 Found 1494 hits with Last Name = 'roques' and Initial = 'bp'
Found 1494 hits with Last Name = 'roques' and Initial = 'bp' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aminopeptidase B
(Mus musculus) | BDBM50078120
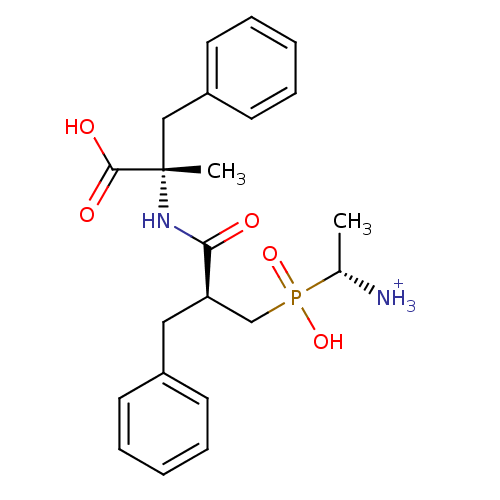
((R)-1-{[(S)-2-((S)-1-Carboxy-1-methyl-2-phenyl-eth...)Show SMILES C[C@H]([NH3+])P(O)(=O)C[C@@H](Cc1ccccc1)C(=O)N[C@@](C)(Cc1ccccc1)C(O)=O Show InChI InChI=1S/C22H29N2O5P/c1-16(23)30(28,29)15-19(13-17-9-5-3-6-10-17)20(25)24-22(2,21(26)27)14-18-11-7-4-8-12-18/h3-12,16,19H,13-15,23H2,1-2H3,(H,24,25)(H,26,27)(H,28,29)/p+1/t16-,19-,22+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| >0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity was measured on Aminopeptidase B using Arg p.NA as substrate |
Bioorg Med Chem Lett 9: 1511-6 (1999)
BindingDB Entry DOI: 10.7270/Q2H132JR |
More data for this
Ligand-Target Pair | |
Aminopeptidase B
(Mus musculus) | BDBM50078122
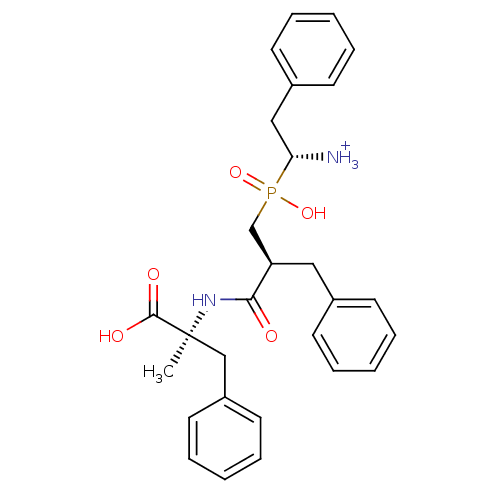
((R)-1-{[(S)-2-((S)-1-Carboxy-1-methyl-2-phenyl-eth...)Show SMILES C[C@@](Cc1ccccc1)(NC(=O)[C@H](Cc1ccccc1)CP(O)(=O)[C@@H]([NH3+])Cc1ccccc1)C(O)=O Show InChI InChI=1S/C28H33N2O5P/c1-28(27(32)33,19-23-15-9-4-10-16-23)30-26(31)24(17-21-11-5-2-6-12-21)20-36(34,35)25(29)18-22-13-7-3-8-14-22/h2-16,24-25H,17-20,29H2,1H3,(H,30,31)(H,32,33)(H,34,35)/p+1/t24-,25-,28+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| >0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity was measured on Aminopeptidase B using Arg p.NA as substrate |
Bioorg Med Chem Lett 9: 1511-6 (1999)
BindingDB Entry DOI: 10.7270/Q2H132JR |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM50078122

((R)-1-{[(S)-2-((S)-1-Carboxy-1-methyl-2-phenyl-eth...)Show SMILES C[C@@](Cc1ccccc1)(NC(=O)[C@H](Cc1ccccc1)CP(O)(=O)[C@@H]([NH3+])Cc1ccccc1)C(O)=O Show InChI InChI=1S/C28H33N2O5P/c1-28(27(32)33,19-23-15-9-4-10-16-23)30-26(31)24(17-21-11-5-2-6-12-21)20-36(34,35)25(29)18-22-13-7-3-8-14-22/h2-16,24-25H,17-20,29H2,1H3,(H,30,31)(H,32,33)(H,34,35)/p+1/t24-,25-,28+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity was measured on pig kidney Aminopeptidase N (activity for C+D stereoisomer) |
Bioorg Med Chem Lett 9: 1511-6 (1999)
BindingDB Entry DOI: 10.7270/Q2H132JR |
More data for this
Ligand-Target Pair | |
Glutamyl aminopeptidase
(Sus scrofa) | BDBM50078120

((R)-1-{[(S)-2-((S)-1-Carboxy-1-methyl-2-phenyl-eth...)Show SMILES C[C@H]([NH3+])P(O)(=O)C[C@@H](Cc1ccccc1)C(=O)N[C@@](C)(Cc1ccccc1)C(O)=O Show InChI InChI=1S/C22H29N2O5P/c1-16(23)30(28,29)15-19(13-17-9-5-3-6-10-17)20(25)24-22(2,21(26)27)14-18-11-7-4-8-12-18/h3-12,16,19H,13-15,23H2,1-2H3,(H,24,25)(H,26,27)(H,28,29)/p+1/t16-,19-,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity was measured on pig kidney Aminopeptidase N (activity for A+B stereoisomer) |
Bioorg Med Chem Lett 9: 1511-6 (1999)
BindingDB Entry DOI: 10.7270/Q2H132JR |
More data for this
Ligand-Target Pair | |
Aminopeptidase N
(Sus scrofa (Pig)) | BDBM50078120

((R)-1-{[(S)-2-((S)-1-Carboxy-1-methyl-2-phenyl-eth...)Show SMILES C[C@H]([NH3+])P(O)(=O)C[C@@H](Cc1ccccc1)C(=O)N[C@@](C)(Cc1ccccc1)C(O)=O Show InChI InChI=1S/C22H29N2O5P/c1-16(23)30(28,29)15-19(13-17-9-5-3-6-10-17)20(25)24-22(2,21(26)27)14-18-11-7-4-8-12-18/h3-12,16,19H,13-15,23H2,1-2H3,(H,24,25)(H,26,27)(H,28,29)/p+1/t16-,19-,22+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity was measured on pig kidney Aminopeptidase N (activity for C+D stereoisomer) |
Bioorg Med Chem Lett 9: 1511-6 (1999)
BindingDB Entry DOI: 10.7270/Q2H132JR |
More data for this
Ligand-Target Pair | |
Glutamyl aminopeptidase
(Sus scrofa) | BDBM50078122

