Bioorg Med Chem Lett 24: 2212-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.068
BindingDB Entry DOI: 10.7270/Q2125V6J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50019682
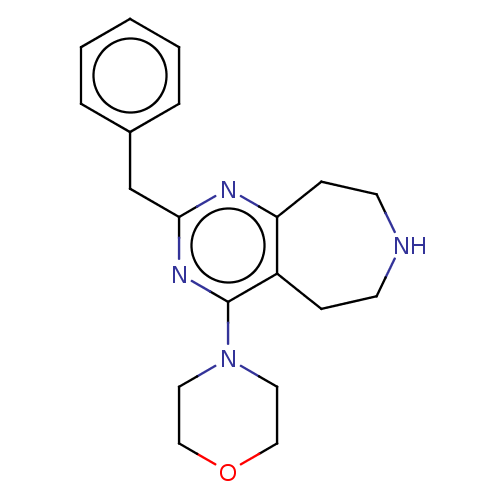
(CHEMBL3286564)Show InChI InChI=1S/C19H24N4O/c1-2-4-15(5-3-1)14-18-21-17-7-9-20-8-6-16(17)19(22-18)23-10-12-24-13-11-23/h1-5,20H,6-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
J Med Chem 57: 5258-69 (2014)
Article DOI: 10.1021/jm5003292
BindingDB Entry DOI: 10.7270/Q2J104RF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50012535
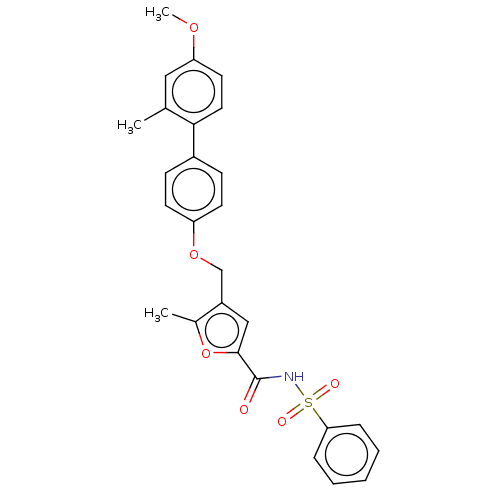
(CHEMBL3260759)Show SMILES COc1ccc(c(C)c1)-c1ccc(OCc2cc(oc2C)C(=O)NS(=O)(=O)c2ccccc2)cc1 Show InChI InChI=1S/C27H25NO6S/c1-18-15-23(32-3)13-14-25(18)20-9-11-22(12-10-20)33-17-21-16-26(34-19(21)2)27(29)28-35(30,31)24-7-5-4-6-8-24/h4-16H,17H2,1-3H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting |
Bioorg Med Chem Lett 24: 2212-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.068
BindingDB Entry DOI: 10.7270/Q2125V6J |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50012503

(CHEMBL3260458)Show SMILES COc1ccc(nc1)-c1ccc(OCc2cc(oc2C)C(=O)NS(=O)(=O)c2ccccc2)cc1 Show InChI InChI=1S/C25H22N2O6S/c1-17-19(14-24(33-17)25(28)27-34(29,30)22-6-4-3-5-7-22)16-32-20-10-8-18(9-11-20)23-13-12-21(31-2)15-26-23/h3-15H,16H2,1-2H3,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting |
Bioorg Med Chem Lett 24: 2212-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.068
BindingDB Entry DOI: 10.7270/Q2125V6J |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50012547

(CHEMBL3260770)Show SMILES COc1ccc(c(C)c1)-c1ccc(OCc2cc(oc2C)C(=O)NS(=O)(=O)c2c(C)noc2C)cc1 Show InChI InChI=1S/C26H26N2O7S/c1-15-12-22(32-5)10-11-23(15)19-6-8-21(9-7-19)33-14-20-13-24(34-17(20)3)26(29)28-36(30,31)25-16(2)27-35-18(25)4/h6-13H,14H2,1-5H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting |
Bioorg Med Chem Lett 24: 2212-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.068
BindingDB Entry DOI: 10.7270/Q2125V6J |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50012500
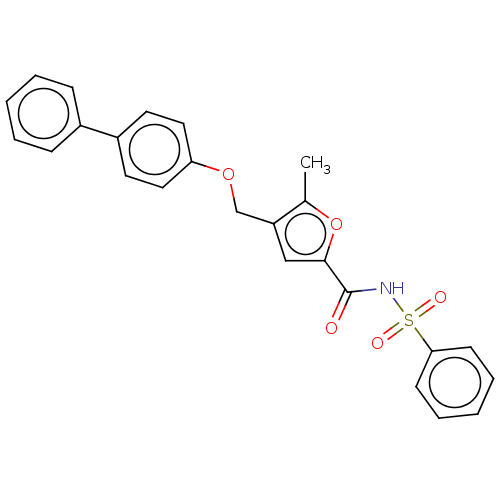
(CHEMBL3260455)Show SMILES Cc1oc(cc1COc1ccc(cc1)-c1ccccc1)C(=O)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C25H21NO5S/c1-18-21(17-30-22-14-12-20(13-15-22)19-8-4-2-5-9-19)16-24(31-18)25(27)26-32(28,29)23-10-6-3-7-11-23/h2-16H,17H2,1H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting |
Bioorg Med Chem Lett 24: 2212-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.068
BindingDB Entry DOI: 10.7270/Q2125V6J |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50019664

