 Found 134 hits with Last Name = 'rossetti' and Initial = 't'
Found 134 hits with Last Name = 'rossetti' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50326858
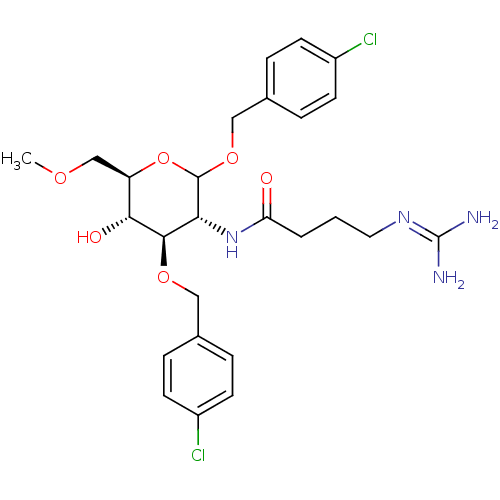
(CHEMBL1254556 | N-((3R,4R,5S,6R)-2,4-bis(4-chlorob...)Show SMILES [#6]-[#8]-[#6]-[#6@H]-1-[#8]-[#6](-[#8]-[#6]-c2ccc(Cl)cc2)-[#6@H](-[#7]-[#6](=O)-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6@@H](-[#8]-[#6]-c2ccc(Cl)cc2)-[#6@@H]-1-[#8] |r| Show InChI InChI=1S/C26H34Cl2N4O6/c1-35-15-20-23(34)24(36-13-16-4-8-18(27)9-5-16)22(32-21(33)3-2-12-31-26(29)30)25(38-20)37-14-17-6-10-19(28)11-7-17/h4-11,20,22-25,34H,2-3,12-15H2,1H3,(H,32,33)(H4,29,30,31)/t20-,22-,23-,24-,25?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemia Ltd
Curated by ChEMBL
| Assay Description
Displacement of [I125I]MCH from human MCH1 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 5576-86 (2010)
Article DOI: 10.1021/jm1002777
BindingDB Entry DOI: 10.7270/Q2QC03QK |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50326859
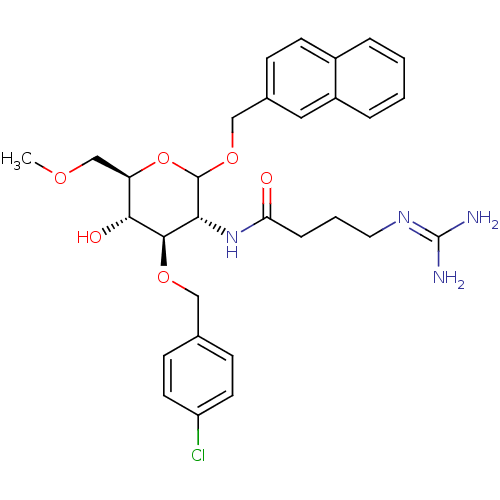
(CHEMBL1254630 | N-((3R,4R,5S,6R)-4-(4-chlorobenzyl...)Show SMILES [#6]-[#8]-[#6]-[#6@H]-1-[#8]-[#6](-[#8]-[#6]-c2ccc3ccccc3c2)-[#6@H](-[#7]-[#6](=O)-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6@@H](-[#8]-[#6]-c2ccc(Cl)cc2)-[#6@@H]-1-[#8] |r| Show InChI InChI=1S/C30H37ClN4O6/c1-38-18-24-27(37)28(39-16-19-9-12-23(31)13-10-19)26(35-25(36)7-4-14-34-30(32)33)29(41-24)40-17-20-8-11-21-5-2-3-6-22(21)15-20/h2-3,5-6,8-13,15,24,26-29,37H,4,7,14,16-18H2,1H3,(H,35,36)(H4,32,33,34)/t24-,26-,27-,28-,29?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemia Ltd
Curated by ChEMBL
| Assay Description
Displacement of [I125I]MCH from human MCH1 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 5576-86 (2010)
Article DOI: 10.1021/jm1002777
BindingDB Entry DOI: 10.7270/Q2QC03QK |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50326860