((R)-1-{[(S)-2-((S)-1-Carboxy-1-methyl-2-phenyl-eth...)Show SMILES C[C@@](Cc1ccccc1)(NC(=O)[C@H](Cc1ccccc1)CP(O)(=O)[C@@H]([NH3+])Cc1ccccc1)C(O)=O Show InChI InChI=1S/C28H33N2O5P/c1-28(27(32)33,19-23-15-9-4-10-16-23)30-26(31)24(17-21-11-5-2-6-12-21)20-36(34,35)25(29)18-22-13-7-3-8-14-22/h2-16,24-25H,17-20,29H2,1H3,(H,30,31)(H,32,33)(H,34,35)/p+1/t24-,25-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity was measured on Aminopeptidase using GluNA as substrate |
Bioorg Med Chem Lett 9: 1511-6 (1999)
BindingDB Entry DOI: 10.7270/Q2H132JR |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50045797
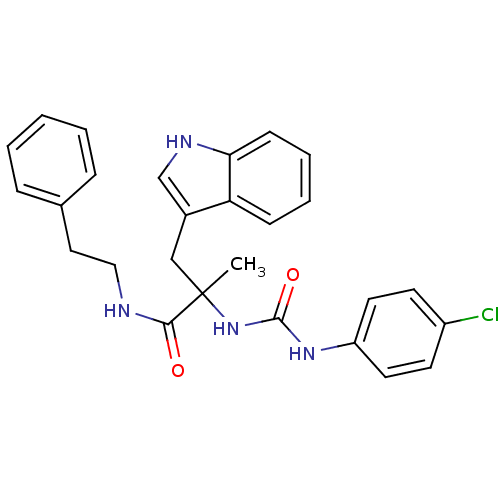
(2-[3-(4-Chloro-phenyl)-ureido]-3-(1H-indol-3-yl)-2...)Show SMILES CC(Cc1c[nH]c2ccccc12)(NC(=O)Nc1ccc(Cl)cc1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C27H27ClN4O2/c1-27(17-20-18-30-24-10-6-5-9-23(20)24,25(33)29-16-15-19-7-3-2-4-8-19)32-26(34)31-22-13-11-21(28)12-14-22/h2-14,18,30H,15-17H2,1H3,(H,29,33)(H2,31,32,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Tested for inhibition of [3H]-pCCK-8 specific binding to cholecystokinin type B receptor in guinea pig brain cortex |
J Med Chem 36: 2868-77 (1993)
BindingDB Entry DOI: 10.7270/Q2MG7NK5 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50281664

(CHEMBL262894 | p-Tyr(SO3Na)-gNle-mGly-Trp-(N-Me)Nl...)Show SMILES CCCC[C@H](NC(=O)[C@@H](N)Cc1ccc(OS([O-])(=O)=O)cc1)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C47H61N9O13S/c1-3-5-15-35(53-43(61)33(48)22-29-18-20-31(21-19-29)69-70(66,67)68)44(62)51-27-40(57)52-38(24-30-26-50-34-17-11-10-14-32(30)34)46(64)54-36(16-6-4-2)45(63)56-39(25-41(58)59)47(65)55-37(42(49)60)23-28-12-8-7-9-13-28/h7-14,17-21,26,33,35-39,50H,3-6,15-16,22-25,27,48H2,1-2H3,(H2,49,60)(H,51,62)(H,52,57)(H,53,61)(H,54,64)(H,55,65)(H,56,63)(H,58,59)(H,66,67,68)/p-1/t33-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the inhibition of [3H]-pCCK-8 to Cholecystokinin type B receptor in guinea pig brain |
Bioorg Med Chem Lett 3: 847-850 (1993)
Article DOI: 10.1016/S0960-894X(00)80678-2
BindingDB Entry DOI: 10.7270/Q2P26Z28 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50046128
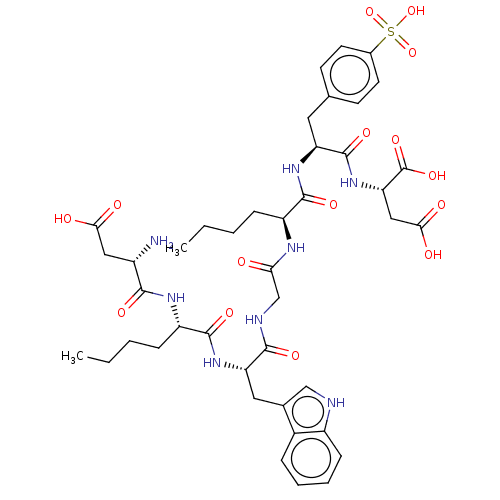
(2-[2-(2-{2-[2-{2-[3-Carboxy-2-(2-methylamino-3-phe...)Show SMILES CCCC[C@H](NC(=O)CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCC)NC(=O)[C@@H](N)CC(O)=O)C(=O)N[C@@H](Cc1ccc(cc1)S(O)(=O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O Show InChI InChI=1S/C42H56N8O15S/c1-3-5-10-29(39(58)48-31(41(60)50-33(42(61)62)20-36(54)55)17-23-13-15-25(16-14-23)66(63,64)65)46-34(51)22-45-38(57)32(18-24-21-44-28-12-8-7-9-26(24)28)49-40(59)30(11-6-4-2)47-37(56)27(43)19-35(52)53/h7-9,12-16,21,27,29-33,44H,3-6,10-11,17-20,22,43H2,1-2H3,(H,45,57)(H,46,51)(H,47,56)(H,48,58)(H,49,59)(H,50,60)(H,52,53)(H,54,55)(H,61,62)(H,63,64,65)/t27-,29-,30-,31-,32-,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]pCCK-8 from cholecystokinin type B receptor in guinea pig brain membrane |
J Med Chem 36: 166-72 (1993)
BindingDB Entry DOI: 10.7270/Q26M37FJ |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50016504

((S)-3-(2-{[(S)-2-(2-{2-[2-tert-Butoxycarbonylamino...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS([O-])(=O)=O)cc1)NC(=O)OC(C)(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C52H69N9O15S/c1-6-8-18-37(57-48(68)40(61-51(71)75-52(3,4)5)26-32-21-23-34(24-22-32)76-77(72,73)74)46(66)55-30-43(62)56-41(27-33-29-54-36-20-14-13-17-35(33)36)49(69)58-38(19-9-7-2)47(67)60-42(28-44(63)64)50(70)59-39(45(53)65)25-31-15-11-10-12-16-31/h10-17,20-24,29,37-42,54H,6-9,18-19,25-28,30H2,1-5H3,(H2,53,65)(H,55,66)(H,56,62)(H,57,68)(H,58,69)(H,59,70)(H,60,67)(H,61,71)(H,63,64)(H,72,73,74)/p-1/t37-,38-,39-,40-,41-,42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the inhibition of [3H]-pCCK-8 binding to Cholecystokinin type B receptor in guinea pig brain |
Bioorg Med Chem Lett 3: 847-850 (1993)
Article DOI: 10.1016/S0960-894X(00)80678-2
BindingDB Entry DOI: 10.7270/Q2P26Z28 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Mus musculus-MOUSE) | BDBM50016425

