 Found 546 hits with Last Name = 'roth' and Initial = 'e'
Found 546 hits with Last Name = 'roth' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dimer of Gag-Pol polyprotein [489-587,V571I]
(Human immunodeficiency virus type 1) | BDBM517

((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0520 | -59.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... |
Proc Natl Acad Sci U S A 91: 4096-100 (1994)
Article DOI: 10.1073/pnas.91.9.4096
BindingDB Entry DOI: 10.7270/Q23F4MS9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [489-587,L565M]
(Human immunodeficiency virus type 1) | BDBM517

((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.114 | -57.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... |
Proc Natl Acad Sci U S A 91: 4096-100 (1994)
Article DOI: 10.1073/pnas.91.9.4096
BindingDB Entry DOI: 10.7270/Q23F4MS9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [489-587,I539L]
(Human immunodeficiency virus type 1) | BDBM517

((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.323 | -55.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... |
Proc Natl Acad Sci U S A 91: 4096-100 (1994)
Article DOI: 10.1073/pnas.91.9.4096
BindingDB Entry DOI: 10.7270/Q23F4MS9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM517

((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.358 | -54.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... |
Proc Natl Acad Sci U S A 91: 4096-100 (1994)
Article DOI: 10.1073/pnas.91.9.4096
BindingDB Entry DOI: 10.7270/Q23F4MS9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [489-587,L512I]
(Human immunodeficiency virus type 1) | BDBM517

((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.585 | -53.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... |
Proc Natl Acad Sci U S A 91: 4096-100 (1994)
Article DOI: 10.1073/pnas.91.9.4096
BindingDB Entry DOI: 10.7270/Q23F4MS9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [489-587,V521I]
(Human immunodeficiency virus type 1) | BDBM517

((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 2.64 | -49.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... |
Proc Natl Acad Sci U S A 91: 4096-100 (1994)
Article DOI: 10.1073/pnas.91.9.4096
BindingDB Entry DOI: 10.7270/Q23F4MS9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [489-587,L512V]
(Human immunodeficiency virus type 1) | BDBM517

((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 2.81 | -49.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... |
Proc Natl Acad Sci U S A 91: 4096-100 (1994)
Article DOI: 10.1073/pnas.91.9.4096
BindingDB Entry DOI: 10.7270/Q23F4MS9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [489-587,I573V]
(Human immunodeficiency virus type 1) | BDBM517

((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 3.11 | -49.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... |
Proc Natl Acad Sci U S A 91: 4096-100 (1994)
Article DOI: 10.1073/pnas.91.9.4096
BindingDB Entry DOI: 10.7270/Q23F4MS9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [514-612]
(Human immunodeficiency virus type 2) | BDBM517

((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3.32 | -49.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... |
Proc Natl Acad Sci U S A 91: 4096-100 (1994)
Article DOI: 10.1073/pnas.91.9.4096
BindingDB Entry DOI: 10.7270/Q23F4MS9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dimer of Gag-Pol polyprotein [489-587,I536L]
(Human immunodeficiency virus type 1) | BDBM517