(CHEMBL3286562)Show InChI InChI=1S/C17H22N4/c1-2-19-17-14-8-10-18-11-9-15(14)20-16(21-17)12-13-6-4-3-5-7-13/h3-7,18H,2,8-12H2,1H3,(H,19,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
J Med Chem 57: 5258-69 (2014)
Article DOI: 10.1021/jm5003292
BindingDB Entry DOI: 10.7270/Q2J104RF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50019691
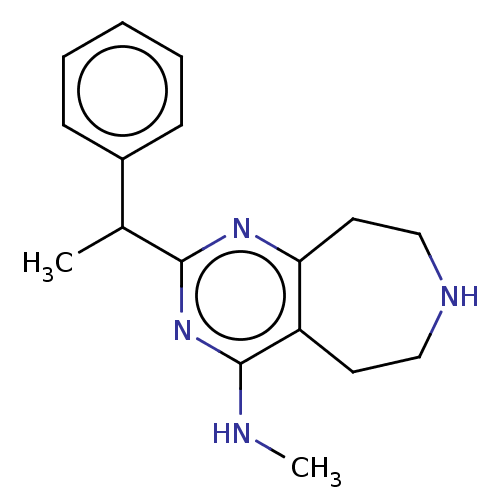
(CHEMBL3286565)Show InChI InChI=1S/C17H22N4/c1-12(13-6-4-3-5-7-13)16-20-15-9-11-19-10-8-14(15)17(18-2)21-16/h3-7,12,19H,8-11H2,1-2H3,(H,18,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
J Med Chem 57: 5258-69 (2014)
Article DOI: 10.1021/jm5003292
BindingDB Entry DOI: 10.7270/Q2J104RF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50019663

(CHEMBL3286561)Show InChI InChI=1S/C16H20N4/c1-17-16-13-7-9-18-10-8-14(13)19-15(20-16)11-12-5-3-2-4-6-12/h2-6,18H,7-11H2,1H3,(H,17,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
J Med Chem 57: 5258-69 (2014)
Article DOI: 10.1021/jm5003292
BindingDB Entry DOI: 10.7270/Q2J104RF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50019662
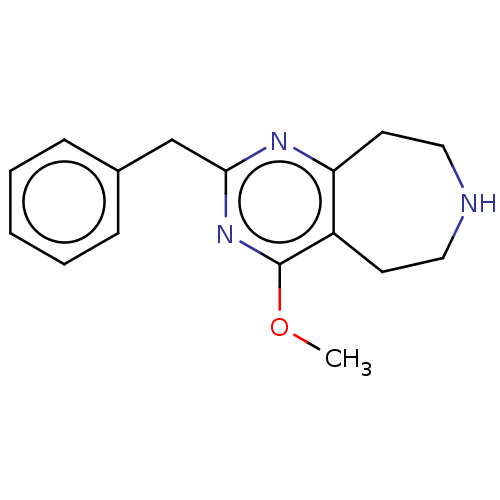
(CHEMBL3286560)Show InChI InChI=1S/C16H19N3O/c1-20-16-13-7-9-17-10-8-14(13)18-15(19-16)11-12-5-3-2-4-6-12/h2-6,17H,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
J Med Chem 57: 5258-69 (2014)
Article DOI: 10.1021/jm5003292
BindingDB Entry DOI: 10.7270/Q2J104RF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50019691