(CHEMBL1254711 | N-((3R,4R,5S,6R)-2-(4-chlorobenzyl...)Show SMILES [#6]-[#8]-[#6]-[#6@H]-1-[#8]-[#6](-[#8]-[#6]-c2ccc(Cl)cc2)-[#6@H](-[#7]-[#6](=O)-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6@@H](-[#8]-[#6]-c2ccc3ccccc3c2)-[#6@@H]-1-[#8] |r| Show InChI InChI=1S/C30H37ClN4O6/c1-38-18-24-27(37)28(39-17-20-8-11-21-5-2-3-6-22(21)15-20)26(35-25(36)7-4-14-34-30(32)33)29(41-24)40-16-19-9-12-23(31)13-10-19/h2-3,5-6,8-13,15,24,26-29,37H,4,7,14,16-18H2,1H3,(H,35,36)(H4,32,33,34)/t24-,26-,27-,28-,29?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemia Ltd
Curated by ChEMBL
| Assay Description
Displacement of [I125I]MCH from human MCH1 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 5576-86 (2010)
Article DOI: 10.1021/jm1002777
BindingDB Entry DOI: 10.7270/Q2QC03QK |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50326861
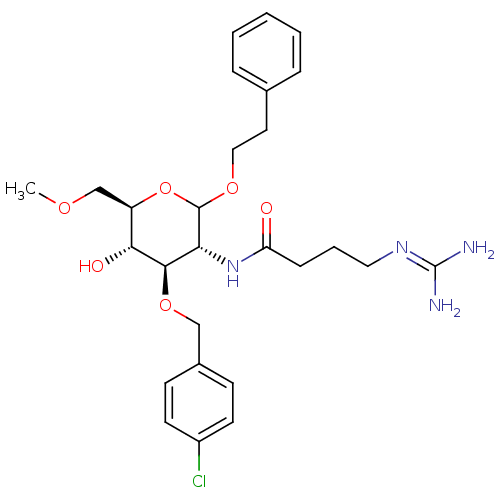
(CHEMBL1254796 | N-((3R,4R,5S,6R)-4-(4-chlorobenzyl...)Show SMILES [#6]-[#8]-[#6]-[#6@H]-1-[#8]-[#6](-[#8]-[#6]-[#6]-c2ccccc2)-[#6@H](-[#7]-[#6](=O)-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6@@H](-[#8]-[#6]-c2ccc(Cl)cc2)-[#6@@H]-1-[#8] |r| Show InChI InChI=1S/C27H37ClN4O6/c1-35-17-21-24(34)25(37-16-19-9-11-20(28)12-10-19)23(32-22(33)8-5-14-31-27(29)30)26(38-21)36-15-13-18-6-3-2-4-7-18/h2-4,6-7,9-12,21,23-26,34H,5,8,13-17H2,1H3,(H,32,33)(H4,29,30,31)/t21-,23-,24-,25-,26?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemia Ltd
Curated by ChEMBL
| Assay Description
Displacement of [I125I]MCH from human MCH1 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 5576-86 (2010)
Article DOI: 10.1021/jm1002777
BindingDB Entry DOI: 10.7270/Q2QC03QK |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50326862
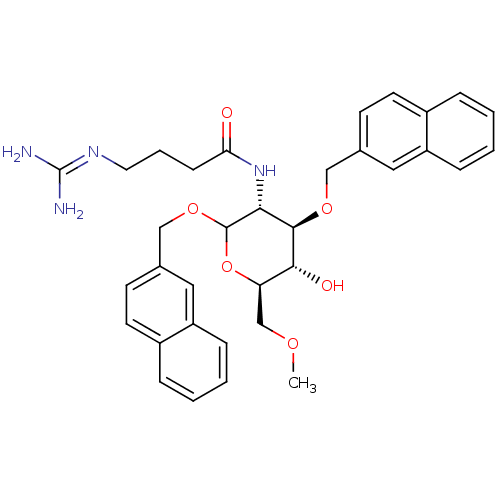
(4-(diaminomethyleneamino)-N-((3R,4R,5S,6R)-5-hydro...)Show SMILES [#6]-[#8]-[#6]-[#6@H]-1-[#8]-[#6](-[#8]-[#6]-c2ccc3ccccc3c2)-[#6@H](-[#7]-[#6](=O)-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6@@H](-[#8]-[#6]-c2ccc3ccccc3c2)-[#6@@H]-1-[#8] |r| Show InChI InChI=1S/C34H40N4O6/c1-41-21-28-31(40)32(42-19-22-12-14-24-7-2-4-9-26(24)17-22)30(38-29(39)11-6-16-37-34(35)36)33(44-28)43-20-23-13-15-25-8-3-5-10-27(25)18-23/h2-5,7-10,12-15,17-18,28,30-33,40H,6,11,16,19-21H2,1H3,(H,38,39)(H4,35,36,37)/t28-,30-,31-,32-,33?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemia Ltd
Curated by ChEMBL
| Assay Description
Displacement of [I125I]MCH from human MCH1 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 5576-86 (2010)
Article DOI: 10.1021/jm1002777
BindingDB Entry DOI: 10.7270/Q2QC03QK |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50326863
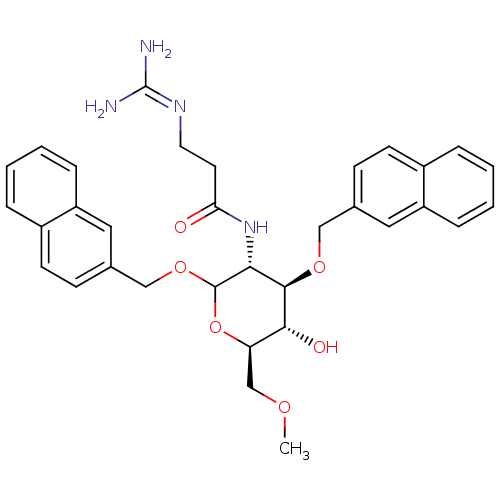
(3-(diaminomethyleneamino)-N-((3R,4R,5S,6R)-5-hydro...)Show SMILES [#6]-[#8]-[#6]-[#6@H]-1-[#8]-[#6](-[#8]-[#6]-c2ccc3ccccc3c2)-[#6@H](-[#7]-[#6](=O)-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6@@H](-[#8]-[#6]-c2ccc3ccccc3c2)-[#6@@H]-1-[#8] |r| Show InChI InChI=1S/C33H38N4O6/c1-40-20-27-30(39)31(41-18-21-10-12-23-6-2-4-8-25(23)16-21)29(37-28(38)14-15-36-33(34)35)32(43-27)42-19-22-11-13-24-7-3-5-9-26(24)17-22/h2-13,16-17,27,29-32,39H,14-15,18-20H2,1H3,(H,37,38)(H4,34,35,36)/t27-,29-,30-,31-,32?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemia Ltd
Curated by ChEMBL
| Assay Description
Displacement of [I125I]MCH from human MCH1 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 5576-86 (2010)
Article DOI: 10.1021/jm1002777
BindingDB Entry DOI: 10.7270/Q2QC03QK |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50326864
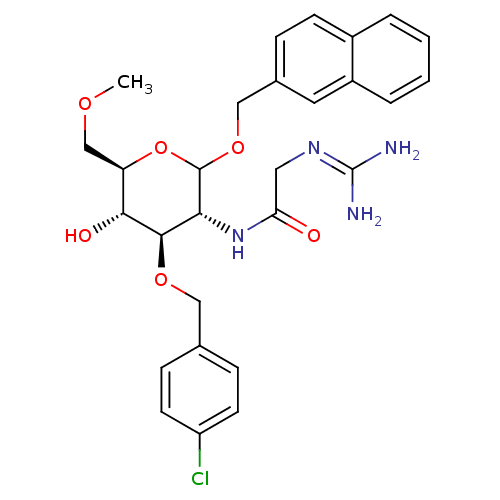
(CHEMBL1254883 | N-((3R,4R,5S,6R)-4-(4-chlorobenzyl...)Show SMILES [#6]-[#8]-[#6]-[#6@H]-1-[#8]-[#6](-[#8]-[#6]-c2ccc3ccccc3c2)-[#6@H](-[#7]-[#6](=O)-[#6]\[#7]=[#6](\[#7])-[#7])-[#6@@H](-[#8]-[#6]-c2ccc(Cl)cc2)-[#6@@H]-1-[#8] |r| Show InChI InChI=1S/C28H33ClN4O6/c1-36-16-22-25(35)26(37-14-17-7-10-21(29)11-8-17)24(33-23(34)13-32-28(30)31)27(39-22)38-15-18-6-9-19-4-2-3-5-20(19)12-18/h2-12,22,24-27,35H,13-16H2,1H3,(H,33,34)(H4,30,31,32)/t22-,24-,25-,26-,27?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemia Ltd
Curated by ChEMBL
| Assay Description
Displacement of [I125I]MCH from human MCH1 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 5576-86 (2010)
Article DOI: 10.1021/jm1002777
BindingDB Entry DOI: 10.7270/Q2QC03QK |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607682
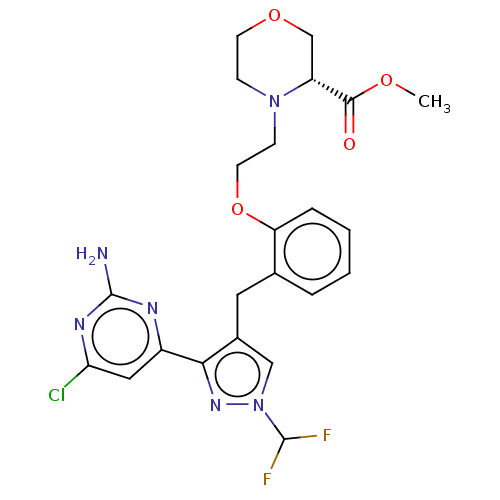
(CHEMBL5220685)Show SMILES COC(=O)[C@H]1COCCN1CCOc1ccccc1Cc1cn(nc1-c1cc(Cl)nc(N)n1)C(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Rattus norvegicus) | BDBM50607676
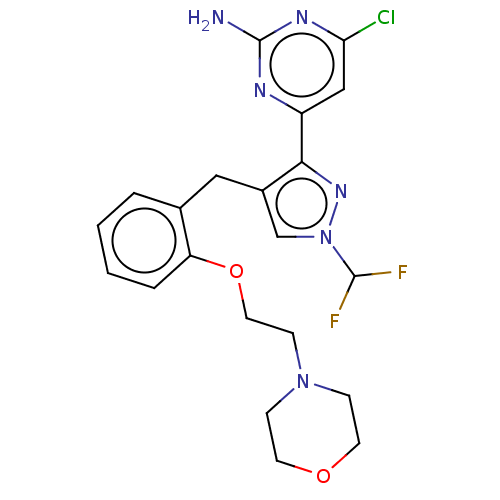
(CHEMBL5218698)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCOCC1)C(F)F | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607678