((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...)Show SMILES CC(C)(C)NC(=O)[C@@H]1CN(Cc2cccnc2)CCN1C[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C36H47N5O4/c1-36(2,3)39-35(45)31-24-40(22-26-12-9-15-37-21-26)16-17-41(31)23-29(42)19-28(18-25-10-5-4-6-11-25)34(44)38-33-30-14-8-7-13-27(30)20-32(33)43/h4-15,21,28-29,31-33,42-43H,16-20,22-24H2,1-3H3,(H,38,44)(H,39,45)/t28-,29+,31+,32-,33+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 6.62 | -47.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Assay of HIV protease inhibition was performed by peptide cleavage using the substrate Val-Ser-Gln-Asn-beta-naphthylalanine*Pro-Ile-Val. Products of ... |
Proc Natl Acad Sci U S A 91: 4096-100 (1994)
Article DOI: 10.1073/pnas.91.9.4096
BindingDB Entry DOI: 10.7270/Q23F4MS9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Macrophage migration inhibitory factor
(Homo sapiens (Human)) | BDBM50240404
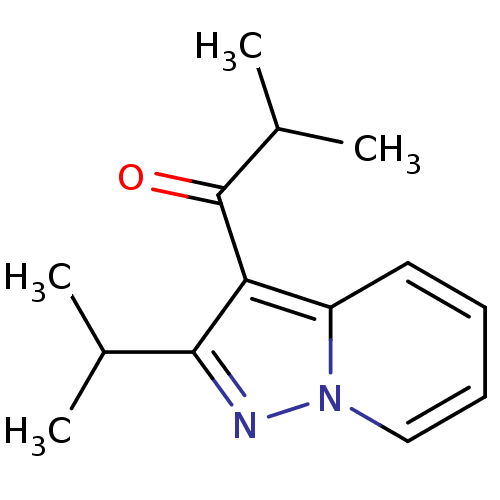
((Ibudilast)1-(2-Isopropyl-pyrazolo[1,5-a]pyridin-3...)Show InChI InChI=1S/C14H18N2O/c1-9(2)13-12(14(17)10(3)4)11-7-5-6-8-16(11)15-13/h5-10H,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 3.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Non-competitive inhibition of recombinant human MIF assessed as inhibition constant using varying levels of p-hydroxyphenylpyruvate as substrate by L... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00351
BindingDB Entry DOI: 10.7270/Q2CJ8J3J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM50118985
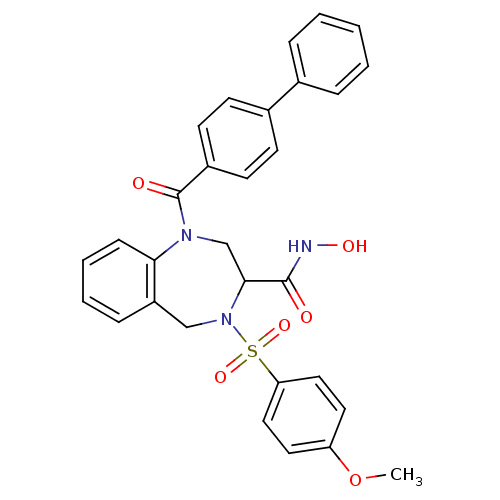
(1-(Biphenyl-4-carbonyl)-4-(4-methoxy-benzenesulfon...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C30H27N3O6S/c1-39-25-15-17-26(18-16-25)40(37,38)33-19-24-9-5-6-10-27(24)32(20-28(33)29(34)31-36)30(35)23-13-11-22(12-14-23)21-7-3-2-4-8-21/h2-18,28,36H,19-20H2,1H3,(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration of the compound required in vitro to inhibit Matrix metalloproteinase-13 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50118983
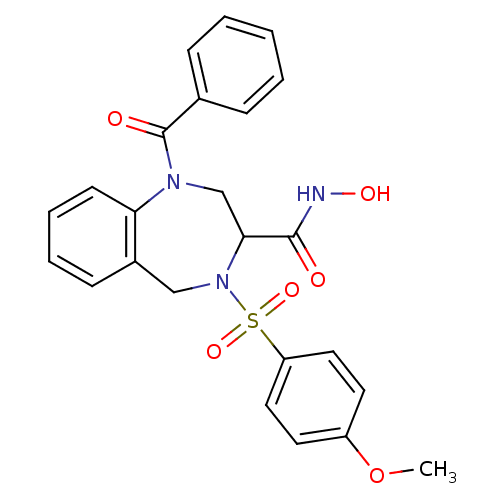
(1-Benzoyl-4-(4-methoxy-benzenesulfonyl)-2,3,4,5-te...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(=O)c1ccccc1 Show InChI InChI=1S/C24H23N3O6S/c1-33-19-11-13-20(14-12-19)34(31,32)27-15-18-9-5-6-10-21(18)26(16-22(27)23(28)25-30)24(29)17-7-3-2-4-8-17/h2-14,22,30H,15-16H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration of the compound required in vitro to inhibit Matrix metalloproteinase-13 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50106133
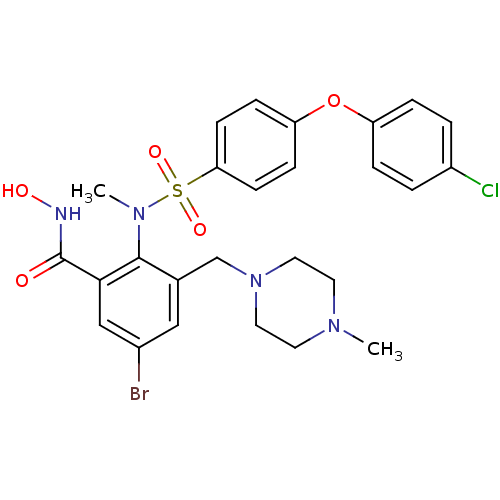
(5-Bromo-2-{[4-(4-chloro-phenoxy)-benzenesulfonyl]-...)Show SMILES CN(c1c(CN2CCN(C)CC2)cc(Br)cc1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C26H28BrClN4O5S/c1-30-11-13-32(14-12-30)17-18-15-19(27)16-24(26(33)29-34)25(18)31(2)38(35,36)23-9-7-22(8-10-23)37-21-5-3-20(28)4-6-21/h3-10,15-16,34H,11-14,17H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of Matrix metalloprotease-9. |
Bioorg Med Chem Lett 11: 2975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2125T64 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50118983