((S)-3-{(S)-2-[(S)-2-(2-{(S)-2-[(S)-2-tert-Butoxyca...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)OC(C)(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C52H69N9O15S/c1-6-8-18-37(57-48(68)40(61-51(71)75-52(3,4)5)26-32-21-23-34(24-22-32)76-77(72,73)74)46(66)55-30-43(62)56-41(27-33-29-54-36-20-14-13-17-35(33)36)49(69)58-38(19-9-7-2)47(67)60-42(28-44(63)64)50(70)59-39(45(53)65)25-31-15-11-10-12-16-31/h10-17,20-24,29,37-42,54H,6-9,18-19,25-28,30H2,1-5H3,(H2,53,65)(H,55,66)(H,56,62)(H,57,68)(H,58,69)(H,59,70)(H,60,67)(H,61,71)(H,63,64)(H,72,73,74)/t37-,38-,39-,40-,41-,42-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity measured by inhibiting [3H]Boc[Nle28,31]CCK27-33 specific binding to Cholecystokinin receptor in mouse brain membranes at a KD conce... |
J Med Chem 30: 962-8 (1987)
BindingDB Entry DOI: 10.7270/Q21J98R1 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50016504

((S)-3-(2-{[(S)-2-(2-{2-[2-tert-Butoxycarbonylamino...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS([O-])(=O)=O)cc1)NC(=O)OC(C)(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C52H69N9O15S/c1-6-8-18-37(57-48(68)40(61-51(71)75-52(3,4)5)26-32-21-23-34(24-22-32)76-77(72,73)74)46(66)55-30-43(62)56-41(27-33-29-54-36-20-14-13-17-35(33)36)49(69)58-38(19-9-7-2)47(67)60-42(28-44(63)64)50(70)59-39(45(53)65)25-31-15-11-10-12-16-31/h10-17,20-24,29,37-42,54H,6-9,18-19,25-28,30H2,1-5H3,(H2,53,65)(H,55,66)(H,56,62)(H,57,68)(H,58,69)(H,59,70)(H,60,67)(H,61,71)(H,63,64)(H,72,73,74)/p-1/t37-,38-,39-,40-,41-,42-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of [3H]-Propionyl specific binding to Cholecystokinin 8 receptor of guinea pig brain |
J Med Chem 32: 445-9 (1989)
BindingDB Entry DOI: 10.7270/Q2S181GT |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50016425

((S)-3-{(S)-2-[(S)-2-(2-{(S)-2-[(S)-2-tert-Butoxyca...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)OC(C)(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C52H69N9O15S/c1-6-8-18-37(57-48(68)40(61-51(71)75-52(3,4)5)26-32-21-23-34(24-22-32)76-77(72,73)74)46(66)55-30-43(62)56-41(27-33-29-54-36-20-14-13-17-35(33)36)49(69)58-38(19-9-7-2)47(67)60-42(28-44(63)64)50(70)59-39(45(53)65)25-31-15-11-10-12-16-31/h10-17,20-24,29,37-42,54H,6-9,18-19,25-28,30H2,1-5H3,(H2,53,65)(H,55,66)(H,56,62)(H,57,68)(H,58,69)(H,59,70)(H,60,67)(H,61,71)(H,63,64)(H,72,73,74)/t37-,38-,39-,40-,41-,42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of 0.2 nM [3H]-pCCK-8 from guinea pig brain membranes |
J Med Chem 32: 1184-90 (1989)
BindingDB Entry DOI: 10.7270/Q2ZG6R7N |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of 0.1 nM [3H]-pCCK-8 from guinea pig pancreatic membranes |
J Med Chem 32: 1184-90 (1989)
BindingDB Entry DOI: 10.7270/Q2ZG6R7N |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of 0.2 nM [3H]-pCCK-8 from guinea pig brain membranes |
J Med Chem 32: 1184-90 (1989)
BindingDB Entry DOI: 10.7270/Q2ZG6R7N |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of [3H]-Propionyl specific binding to CCK-8 receptor of guinea pig brain |
J Med Chem 32: 445-9 (1989)
BindingDB Entry DOI: 10.7270/Q2S181GT |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of [3H]pCCK-8 binding to Cholecystokinin type A receptor of Guinea pig pancreatic membranes |
J Med Chem 40: 647-58 (1997)
Article DOI: 10.1021/jm9603072
BindingDB Entry DOI: 10.7270/Q2PG1SDX |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Mus musculus-MOUSE) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Potency in displacing [3H]propionyl-Cholecystokinin from mouse brain membranes. |
J Med Chem 31: 966-70 (1988)
BindingDB Entry DOI: 10.7270/Q2XP775P |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]pCCK-8 from cholecystokinin type B receptor in guinea pig brain membrane |
J Med Chem 36: 166-72 (1993)
BindingDB Entry DOI: 10.7270/Q26M37FJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50021325