(CHEMBL5218878)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCNC(=O)C1)C(F)F | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607681
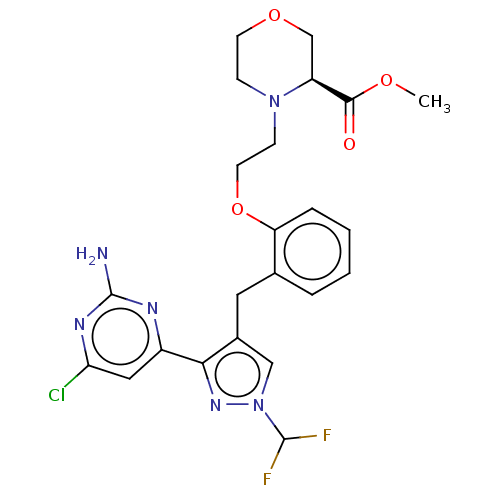
(CHEMBL5218552)Show SMILES COC(=O)[C@@H]1COCCN1CCOc1ccccc1Cc1cn(nc1-c1cc(Cl)nc(N)n1)C(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607679
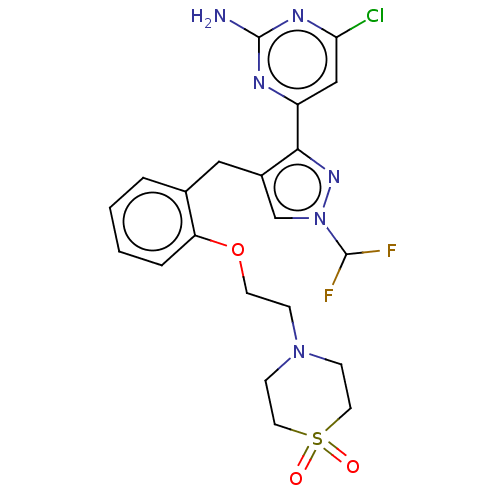
(CHEMBL5218615)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCS(=O)(=O)CC1)C(F)F | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Rattus norvegicus) | BDBM50607682