(1-Benzoyl-4-(4-methoxy-benzenesulfonyl)-2,3,4,5-te...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(=O)c1ccccc1 Show InChI InChI=1S/C24H23N3O6S/c1-33-19-11-13-20(14-12-19)34(31,32)27-15-18-9-5-6-10-21(18)26(16-22(27)23(28)25-30)24(29)17-7-3-2-4-8-17/h2-14,22,30H,15-16H2,1H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration required in vitro to inhibit Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50118985

(1-(Biphenyl-4-carbonyl)-4-(4-methoxy-benzenesulfon...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C30H27N3O6S/c1-39-25-15-17-26(18-16-25)40(37,38)33-19-24-9-5-6-10-27(24)32(20-28(33)29(34)31-36)30(35)23-13-11-22(12-14-23)21-7-3-2-4-8-21/h2-18,28,36H,19-20H2,1H3,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration required in vitro to inhibit Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50106133

(5-Bromo-2-{[4-(4-chloro-phenoxy)-benzenesulfonyl]-...)Show SMILES CN(c1c(CN2CCN(C)CC2)cc(Br)cc1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C26H28BrClN4O5S/c1-30-11-13-32(14-12-30)17-18-15-19(27)16-24(26(33)29-34)25(18)31(2)38(35,36)23-9-7-22(8-10-23)37-21-5-3-20(28)4-6-21/h3-10,15-16,34H,11-14,17H2,1-2H3,(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-13 |
Bioorg Med Chem Lett 11: 2975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2125T64 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50106136
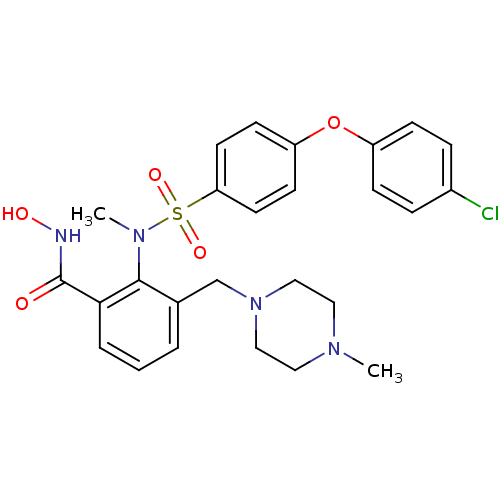
(2-{[4-(4-Chloro-phenoxy)-benzenesulfonyl]-methyl-a...)Show SMILES CN(c1c(CN2CCN(C)CC2)cccc1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C26H29ClN4O5S/c1-29-14-16-31(17-15-29)18-19-4-3-5-24(26(32)28-33)25(19)30(2)37(34,35)23-12-10-22(11-13-23)36-21-8-6-20(27)7-9-21/h3-13,33H,14-18H2,1-2H3,(H,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of Matrix metalloprotease-13. |
Bioorg Med Chem Lett 11: 2975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2125T64 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM205093
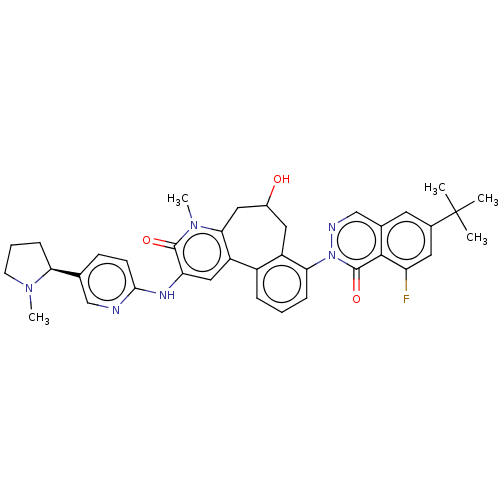