(4-methyl-(1S,5R,13R,14S)-12-oxa-4-azapentacyclo[9....)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1C3CC[C@@H]4O)ccc5O |TLB:13:12:8.9.10:1.3.2| Show InChI InChI=1S/C17H21NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2,4,10-11,13,16,19-20H,3,5-8H2,1H3/t10?,11-,13+,16+,17+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungarian Academy of Sciences
Curated by PDSP Ki Database
| |
Eur J Pharmacol 383: 209-14 (1999)
Article DOI: 10.1016/s0014-2999(99)00610-x
BindingDB Entry DOI: 10.7270/Q2TT4PJ9 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50449787
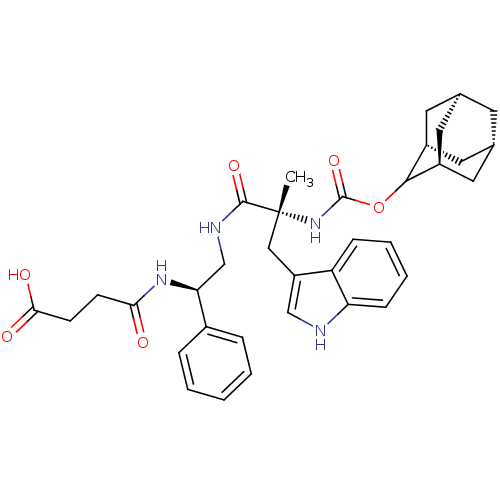
(CHEMBL2062154 | PD-134308)Show SMILES [H][C@@]12C[C@@]3([H])C[C@@]([H])(C1)C(OC(=O)N[C@](C)(Cc1c[nH]c4ccccc14)C(=O)NC[C@H](NC(=O)CCC(O)=O)c1ccccc1)[C@@]([H])(C2)C3 |wU:30.41,14.15,45.49,3.3,wD:6.6,1.0,TLB:5:3:47:9.6.8,10:9:47:3.48.2,THB:5:6:47:3.48.2,10:9:1.47.8:3.5.48,2:3:9:1.47.8,2:1:9:3.5.48,(-14.99,-2.1,;-13.56,-2.66,;-14.77,-3.94,;-13.26,-3.53,;-13.35,-5.06,;-11.86,-4.09,;-10.83,-2.82,;-9.38,-3.33,;-12.24,-3.16,;-10.83,-1.28,;-9.29,-1.31,;-8.5,.01,;-9.25,1.36,;-6.96,-.01,;-6.19,1.3,;-5.42,-.02,;-7.44,2.2,;-7.28,3.74,;-8.44,4.76,;-7.81,6.18,;-6.29,6.03,;-5.14,7.07,;-3.69,6.6,;-3.34,5.08,;-4.49,4.06,;-5.94,4.52,;-4.66,1.42,;-3.99,2.8,;-3.79,.15,;-2.27,.27,;-1.4,-1.02,;-2.08,-2.4,;-1.22,-3.69,;.32,-3.58,;-1.9,-5.07,;-3.43,-5.16,;-4.1,-6.57,;-5.64,-6.67,;-3.25,-7.83,;.14,-.91,;.99,-2.2,;2.51,-2.08,;3.19,-.7,;2.32,.59,;.8,.47,;-12.23,-.7,;-12.2,.82,;-13.58,-1.18,;-13.27,-1.94,)| Show InChI InChI=1S/C35H42N4O6/c1-35(18-26-19-36-28-10-6-5-9-27(26)28,39-34(44)45-32-24-14-21-13-22(16-24)17-25(32)15-21)33(43)37-20-29(23-7-3-2-4-8-23)38-30(40)11-12-31(41)42/h2-10,19,21-22,24-25,29,32,36H,11-18,20H2,1H3,(H,37,43)(H,38,40)(H,39,44)(H,41,42)/t21-,22+,24-,25+,29-,32?,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Compound was tested for the affinity against Cholecystokinin type B receptor on guinea pig cortex. |
J Med Chem 40: 3947-56 (1998)
Article DOI: 10.1021/jm970439a
BindingDB Entry DOI: 10.7270/Q27H1K8Z |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50281665
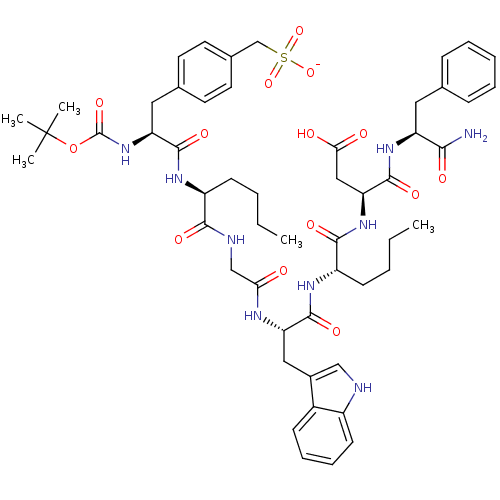
(Boc-Phe(CH2-SO3Na)-gNle-mCIy-Trp-(N-Me)Nle-Asp-Phe...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(CS([O-])(=O)=O)cc1)NC(=O)OC(C)(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C53H71N9O14S/c1-6-8-18-38(58-49(69)41(62-52(72)76-53(3,4)5)26-33-21-23-34(24-22-33)31-77(73,74)75)47(67)56-30-44(63)57-42(27-35-29-55-37-20-14-13-17-36(35)37)50(70)59-39(19-9-7-2)48(68)61-43(28-45(64)65)51(71)60-40(46(54)66)25-32-15-11-10-12-16-32/h10-17,20-24,29,38-43,55H,6-9,18-19,25-28,30-31H2,1-5H3,(H2,54,66)(H,56,67)(H,57,63)(H,58,69)(H,59,70)(H,60,71)(H,61,68)(H,62,72)(H,64,65)(H,73,74,75)/p-1/t38-,39-,40-,41-,42-,43-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the inhibition of [3H]-pCCK-8 to Cholecystokinin type B receptor in guinea pig brain |
Bioorg Med Chem Lett 3: 847-850 (1993)
Article DOI: 10.1016/S0960-894X(00)80678-2
BindingDB Entry DOI: 10.7270/Q2P26Z28 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor
(Mus musculus-MOUSE) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.491 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity measured by inhibiting [3H]Boc[Nle28,31]CCK27-33 specific binding to Cholecystokinin receptor in mouse brain membranes at a KD conce... |
J Med Chem 30: 962-8 (1987)
BindingDB Entry DOI: 10.7270/Q21J98R1 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50061266
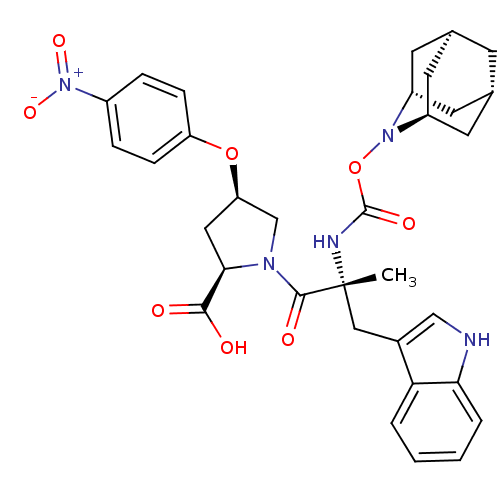
((2R,4R)-1-[(R)-2-(2-Aza-tricyclo[3.3.1.1*3,7*]dec-...)Show SMILES C[C@](Cc1c[nH]c2ccccc12)(NC(=O)ON1[C@H]2C[C@@H]3C[C@@H](C[C@H]1C3)C2)C(=O)N1C[C@@H](C[C@@H]1C(O)=O)Oc1ccc(cc1)[N+]([O-])=O |TLB:24:23:25:19.18.20,15:16:18:21.25.20,THB:22:23:18:21.25.20,22:21:18:16.23.24,15:16:25:19.18.20| Show InChI InChI=1S/C33H37N5O8/c1-33(16-21-17-34-28-5-3-2-4-27(21)28,35-32(42)46-37-23-11-19-10-20(13-23)14-24(37)12-19)31(41)36-18-26(15-29(36)30(39)40)45-25-8-6-22(7-9-25)38(43)44/h2-9,17,19-20,23-24,26,29,34H,10-16,18H2,1H3,(H,35,42)(H,39,40)/t19-,20+,23-,24+,26-,29-,33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity (affinity state 1) for Cholecystokinin type B receptor, was determined using CHO cells |
J Med Chem 40: 3947-56 (1998)
Article DOI: 10.1021/jm970439a
BindingDB Entry DOI: 10.7270/Q27H1K8Z |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50018060
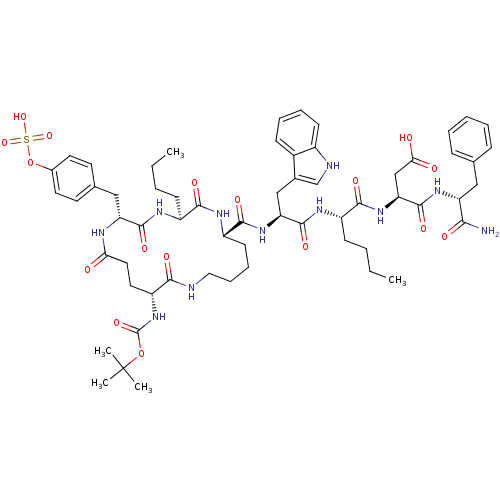
(Boc-cyclo-(gama-D-Glu-Tyr(SO3H)-Nle-D-Lys)-Trp-Nle...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@@H]1CCCCNC(=O)[C@@H](CCC(=O)N[C@H](Cc2ccc(OS(O)(=O)=O)cc2)C(=O)N[C@@H](CCCC)C(=O)N1)NC(=O)OC(C)(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C61H83N11O17S/c1-6-8-20-42-54(78)66-44(23-15-16-30-63-53(77)45(72-60(84)88-61(3,4)5)28-29-50(73)65-47(57(81)67-42)32-37-24-26-39(27-25-37)89-90(85,86)87)56(80)70-48(33-38-35-64-41-22-14-13-19-40(38)41)58(82)68-43(21-9-7-2)55(79)71-49(34-51(74)75)59(83)69-46(52(62)76)31-36-17-11-10-12-18-36/h10-14,17-19,22,24-27,35,42-49,64H,6-9,15-16,20-21,23,28-34H2,1-5H3,(H2,62,76)(H,63,77)(H,65,73)(H,66,78)(H,67,81)(H,68,82)(H,69,83)(H,70,80)(H,71,79)(H,72,84)(H,74,75)(H,85,86,87)/t42-,43-,44-,45+,46+,47+,48-,49-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of 0.2 nM [3H]-pCCK-8 from guinea pig brain membranes |
J Med Chem 32: 1184-90 (1989)
BindingDB Entry DOI: 10.7270/Q2ZG6R7N |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(RAT) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Affinity to inhibit [3H]pCCK-8 specific binding on rat brain Cholecystokinin type B receptor expressed in CHO cells |
J Med Chem 40: 647-58 (1997)
Article DOI: 10.1021/jm9603072
BindingDB Entry DOI: 10.7270/Q2PG1SDX |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of [3H]-Propionyl specific binding to Cholecystokinin 8 receptor of guinea pig brain |
J Med Chem 32: 445-9 (1989)
BindingDB Entry DOI: 10.7270/Q2S181GT |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of [3H]pCCK-8 binding to Guinea pig cortex membrane Cholecystokinin type B receptor |
J Med Chem 40: 647-58 (1997)
Article DOI: 10.1021/jm9603072
BindingDB Entry DOI: 10.7270/Q2PG1SDX |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the inhibition of [3H]-pCCK-8 binding to Cholecystokinin type A receptor in pancreatic membranes of guinea-pig |
Bioorg Med Chem Lett 3: 847-850 (1993)
Article DOI: 10.1016/S0960-894X(00)80678-2
BindingDB Entry DOI: 10.7270/Q2P26Z28 |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for the inhibition of [3H]-pCCK-8 binding to Cholecystokinin type B receptor in guinea pig brain |
Bioorg Med Chem Lett 3: 847-850 (1993)
Article DOI: 10.1016/S0960-894X(00)80678-2
BindingDB Entry DOI: 10.7270/Q2P26Z28 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50024101
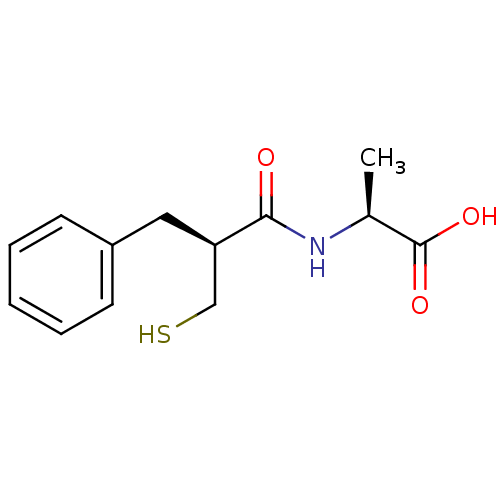
((S)-2-((S)-2-Mercaptomethyl-3-phenyl-propionylamin...)Show InChI InChI=1S/C13H17NO3S/c1-9(13(16)17)14-12(15)11(8-18)7-10-5-3-2-4-6-10/h2-6,9,11,18H,7-8H2,1H3,(H,14,15)(H,16,17)/t9-,11+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaleads , Paris BioPark, 11 Rue Watt, 75013 Paris, France.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NEP using Suc-Ala-Ala-Phe-AMC as substrate after 30 mins by fluorimetry |
J Med Chem 57: 5748-63 (2014)
Article DOI: 10.1021/jm500602h
BindingDB Entry DOI: 10.7270/Q2Q241TH |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM21147