(CHEMBL5220685)Show SMILES COC(=O)[C@H]1COCCN1CCOc1ccccc1Cc1cn(nc1-c1cc(Cl)nc(N)n1)C(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607683
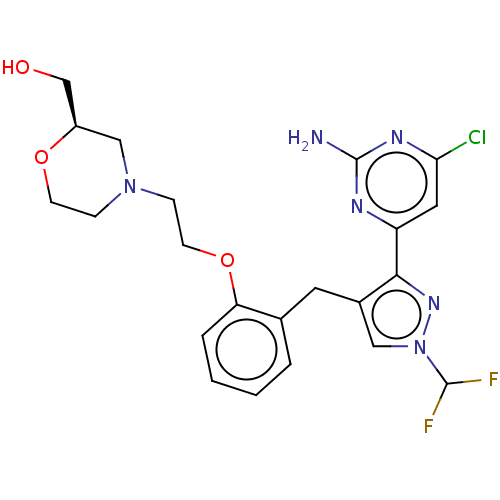
(CHEMBL5219579)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCO[C@@H](CO)C1)C(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607686
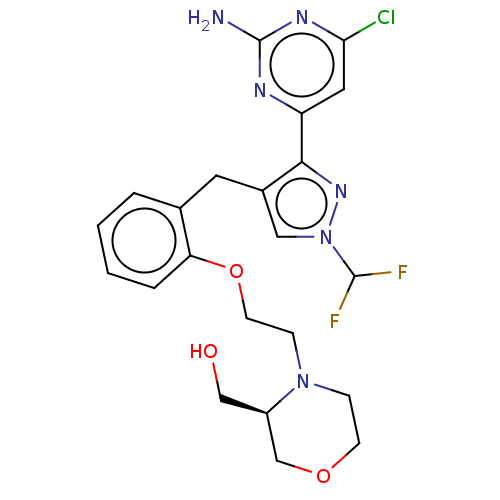
(CHEMBL5219830)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCOC[C@H]1CO)C(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607676

(CHEMBL5218698)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCOCC1)C(F)F | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607680
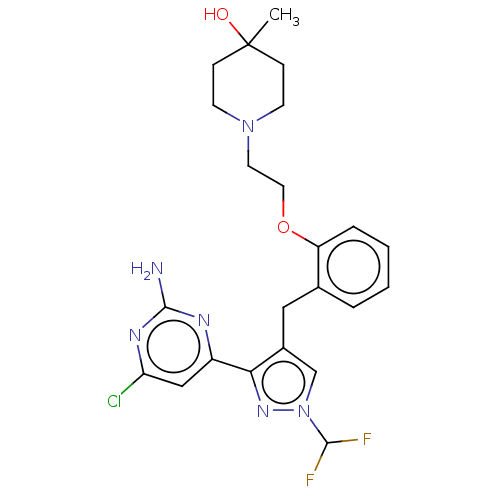
(CHEMBL5218702)Show SMILES CC1(O)CCN(CCOc2ccccc2Cc2cn(nc2-c2cc(Cl)nc(N)n2)C(F)F)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607685
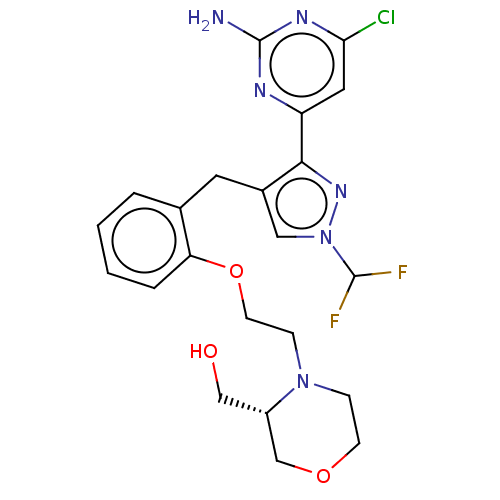
(CHEMBL5220647)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCOC[C@@H]1CO)C(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Rattus norvegicus) | BDBM50607686

(CHEMBL5219830)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCOC[C@H]1CO)C(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenylate cyclase type 10
(Rattus norvegicus) | BDBM50607678

(CHEMBL5218878)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCNC(=O)C1)C(F)F | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607684
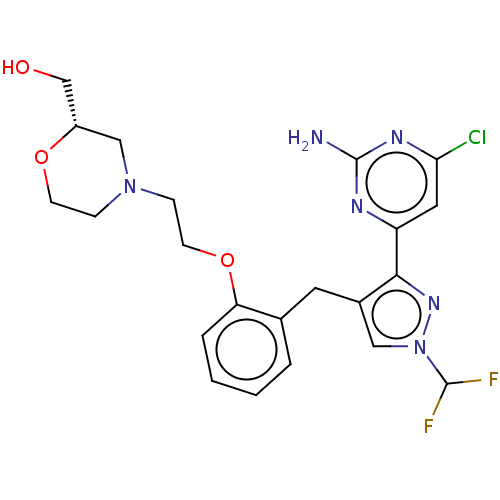
(CHEMBL5219443)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCO[C@H](CO)C1)C(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607673
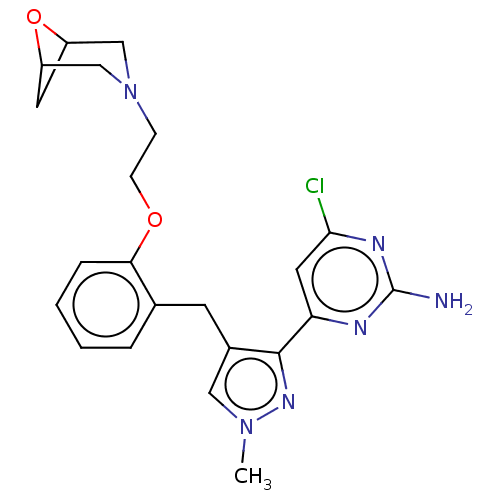
(CHEMBL5220895)Show SMILES Cn1cc(Cc2ccccc2OCCN2CC3CC(C2)O3)c(n1)-c1cc(Cl)nc(N)n1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607674
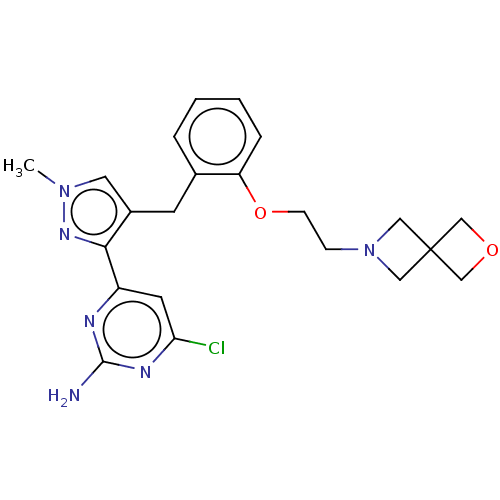
(CHEMBL5220347)Show SMILES Cn1cc(Cc2ccccc2OCCN2CC3(COC3)C2)c(n1)-c1cc(Cl)nc(N)n1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Rattus norvegicus) | BDBM50607685