(US9556147, 2)Show SMILES CN1CCC[C@H]1c1ccc(Nc2cc-3c(CC(O)Cc4c-3cccc4-n3ncc4cc(cc(F)c4c3=O)C(C)(C)C)n(C)c2=O)nc1 |r| Show InChI InChI=1S/C37H39FN6O3/c1-37(2,3)23-14-22-20-40-44(36(47)34(22)28(38)15-23)31-9-6-8-25-26(31)16-24(45)17-32-27(25)18-29(35(46)43(32)5)41-33-12-11-21(19-39-33)30-10-7-13-42(30)4/h6,8-9,11-12,14-15,18-20,24,30,45H,7,10,13,16-17H2,1-5H3,(H,39,41)/t24?,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | 7.2 | n/a |
HOFFMANN-LA INC.
US Patent
| Assay Description
The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src... |
US Patent US9556147 (2017)
BindingDB Entry DOI: 10.7270/Q2HX1FP9 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM205094
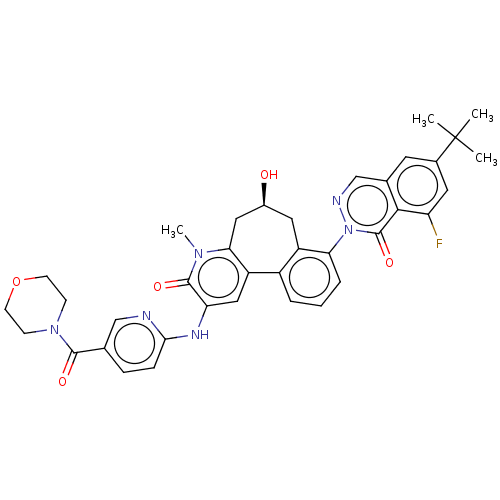
(US9556147, 3)Show SMILES Cn1c2C[C@@H](O)Cc3c(cccc3-n3ncc4cc(cc(F)c4c3=O)C(C)(C)C)-c2cc(Nc2ccc(cn2)C(=O)N2CCOCC2)c1=O |r| Show InChI InChI=1S/C37H37FN6O5/c1-37(2,3)23-14-22-20-40-44(36(48)33(22)28(38)15-23)30-7-5-6-25-26(30)16-24(45)17-31-27(25)18-29(35(47)42(31)4)41-32-9-8-21(19-39-32)34(46)43-10-12-49-13-11-43/h5-9,14-15,18-20,24,45H,10-13,16-17H2,1-4H3,(H,39,41)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
US Patent
| n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | 7.2 | n/a |
HOFFMANN-LA INC.
US Patent
| Assay Description
The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src... |
US Patent US9556147 (2017)
BindingDB Entry DOI: 10.7270/Q2HX1FP9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM205082
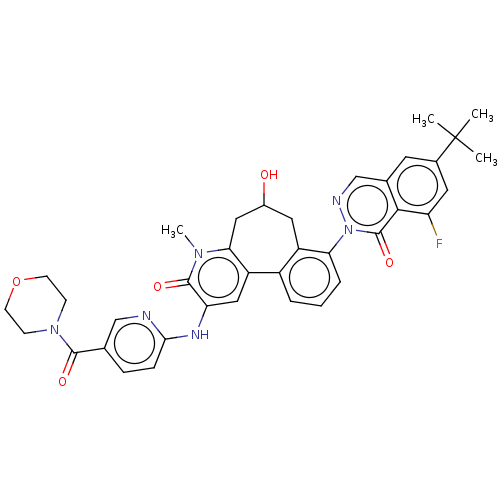
(US9556147, 1)Show SMILES Cn1c2CC(O)Cc3c(cccc3-n3ncc4cc(cc(F)c4c3=O)C(C)(C)C)-c2cc(Nc2ccc(cn2)C(=O)N2CCOCC2)c1=O Show InChI InChI=1S/C37H37FN6O5/c1-37(2,3)23-14-22-20-40-44(36(48)33(22)28(38)15-23)30-7-5-6-25-26(30)16-24(45)17-31-27(25)18-29(35(47)42(31)4)41-32-9-8-21(19-39-32)34(46)43-10-12-49-13-11-43/h5-9,14-15,18-20,24,45H,10-13,16-17H2,1-4H3,(H,39,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
US Patent
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | 7.2 | n/a |
HOFFMANN-LA INC.
US Patent
| Assay Description
The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src... |
US Patent US9556147 (2017)
BindingDB Entry DOI: 10.7270/Q2HX1FP9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM50096472
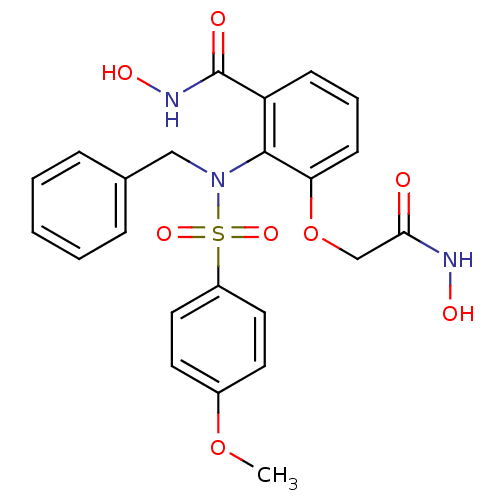