((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...)Show SMILES CSCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)[C@@H](N)CC(O)=O)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCSC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H62N10O16S3/c1-76-18-16-34(55-47(69)37(58-44(66)32(50)23-41(61)62)21-28-12-14-30(15-13-28)75-78(72,73)74)45(67)53-26-40(60)54-38(22-29-25-52-33-11-7-6-10-31(29)33)48(70)56-35(17-19-77-2)46(68)59-39(24-42(63)64)49(71)57-36(43(51)65)20-27-8-4-3-5-9-27/h3-15,25,32,34-39,52H,16-24,26,50H2,1-2H3,(H2,51,65)(H,53,67)(H,54,60)(H,55,69)(H,56,70)(H,57,71)(H,58,66)(H,59,68)(H,61,62)(H,63,64)(H,72,73,74)/t32-,34-,35-,36-,37-,38-,39-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]-pCCK-8 from cholecystokinin-A receptor in guinea pig pancreatic membrane |
J Med Chem 36: 166-72 (1993)
BindingDB Entry DOI: 10.7270/Q26M37FJ |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50407297
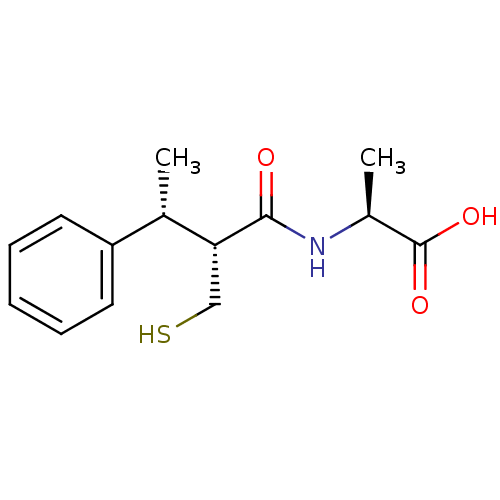
(CHEMBL2052008)Show SMILES C[C@H](NC(=O)[C@H](CS)[C@@H](C)c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C14H19NO3S/c1-9(11-6-4-3-5-7-11)12(8-19)13(16)15-10(2)14(17)18/h3-7,9-10,12,19H,8H2,1-2H3,(H,15,16)(H,17,18)/t9-,10-,12+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney |
J Med Chem 37: 1070-83 (1994)
BindingDB Entry DOI: 10.7270/Q26W995T |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM21653
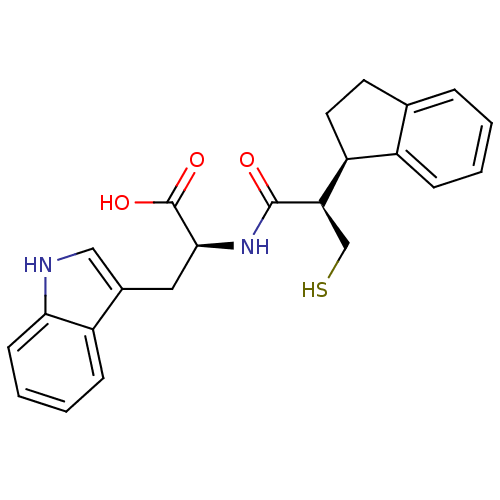
((2S)-2-[(2R)-2-[(1R)-2,3-dihydro-1H-inden-1-yl]-3-...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CS)[C@H]1CCc2ccccc12 |r| Show InChI InChI=1S/C23H24N2O3S/c26-22(19(13-29)18-10-9-14-5-1-2-6-16(14)18)25-21(23(27)28)11-15-12-24-20-8-4-3-7-17(15)20/h1-8,12,18-19,21,24,29H,9-11,13H2,(H,25,26)(H,27,28)/t18-,19+,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | -54.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
CNRS
| Assay Description
NEP was preincubated in black 96-well microplates with or without increasing concentrations of inhibitors. DGPA was added, and the reaction was stopp... |
J Med Chem 45: 1477-86 (2002)
Article DOI: 10.1021/jm0005454
BindingDB Entry DOI: 10.7270/Q2T72FQ1 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM21653