(CHEMBL5220647)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCOC[C@@H]1CO)C(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607672
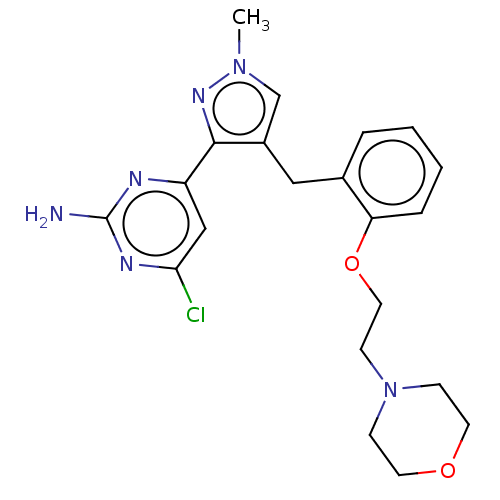
(CHEMBL5220723)Show SMILES Cn1cc(Cc2ccccc2OCCN2CCOCC2)c(n1)-c1cc(Cl)nc(N)n1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Rattus norvegicus) | BDBM50607679

(CHEMBL5218615)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCS(=O)(=O)CC1)C(F)F | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607675
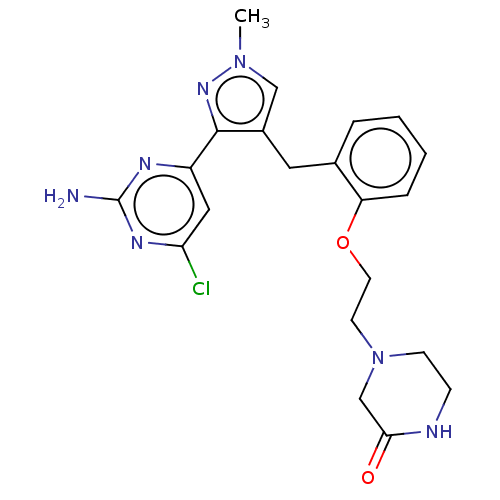
(CHEMBL5219922)Show SMILES Cn1cc(Cc2ccccc2OCCN2CCNC(=O)C2)c(n1)-c1cc(Cl)nc(N)n1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Rattus norvegicus) | BDBM50607680

(CHEMBL5218702)Show SMILES CC1(O)CCN(CCOc2ccccc2Cc2cn(nc2-c2cc(Cl)nc(N)n2)C(F)F)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Rattus norvegicus) | BDBM50607681

(CHEMBL5218552)Show SMILES COC(=O)[C@@H]1COCCN1CCOc1ccccc1Cc1cn(nc1-c1cc(Cl)nc(N)n1)C(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607667
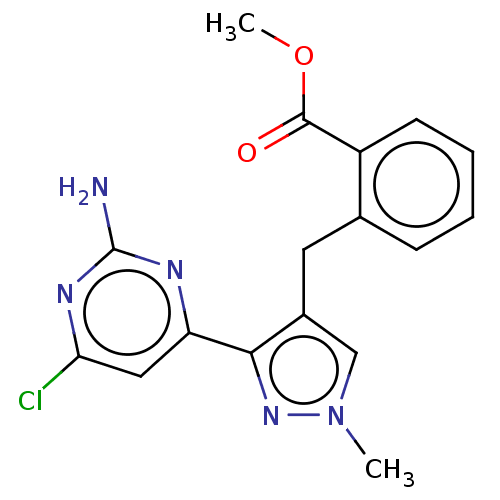
(CHEMBL5219530) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenylate cyclase type 10
(Rattus norvegicus) | BDBM50607684

(CHEMBL5219443)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCO[C@H](CO)C1)C(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607677
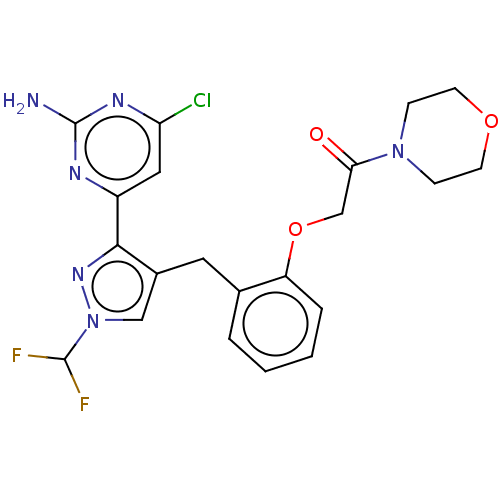
(CHEMBL5220632)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCC(=O)N1CCOCC1)C(F)F | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50577248
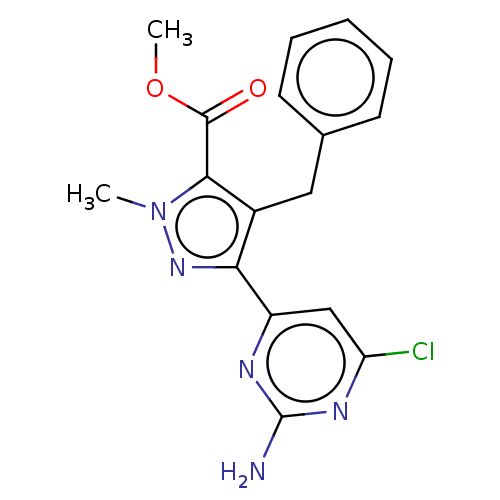
(CHEMBL4878379)Show SMILES COC(=O)c1c(Cc2ccccc2)c(nn1C)-c1cc(Cl)nc(N)n1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00273
BindingDB Entry DOI: 10.7270/Q29S1VWQ |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Rattus norvegicus) | BDBM50607683