(2-(N-benzyl-4-methoxyphenylsulfonamido)-N-hydroxy-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccccc1)c1c(OCC(=O)NO)cccc1C(=O)NO Show InChI InChI=1S/C23H23N3O8S/c1-33-17-10-12-18(13-11-17)35(31,32)26(14-16-6-3-2-4-7-16)22-19(23(28)25-30)8-5-9-20(22)34-15-21(27)24-29/h2-13,29-30H,14-15H2,1H3,(H,24,27)(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Matrix metalloprotease-13 |
Bioorg Med Chem Lett 11: 235-8 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8N78 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50126612
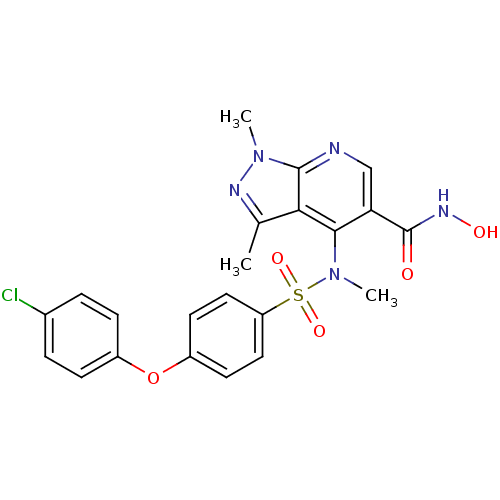
(4-{[4-(4-Chloro-phenoxy)-benzenesulfonyl]-methyl-a...)Show SMILES CN(c1c(cnc2n(C)nc(C)c12)C(=O)NO)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C22H20ClN5O5S/c1-13-19-20(18(22(29)26-30)12-24-21(19)27(2)25-13)28(3)34(31,32)17-10-8-16(9-11-17)33-15-6-4-14(23)5-7-15/h4-12,30H,1-3H3,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 9 (MMP-9). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50118974
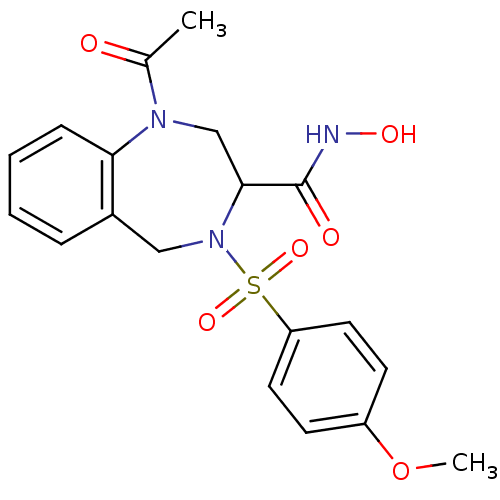
(1-Acetyl-4-(4-methoxy-benzenesulfonyl)-2,3,4,5-tet...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(C)=O Show InChI InChI=1S/C19H21N3O6S/c1-13(23)21-12-18(19(24)20-25)22(11-14-5-3-4-6-17(14)21)29(26,27)16-9-7-15(28-2)8-10-16/h3-10,18,25H,11-12H2,1-2H3,(H,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration of the compound required in vitro to inhibit Matrix metalloproteinase-13 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126600
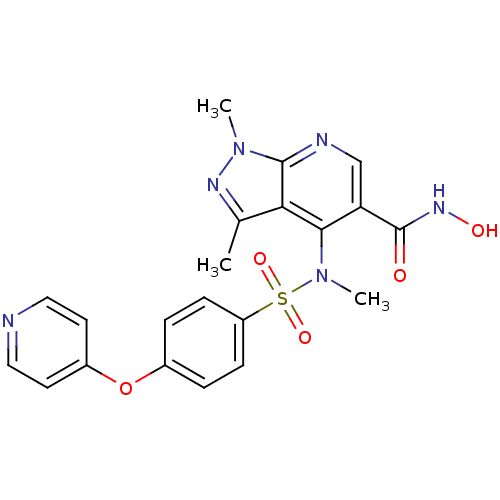
(1,3-Dimethyl-4-{methyl-[4-(pyridin-4-yloxy)-benzen...)Show SMILES CN(c1c(cnc2n(C)nc(C)c12)C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C21H20N6O5S/c1-13-18-19(17(21(28)25-29)12-23-20(18)26(2)24-13)27(3)33(30,31)16-6-4-14(5-7-16)32-15-8-10-22-11-9-15/h4-12,29H,1-3H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50118975
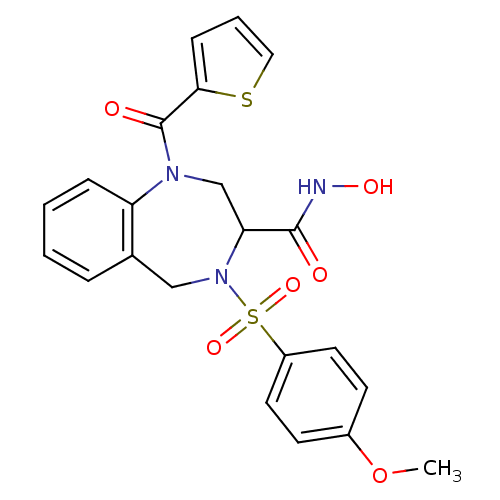
(4-(4-Methoxy-benzenesulfonyl)-1-(thiophene-2-carbo...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(=O)c1cccs1 Show InChI InChI=1S/C22H21N3O6S2/c1-31-16-8-10-17(11-9-16)33(29,30)25-13-15-5-2-3-6-18(15)24(14-19(25)21(26)23-28)22(27)20-7-4-12-32-20/h2-12,19,28H,13-14H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration required in vitro to inhibit Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50106136