((2S)-2-[(2R)-2-[(1R)-2,3-dihydro-1H-inden-1-yl]-3-...)Show SMILES OC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CS)[C@H]1CCc2ccccc12 |r| Show InChI InChI=1S/C23H24N2O3S/c26-22(19(13-29)18-10-9-14-5-1-2-6-16(14)18)25-21(23(27)28)11-15-12-24-20-8-4-3-7-17(15)20/h1-8,12,18-19,21,24,29H,9-11,13H2,(H,25,26)(H,27,28)/t18-,19+,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of Neutral endopeptidase. |
Bioorg Med Chem Lett 12: 2001-5 (2002)
BindingDB Entry DOI: 10.7270/Q27080S4 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50407297

(CHEMBL2052008)Show SMILES C[C@H](NC(=O)[C@H](CS)[C@@H](C)c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C14H19NO3S/c1-9(11-6-4-3-5-7-11)12(8-19)13(16)15-10(2)14(17)18/h3-7,9-10,12,19H,8H2,1-2H3,(H,15,16)(H,17,18)/t9-,10-,12+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney |
J Med Chem 37: 1070-83 (1994)
BindingDB Entry DOI: 10.7270/Q26W995T |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50046121
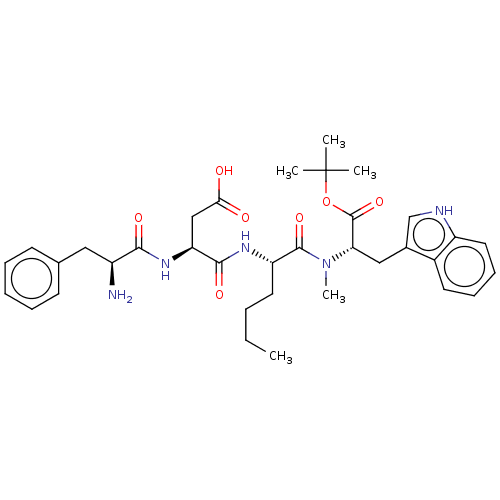
(3-(2-Amino-3-phenyl-propionylamino)-N-(1-{[1-tert-...)Show SMILES CCCC[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](N)Cc1ccccc1)C(=O)N(C)[C@@H](Cc1c[nH]c2ccccc12)C(=O)OC(C)(C)C Show InChI InChI=1S/C35H47N5O7/c1-6-7-16-27(38-32(44)28(20-30(41)42)39-31(43)25(36)18-22-13-9-8-10-14-22)33(45)40(5)29(34(46)47-35(2,3)4)19-23-21-37-26-17-12-11-15-24(23)26/h8-15,17,21,25,27-29,37H,6-7,16,18-20,36H2,1-5H3,(H,38,44)(H,39,43)(H,41,42)/t25-,27-,28-,29-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]pCCK-8 from cholecystokinin type B receptor in guinea pig brain membrane |
J Med Chem 36: 166-72 (1993)
BindingDB Entry DOI: 10.7270/Q26M37FJ |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50056453
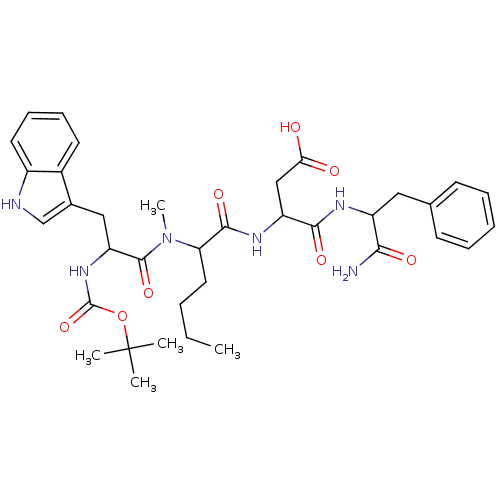
(3-(2-{[2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl...)Show SMILES CCCCC(N(C)C(=O)C(Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C)C(=O)NC(CC(O)=O)C(=O)NC(Cc1ccccc1)C(N)=O Show InChI InChI=1S/C36H48N6O8/c1-6-7-17-29(33(47)40-27(20-30(43)44)32(46)39-26(31(37)45)18-22-13-9-8-10-14-22)42(5)34(48)28(41-35(49)50-36(2,3)4)19-23-21-38-25-16-12-11-15-24(23)25/h8-16,21,26-29,38H,6-7,17-20H2,1-5H3,(H2,37,45)(H,39,46)(H,40,47)(H,41,49)(H,43,44) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of [3H]pCCK-8 binding to Guinea pig cortex membrane Cholecystokinin type B receptor |
J Med Chem 40: 647-58 (1997)
Article DOI: 10.1021/jm9603072
BindingDB Entry DOI: 10.7270/Q2PG1SDX |
More data for this
Ligand-Target Pair | |
Glutamyl aminopeptidase
(Homo sapiens (Human)) | BDBM50083386