(CHEMBL5219579)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCCN1CCO[C@@H](CO)C1)C(F)F |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Rattus norvegicus) | BDBM50607677

(CHEMBL5220632)Show SMILES Nc1nc(Cl)cc(n1)-c1nn(cc1Cc1ccccc1OCC(=O)N1CCOCC1)C(F)F | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50326854
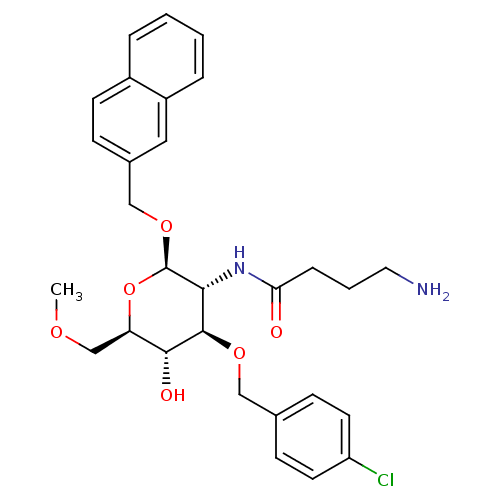
((Naphth-2-yl)methyl-2-(4'-Aminobutyrylamido)-3-O-(...)Show SMILES COC[C@H]1O[C@@H](OCc2ccc3ccccc3c2)[C@H](NC(=O)CCCN)[C@@H](OCc2ccc(Cl)cc2)[C@@H]1O |r| Show InChI InChI=1S/C29H35ClN2O6/c1-35-18-24-27(34)28(36-16-19-9-12-23(30)13-10-19)26(32-25(33)7-4-14-31)29(38-24)37-17-20-8-11-21-5-2-3-6-22(21)15-20/h2-3,5-6,8-13,15,24,26-29,34H,4,7,14,16-18,31H2,1H3,(H,32,33)/t24-,26-,27-,28-,29-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemia Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodotyrosyl from human SST5 receptor expressed in CHO cells |
J Med Chem 53: 5576-86 (2010)
Article DOI: 10.1021/jm1002777
BindingDB Entry DOI: 10.7270/Q2QC03QK |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607660
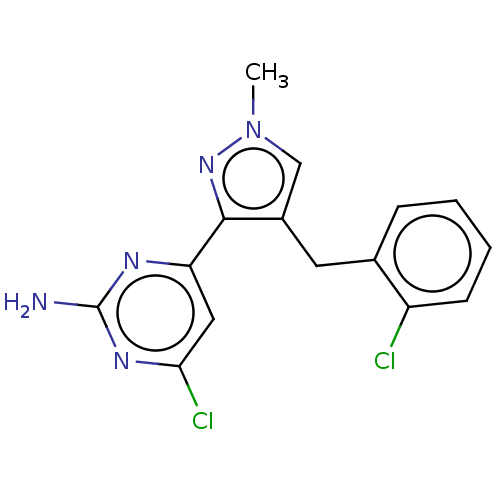
(CHEMBL5221008) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607666
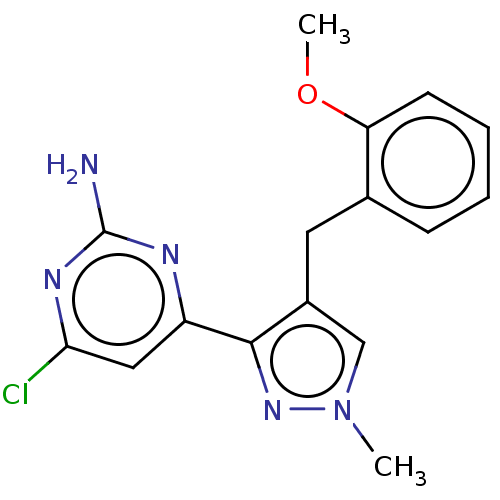
(CHEMBL5220075) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50326878
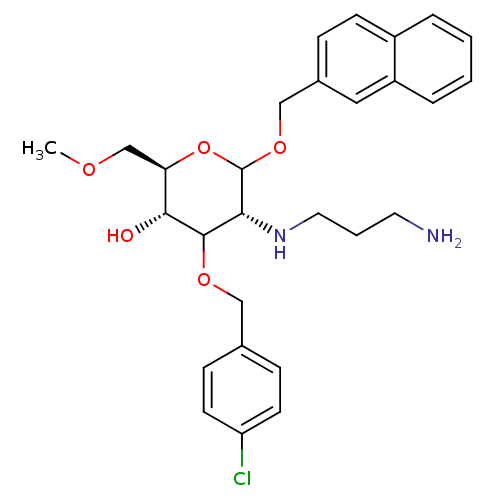
((2R,3S,5R)-5-(3-aminopropylamino)-4-(4-chlorobenzy...)Show SMILES COC[C@H]1OC(OCc2ccc3ccccc3c2)[C@H](NCCCN)C(OCc2ccc(Cl)cc2)[C@@H]1O |r| Show InChI InChI=1S/C28H35ClN2O5/c1-33-18-24-26(32)27(34-16-19-8-11-23(29)12-9-19)25(31-14-4-13-30)28(36-24)35-17-20-7-10-21-5-2-3-6-22(21)15-20/h2-3,5-12,15,24-28,31-32H,4,13-14,16-18,30H2,1H3/t24-,25-,26-,27?,28?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemia Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodotyrosyl from human SST5 receptor expressed in CHO cells |
J Med Chem 53: 5576-86 (2010)
Article DOI: 10.1021/jm1002777
BindingDB Entry DOI: 10.7270/Q2QC03QK |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50326872
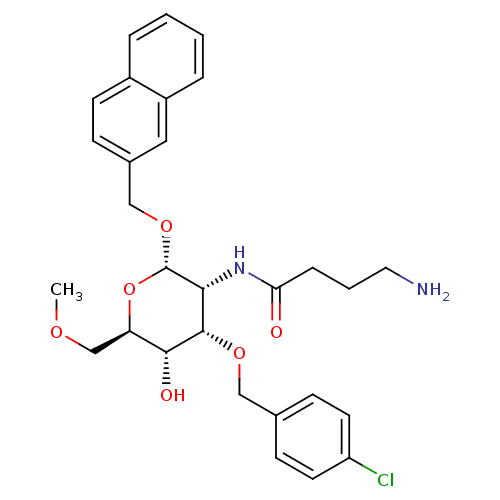