(2-{[4-(4-Chloro-phenoxy)-benzenesulfonyl]-methyl-a...)Show SMILES CN(c1c(CN2CCN(C)CC2)cccc1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C26H29ClN4O5S/c1-29-14-16-31(17-15-29)18-19-4-3-5-24(26(32)28-33)25(19)30(2)37(34,35)23-12-10-22(11-13-23)36-21-8-6-20(27)7-9-21/h3-13,33H,14-18H2,1-2H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of Matrix metalloprotease-9. |
Bioorg Med Chem Lett 11: 2975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2125T64 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50106130
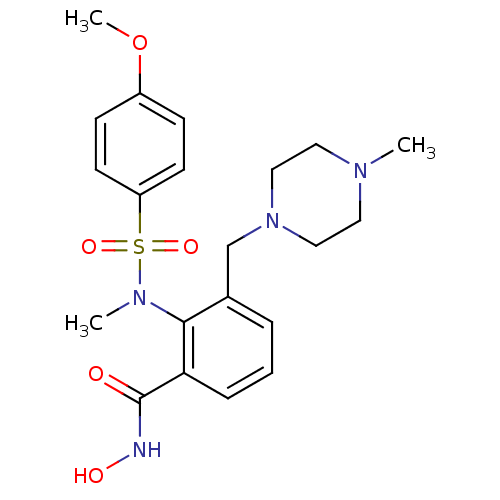
(CHEMBL100512 | N-Hydroxy-2-[(4-methoxy-benzenesulf...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(C)c1c(CN2CCN(C)CC2)cccc1C(=O)NO Show InChI InChI=1S/C21H28N4O5S/c1-23-11-13-25(14-12-23)15-16-5-4-6-19(21(26)22-27)20(16)24(2)31(28,29)18-9-7-17(30-3)8-10-18/h4-10,27H,11-15H2,1-3H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of Matrix metalloprotease-9. |
Bioorg Med Chem Lett 11: 2975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2125T64 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126612