(1-{[1-(2,3-Dicarboxy-pyrrolidine-1-carbonyl)-2-met...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](S)[C@H]([NH3+])CCS([O-])(=O)=O)C(=O)N1CCC([C@H]1C(O)=O)C(O)=O Show InChI InChI=1S/C17H29N3O9S2/c1-3-8(2)11(19-14(21)13(30)10(18)5-7-31(27,28)29)15(22)20-6-4-9(16(23)24)12(20)17(25)26/h8-13,30H,3-7,18H2,1-2H3,(H,19,21)(H,23,24)(H,25,26)(H,27,28,29)/t8-,9?,10-,11+,12+,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.873 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Binding affinity against recombinant Aminopeptidase A |
J Med Chem 42: 5197-211 (2000)
BindingDB Entry DOI: 10.7270/Q2028S8F |
More data for this
Ligand-Target Pair | |
Aminopeptidase B
(Homo sapiens (Human)) | BDBM50036831
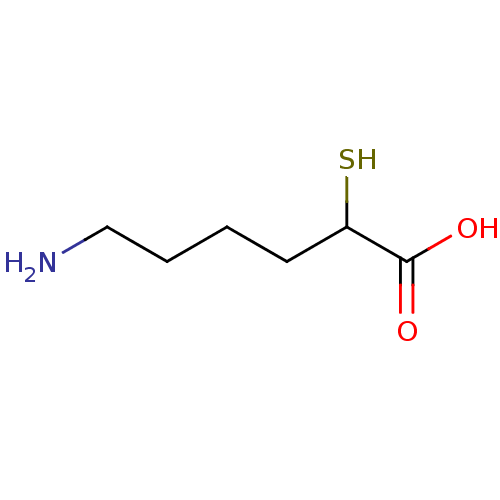
(6-Amino-2-mercapto-hexanoic acid | CHEMBL432852)Show InChI InChI=1S/C6H13NO2S/c7-4-2-1-3-5(10)6(8)9/h5,10H,1-4,7H2,(H,8,9) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of arginylaminopeptidase (aminopeptidase B) |
J Med Chem 37: 1339-46 (1994)
BindingDB Entry DOI: 10.7270/Q2K074ZR |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50000788

((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungarian Academy of Sciences
Curated by PDSP Ki Database
| |
Eur J Pharmacol 383: 209-14 (1999)
Article DOI: 10.1016/s0014-2999(99)00610-x
BindingDB Entry DOI: 10.7270/Q2TT4PJ9 |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50016504

((S)-3-(2-{[(S)-2-(2-{2-[2-tert-Butoxycarbonylamino...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS([O-])(=O)=O)cc1)NC(=O)OC(C)(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C52H69N9O15S/c1-6-8-18-37(57-48(68)40(61-51(71)75-52(3,4)5)26-32-21-23-34(24-22-32)76-77(72,73)74)46(66)55-30-43(62)56-41(27-33-29-54-36-20-14-13-17-35(33)36)49(69)58-38(19-9-7-2)47(67)60-42(28-44(63)64)50(70)59-39(45(53)65)25-31-15-11-10-12-16-31/h10-17,20-24,29,37-42,54H,6-9,18-19,25-28,30H2,1-5H3,(H2,53,65)(H,55,66)(H,56,62)(H,57,68)(H,58,69)(H,59,70)(H,60,67)(H,61,71)(H,63,64)(H,72,73,74)/p-1/t37-,38-,39-,40-,41-,42-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
The compound was tested for the inhibition of [3H]-Propionyl specific binding to Cholecystokinin 8 receptor of guinea pig pancreatic membrane |
J Med Chem 32: 445-9 (1989)
BindingDB Entry DOI: 10.7270/Q2S181GT |
More data for this
Ligand-Target Pair | |
Cholecystokinin receptor type A
(Cavia porcellus) | BDBM50016425

((S)-3-{(S)-2-[(S)-2-(2-{(S)-2-[(S)-2-tert-Butoxyca...)Show SMILES CCCC[C@H](NC(=O)[C@H](Cc1ccc(OS(O)(=O)=O)cc1)NC(=O)OC(C)(C)C)C(=O)NCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCC)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C52H69N9O15S/c1-6-8-18-37(57-48(68)40(61-51(71)75-52(3,4)5)26-32-21-23-34(24-22-32)76-77(72,73)74)46(66)55-30-43(62)56-41(27-33-29-54-36-20-14-13-17-35(33)36)49(69)58-38(19-9-7-2)47(67)60-42(28-44(63)64)50(70)59-39(45(53)65)25-31-15-11-10-12-16-31/h10-17,20-24,29,37-42,54H,6-9,18-19,25-28,30H2,1-5H3,(H2,53,65)(H,55,66)(H,56,62)(H,57,68)(H,58,69)(H,59,70)(H,60,67)(H,61,71)(H,63,64)(H,72,73,74)/t37-,38-,39-,40-,41-,42-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of 0.1 nM [3H]-pCCK-8 from guinea pig pancreatic membranes |
J Med Chem 32: 1184-90 (1989)
BindingDB Entry DOI: 10.7270/Q2ZG6R7N |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50087104
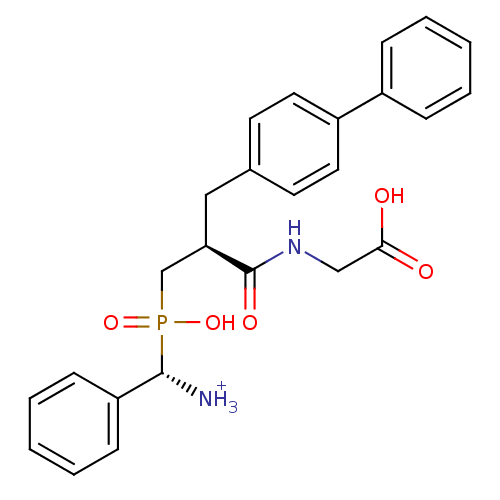
(C-{[3-Biphenyl-4-yl-2-(carboxymethyl-carbamoyl)-pr...)Show SMILES [NH3+][C@H](c1ccccc1)P(O)(=O)C[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)NCC(O)=O Show InChI InChI=1S/C25H27N2O5P/c26-24(21-9-5-2-6-10-21)33(31,32)17-22(25(30)27-16-23(28)29)15-18-11-13-20(14-12-18)19-7-3-1-4-8-19/h1-14,22,24H,15-17,26H2,(H,27,30)(H,28,29)(H,31,32)/p+1/t22-,24+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity on neutral endopeptidase (NEP) using DGNPA as substrate. |
J Med Chem 43: 1398-408 (2001)
BindingDB Entry DOI: 10.7270/Q2TQ627J |
More data for this
Ligand-Target Pair | |
Gastrin/cholecystokinin type B receptor
(Homo sapiens (Human)) | BDBM50046115
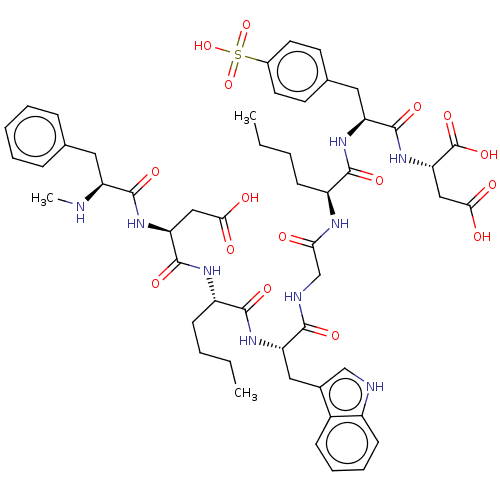
(2-[2-(2-{2-[2-[2-(2-Amino-3-carboxy-propionylamino...)Show SMILES CCCC[C@H](NC(=O)CNC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCCC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1ccccc1)NC)C(=O)N[C@@H](Cc1ccc(cc1)S(O)(=O)=O)C(=O)N[C@@H](CC(O)=O)C(O)=O Show InChI InChI=1S/C52H67N9O16S/c1-4-6-16-36(47(68)58-39(50(71)61-42(52(73)74)27-45(65)66)24-31-19-21-33(22-20-31)78(75,76)77)56-43(62)29-55-46(67)40(25-32-28-54-35-18-12-11-15-34(32)35)59-48(69)37(17-7-5-2)57-51(72)41(26-44(63)64)60-49(70)38(53-3)23-30-13-9-8-10-14-30/h8-15,18-22,28,36-42,53-54H,4-7,16-17,23-27,29H2,1-3H3,(H,55,67)(H,56,62)(H,57,72)(H,58,68)(H,59,69)(H,60,70)(H,61,71)(H,63,64)(H,65,66)(H,73,74)(H,75,76,77)/t36-,37-,38-,39-,40-,41-,42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Displacement of [3H]pCCK-8 from cholecystokinin type B receptor in guinea pig brain membrane |
J Med Chem 36: 166-72 (1993)
BindingDB Entry DOI: 10.7270/Q26M37FJ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM21015