(CHEMBL1254140 | Naphth-2-yl)methyl-2-(4'-Aminobuty...)Show SMILES COC[C@H]1O[C@H](OCc2ccc3ccccc3c2)[C@H](NC(=O)CCCN)[C@H](OCc2ccc(Cl)cc2)[C@@H]1O |r| Show InChI InChI=1S/C29H35ClN2O6/c1-35-18-24-27(34)28(36-16-19-9-12-23(30)13-10-19)26(32-25(33)7-4-14-31)29(38-24)37-17-20-8-11-21-5-2-3-6-22(21)15-20/h2-3,5-6,8-13,15,24,26-29,34H,4,7,14,16-18,31H2,1H3,(H,32,33)/t24-,26-,27-,28+,29+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemia Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodotyrosyl from human SST5 receptor expressed in CHO cells |
J Med Chem 53: 5576-86 (2010)
Article DOI: 10.1021/jm1002777
BindingDB Entry DOI: 10.7270/Q2QC03QK |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50577241
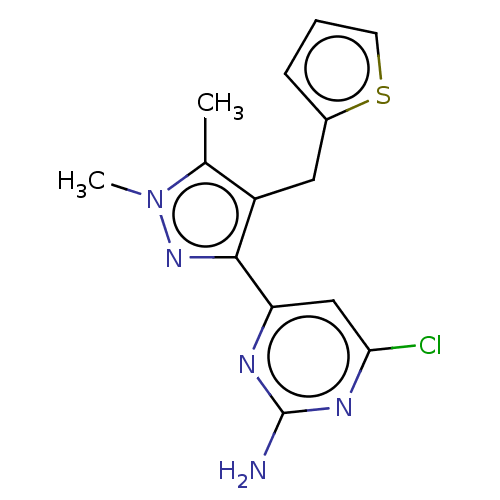
(CHEMBL4848035) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00273
BindingDB Entry DOI: 10.7270/Q29S1VWQ |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607671
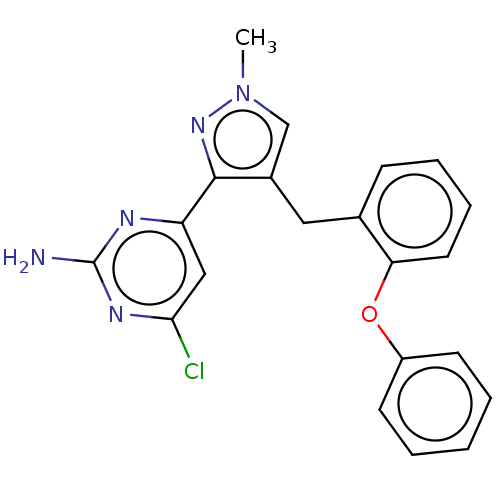
(CHEMBL5221093)Show SMILES Cn1cc(Cc2ccccc2Oc2ccccc2)c(n1)-c1cc(Cl)nc(N)n1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50577242
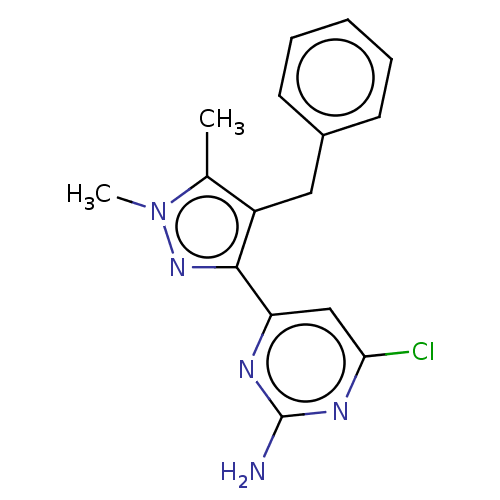
(CHEMBL4854762) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human soluble adenylyl cyclase transfected (4-4 clones)in human HEK293 cells assessed as reduction in cAMP levels preincubated for 5 mi... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00273
BindingDB Entry DOI: 10.7270/Q29S1VWQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50326879

((2R,3S,5R)-5-(2-aminoethylamino)-2-(methoxymethyl)...)Show SMILES COC[C@H]1OC(OCc2ccc3ccccc3c2)[C@H](NCCN)C(OCc2cccc3ccccc23)[C@@H]1O |r| Show InChI InChI=1S/C31H36N2O5/c1-35-20-27-29(34)30(36-19-25-11-6-10-23-8-4-5-12-26(23)25)28(33-16-15-32)31(38-27)37-18-21-13-14-22-7-2-3-9-24(22)17-21/h2-14,17,27-31,33-34H,15-16,18-20,32H2,1H3/t27-,28-,29-,30?,31?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemia Ltd
Curated by ChEMBL
| Assay Description
Displacement of [125I]iodotyrosyl from human SST5 receptor expressed in CHO cells |
J Med Chem 53: 5576-86 (2010)
Article DOI: 10.1021/jm1002777
BindingDB Entry DOI: 10.7270/Q2QC03QK |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50577241