(4-{[4-(4-Chloro-phenoxy)-benzenesulfonyl]-methyl-a...)Show SMILES CN(c1c(cnc2n(C)nc(C)c12)C(=O)NO)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C22H20ClN5O5S/c1-13-19-20(18(22(29)26-30)12-24-21(19)27(2)25-13)28(3)34(31,32)17-10-8-16(9-11-17)33-15-6-4-14(23)5-7-15/h4-12,30H,1-3H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126622
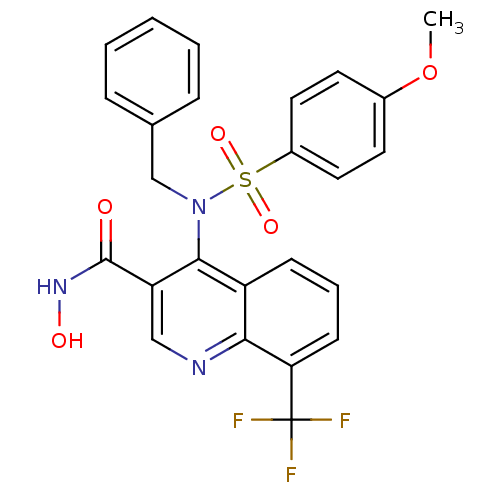
(4-(N-benzyl-4-methoxyphenylsulfonamido)-N-hydroxy-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccccc1)c1c(cnc2c(cccc12)C(F)(F)F)C(=O)NO Show InChI InChI=1S/C25H20F3N3O5S/c1-36-17-10-12-18(13-11-17)37(34,35)31(15-16-6-3-2-4-7-16)23-19-8-5-9-21(25(26,27)28)22(19)29-14-20(23)24(32)30-33/h2-14,33H,15H2,1H3,(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126626
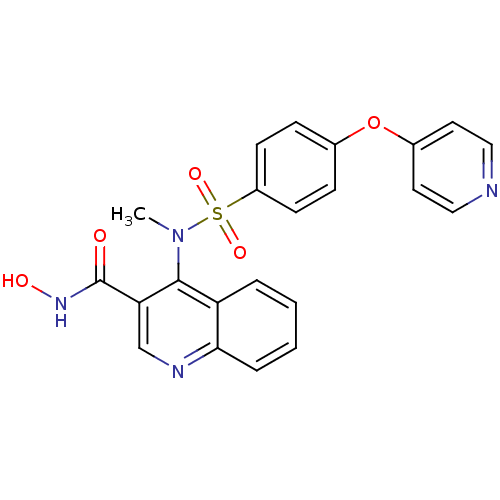
(4-{Methyl-[4-(pyridin-4-yloxy)-benzenesulfonyl]-am...)Show SMILES CN(c1c(cnc2ccccc12)C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C22H18N4O5S/c1-26(21-18-4-2-3-5-20(18)24-14-19(21)22(27)25-28)32(29,30)17-8-6-15(7-9-17)31-16-10-12-23-13-11-16/h2-14,28H,1H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50126626