(CHEMBL3286565)Show InChI InChI=1S/C17H22N4/c1-12(13-6-4-3-5-7-13)16-20-15-9-11-19-10-8-14(15)17(18-2)21-16/h3-7,12,19H,8-11H2,1-2H3,(H,18,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
J Med Chem 57: 5258-69 (2014)
Article DOI: 10.1021/jm5003292
BindingDB Entry DOI: 10.7270/Q2J104RF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400788
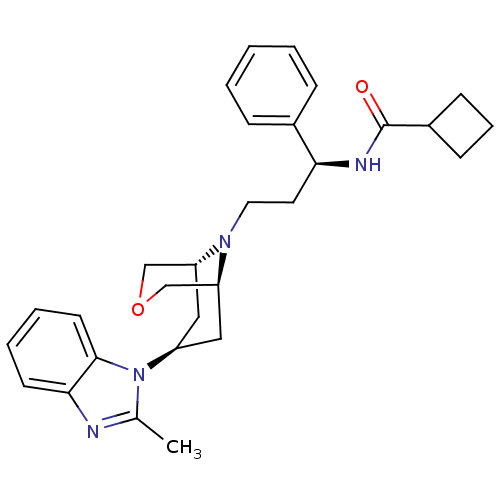
(CHEMBL2203612)Show SMILES Cc1nc2ccccc2n1[C@H]1C[C@@H]2COC[C@H](C1)N2CC[C@H](NC(=O)C1CCC1)c1ccccc1 |r,THB:19:18:17.10.11:15.13.14| Show InChI InChI=1S/C29H36N4O2/c1-20-30-27-12-5-6-13-28(27)33(20)23-16-24-18-35-19-25(17-23)32(24)15-14-26(21-8-3-2-4-9-21)31-29(34)22-10-7-11-22/h2-6,8-9,12-13,22-26H,7,10-11,14-19H2,1H3,(H,31,34)/t23-,24+,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]dofetilide from human ERG |
Chem Biol Drug Des 67: 305-8 (2006)
Article DOI: 10.1111/j.1747-0285.2006.00376.x
BindingDB Entry DOI: 10.7270/Q2D50P44 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50019692
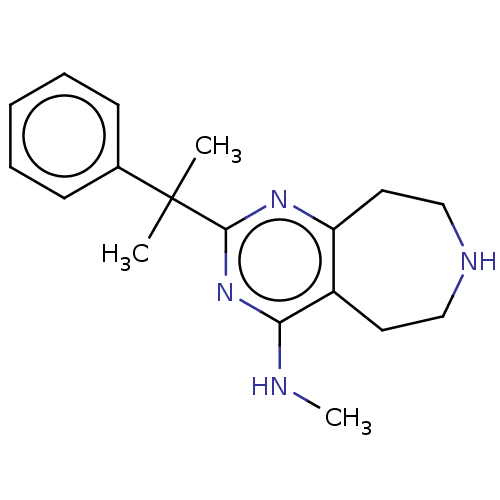
(CHEMBL3286566)Show InChI InChI=1S/C18H24N4/c1-18(2,13-7-5-4-6-8-13)17-21-15-10-12-20-11-9-14(15)16(19-3)22-17/h4-8,20H,9-12H2,1-3H3,(H,19,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
J Med Chem 57: 5258-69 (2014)
Article DOI: 10.1021/jm5003292
BindingDB Entry DOI: 10.7270/Q2J104RF |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
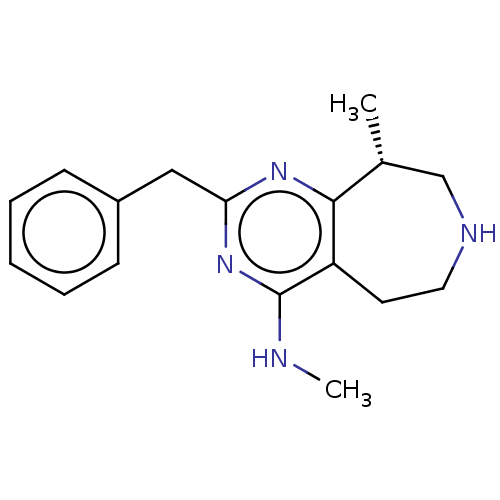
(Homo sapiens (Human)) | BDBM50019695
(CHEMBL3286555)Show InChI InChI=1S/C17H22N4/c1-12-11-19-9-8-14-16(12)20-15(21-17(14)18-2)10-13-6-4-3-5-7-13/h3-7,12,19H,8-11H2,1-2H3,(H,18,20,21)/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]-mesulergine from human recombinant 5-HT2C receptor expressed in Swiss mouse 3T3 cells by scintillation proximity assay |
J Med Chem 57: 5258-69 (2014)
Article DOI: 10.1021/jm5003292
BindingDB Entry DOI: 10.7270/Q2J104RF |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50012324

(CHEMBL3260430)Show InChI InChI=1S/C20H18O4/c1-14-17(11-19(24-14)12-20(21)22)13-23-18-9-7-16(8-10-18)15-5-3-2-4-6-15/h2-11H,12-13H2,1H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting |
Bioorg Med Chem Lett 24: 2212-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.068
BindingDB Entry DOI: 10.7270/Q2125V6J |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50012325
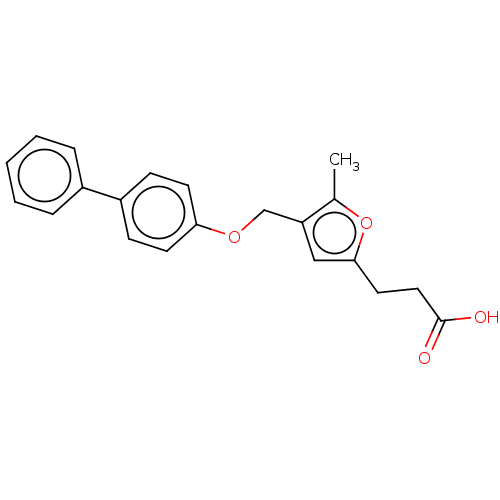
(CHEMBL3260431)Show InChI InChI=1S/C21H20O4/c1-15-18(13-20(25-15)11-12-21(22)23)14-24-19-9-7-17(8-10-19)16-5-3-2-4-6-16/h2-10,13H,11-12,14H2,1H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human prostanoid EP4 receptor expressed in cell membranes by scintillation counting |
Bioorg Med Chem Lett 24: 2212-21 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.068
BindingDB Entry DOI: 10.7270/Q2125V6J |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data