(CHEMBL4848035) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human soluble adenylyl cyclase transfected (4-4 clones)in human HEK293 cells assessed as reduction in cAMP levels preincubated for 5 mi... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00273
BindingDB Entry DOI: 10.7270/Q29S1VWQ |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50577243
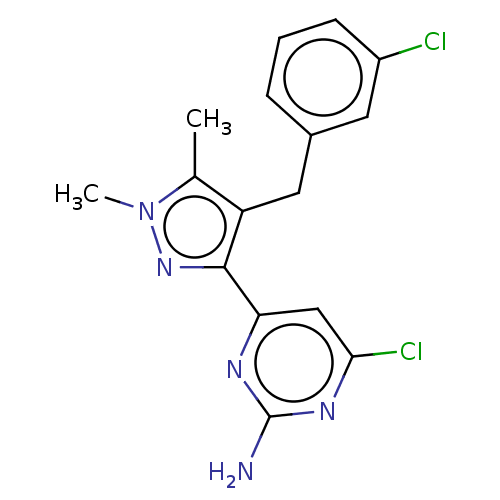
(CHEMBL4868801) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00273
BindingDB Entry DOI: 10.7270/Q29S1VWQ |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50607662

(CHEMBL5219193) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 112 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01133
BindingDB Entry DOI: 10.7270/Q2057M2B |
More data for this
Ligand-Target Pair | |
Melanin-concentrating hormone receptor 1
(Homo sapiens (Human)) | BDBM50326865
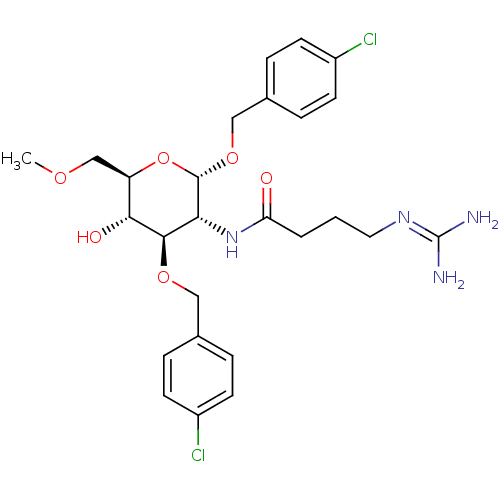
((4'-Chlorobenzyl)3-O-(4'-Chlorobenzyl)-2-deoxy-2-(...)Show SMILES [#6]-[#8]-[#6]-[#6@H]-1-[#8]-[#6@H](-[#8]-[#6]-c2ccc(Cl)cc2)-[#6@H](-[#7]-[#6](=O)-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6@@H](-[#8]-[#6]-c2ccc(Cl)cc2)-[#6@@H]-1-[#8] |r| Show InChI InChI=1S/C26H34Cl2N4O6/c1-35-15-20-23(34)24(36-13-16-4-8-18(27)9-5-16)22(32-21(33)3-2-12-31-26(29)30)25(38-20)37-14-17-6-10-19(28)11-7-17/h4-11,20,22-25,34H,2-3,12-15H2,1H3,(H,32,33)(H4,29,30,31)/t20-,22-,23-,24-,25+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Alchemia Ltd
Curated by ChEMBL
| Assay Description
Displacement of [I125I]MCH from human MCH1 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 5576-86 (2010)
Article DOI: 10.1021/jm1002777
BindingDB Entry DOI: 10.7270/Q2QC03QK |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50577244
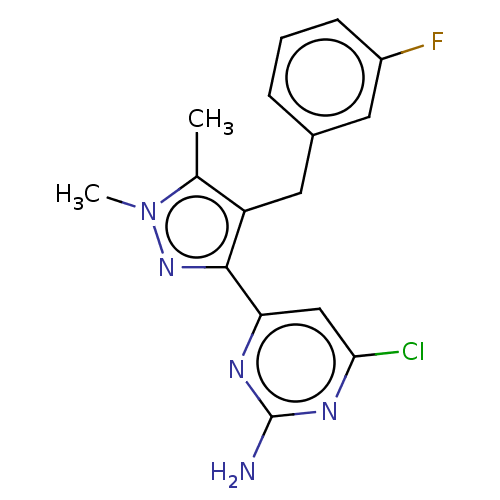
(CHEMBL4864668) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00273
BindingDB Entry DOI: 10.7270/Q29S1VWQ |
More data for this
Ligand-Target Pair | |
Adenylate cyclase type 10
(Homo sapiens (Human)) | BDBM50577236
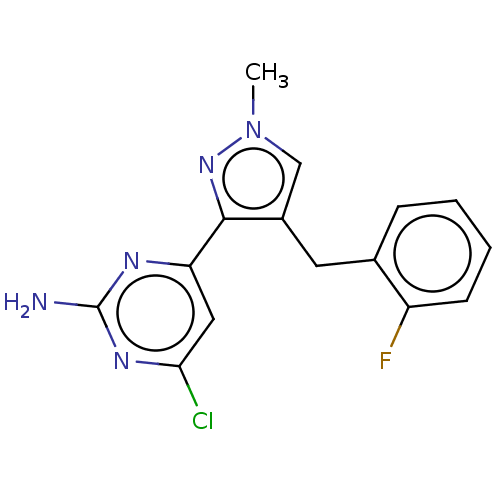
(CHEMBL4857305) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human soluble adenylyl cyclase assessed as reduction in cAMP levels in the presence of alpha-32p labeled ATP by biochemical assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00273
BindingDB Entry DOI: 10.7270/Q29S1VWQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data