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hungarian Academy of Sciences
Curated by PDSP Ki Database
| |
Eur J Pharmacol 383: 209-14 (1999)
Article DOI: 10.1016/s0014-2999(99)00610-x
BindingDB Entry DOI: 10.7270/Q2TT4PJ9 |
More data for this
Ligand-Target Pair | |
EEF1AKMT4-ECE2 readthrough transcript protein
(Homo sapiens (Human)) | BDBM50251742

((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...)Show SMILES CC(C)C[C@H](NP(O)(=O)O[C@@H]1O[C@@H](C)[C@H](O)[C@@H](O)[C@H]1O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O |r| Show InChI InChI=1S/C23H34N3O10P/c1-11(2)8-16(26-37(33,34)36-23-20(29)19(28)18(27)12(3)35-23)21(30)25-17(22(31)32)9-13-10-24-15-7-5-4-6-14(13)15/h4-7,10-12,16-20,23-24,27-29H,8-9H2,1-3H3,(H,25,30)(H,31,32)(H2,26,33,34)/t12-,16-,17-,18-,19+,20+,23-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | 2.33 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaleads
| Assay Description
Inhibitory assay against ECE-2. |
J Biol Chem 285: 34390-400 (2010)
Article DOI: 10.1074/jbc.M110.120576
BindingDB Entry DOI: 10.7270/Q29P307K |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50105262
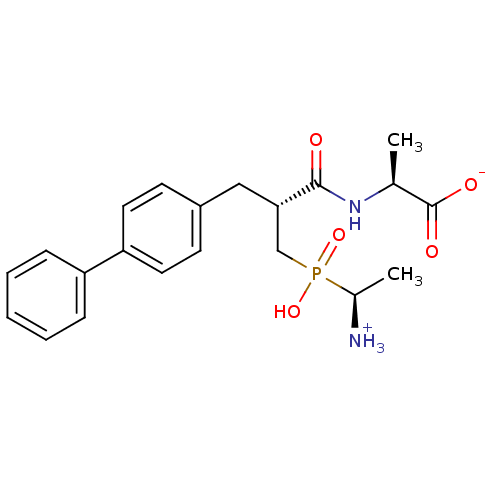
(2-{3-[(1-Amino-ethyl)-hydroxy-phosphinoyl]-2-biphe...)Show SMILES C[C@@H]([NH3+])P(O)(=O)C[C@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N[C@@H](C)C([O-])=O Show InChI InChI=1S/C21H27N2O5P/c1-14(21(25)26)23-20(24)19(13-29(27,28)15(2)22)12-16-8-10-18(11-9-16)17-6-4-3-5-7-17/h3-11,14-15,19H,12-13,22H2,1-2H3,(H,23,24)(H,25,26)(H,27,28)/t14-,15-,19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of enkephalin degrading enzyme, neutral endopeptidase (NEP) |
J Med Chem 44: 3523-30 (2001)
BindingDB Entry DOI: 10.7270/Q2QN67HK |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50087106
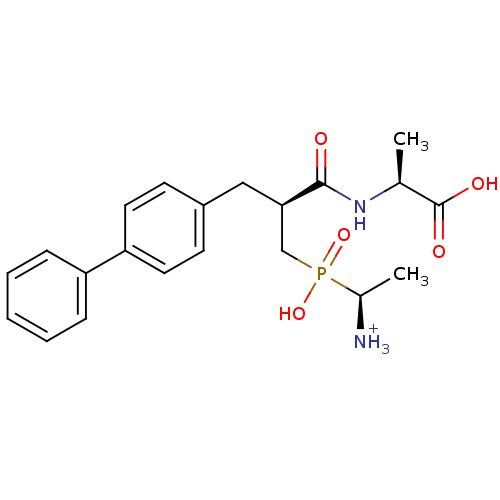
((2S)-2-[(2S)-3-{[(1S)-1-azaniumylethyl](hydroxy)ph...)Show SMILES C[C@@H]([NH3+])P(O)(=O)C[C@@H](Cc1ccc(cc1)-c1ccccc1)C(=O)N[C@@H](C)C(O)=O Show InChI InChI=1S/C21H27N2O5P/c1-14(21(25)26)23-20(24)19(13-29(27,28)15(2)22)12-16-8-10-18(11-9-16)17-6-4-3-5-7-17/h3-11,14-15,19H,12-13,22H2,1-2H3,(H,23,24)(H,25,26)(H,27,28)/p+1/t14-,15-,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibitory activity on neutral endopeptidase (NEP) using DGNPA as substrate. |
J Med Chem 43: 1398-408 (2001)
BindingDB Entry DOI: 10.7270/Q2TQ627J |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50115846
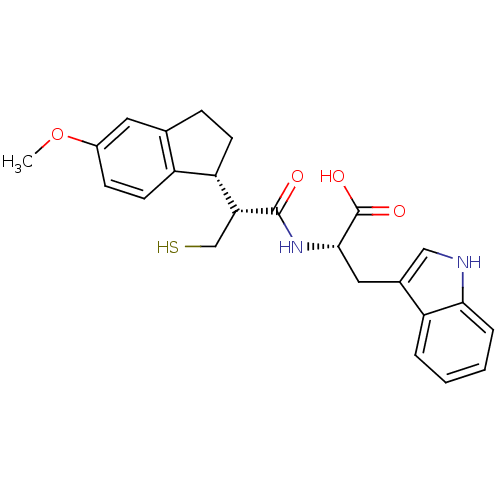
((S)-3-(1H-Indol-3-yl)-2-[(R)-3-mercapto-2-((R)-5-m...)Show SMILES COc1ccc2[C@H](CCc2c1)[C@@H](CS)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C24H26N2O4S/c1-30-16-7-9-17-14(10-16)6-8-19(17)20(13-31)23(27)26-22(24(28)29)11-15-12-25-21-5-3-2-4-18(15)21/h2-5,7,9-10,12,19-20,22,25,31H,6,8,11,13H2,1H3,(H,26,27)(H,28,29)/t19-,20+,22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
In vitro inhibition of Neutral endopeptidase. |
Bioorg Med Chem Lett 12: 2001-5 (2002)
BindingDB Entry DOI: 10.7270/Q27080S4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data