(4-{Methyl-[4-(pyridin-4-yloxy)-benzenesulfonyl]-am...)Show SMILES CN(c1c(cnc2ccccc12)C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C22H18N4O5S/c1-26(21-18-4-2-3-5-20(18)24-14-19(21)22(27)25-28)32(29,30)17-8-6-15(7-9-17)31-16-10-12-23-13-11-16/h2-14,28H,1H3,(H,25,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 9 (MMP-9). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50118975

(4-(4-Methoxy-benzenesulfonyl)-1-(thiophene-2-carbo...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(=O)c1cccs1 Show InChI InChI=1S/C22H21N3O6S2/c1-31-16-8-10-17(11-9-16)33(29,30)25-13-15-5-2-3-6-18(15)24(14-19(25)21(26)23-28)22(27)20-7-4-12-32-20/h2-12,19,28H,13-14H2,1H3,(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration of the compound required in vitro to inhibit Matrix metalloproteinase-13 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126623
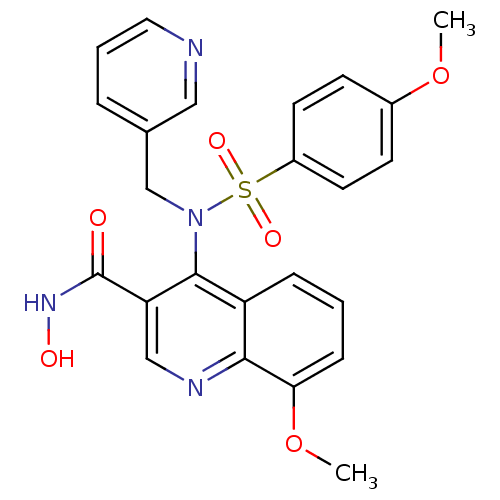
(8-Methoxy-4-[(4-methoxy-benzenesulfonyl)-pyridin-3...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2c(OC)cccc12)C(=O)NO Show InChI InChI=1S/C24H22N4O6S/c1-33-17-8-10-18(11-9-17)35(31,32)28(15-16-5-4-12-25-13-16)23-19-6-3-7-21(34-2)22(19)26-14-20(23)24(29)27-30/h3-14,30H,15H2,1-2H3,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126620
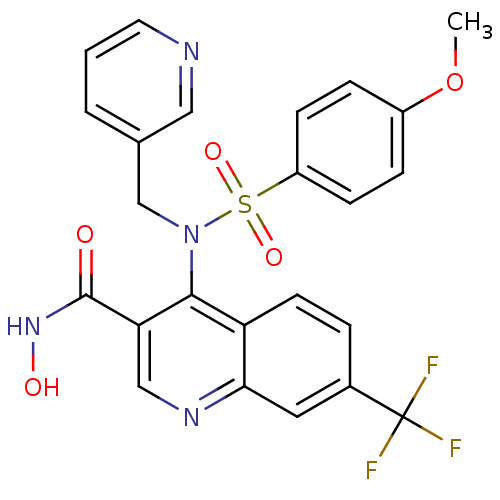
(4-[(4-Methoxy-benzenesulfonyl)-pyridin-3-ylmethyl-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2cc(ccc12)C(F)(F)F)C(=O)NO Show InChI InChI=1S/C24H19F3N4O5S/c1-36-17-5-7-18(8-6-17)37(34,35)31(14-15-3-2-10-28-12-15)22-19-9-4-16(24(25,26)27)11-21(19)29-13-20(22)23(32)30-33/h2-13,33H,14H2,1H3,(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50126624
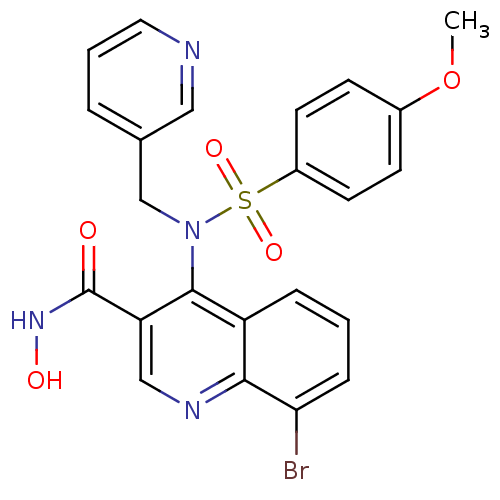
(8-Bromo-4-[(4-methoxy-benzenesulfonyl)-pyridin-3-y...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2c(Br)cccc12)C(=O)NO Show InChI InChI=1S/C23H19BrN4O5S/c1-33-16-7-9-17(10-8-16)34(31,32)28(14-15-4-3-11-25-12-15)22-18-5-2-6-20(24)21(18)26-13-19(22)23(29)27-30/h2-13,30H,14H2,1H3,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 13 (MMP-13). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50071839
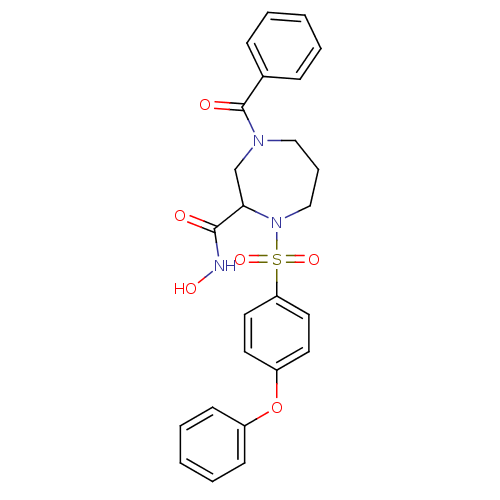
(4-Benzoyl-1-(4-phenoxy-benzenesulfonyl)-[1,4]diaze...)Show SMILES ONC(=O)C1CN(CCCN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)c1ccccc1 Show InChI InChI=1S/C25H25N3O6S/c29-24(26-31)23-18-27(25(30)19-8-3-1-4-9-19)16-7-17-28(23)35(32,33)22-14-12-21(13-15-22)34-20-10-5-2-6-11-20/h1-6,8-15,23,31H,7,16-18H2,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-9 (MMP-9) |
Bioorg Med Chem Lett 8: 2657-62 (1999)
BindingDB Entry DOI: 10.7270/Q28K79KZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM205095
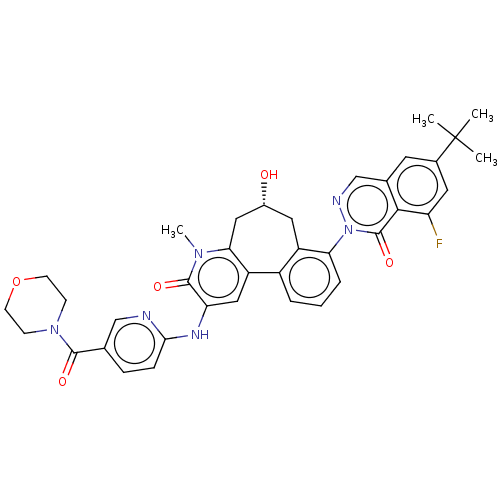
(US9556147, 4)Show SMILES Cn1c2C[C@H](O)Cc3c(cccc3-n3ncc4cc(cc(F)c4c3=O)C(C)(C)C)-c2cc(Nc2ccc(cn2)C(=O)N2CCOCC2)c1=O |r| Show InChI InChI=1S/C37H37FN6O5/c1-37(2,3)23-14-22-20-40-44(36(48)33(22)28(38)15-23)30-7-5-6-25-26(30)16-24(45)17-31-27(25)18-29(35(47)42(31)4)41-32-9-8-21(19-39-32)34(46)43-10-12-49-13-11-43/h5-9,14-15,18-20,24,45H,10-13,16-17H2,1-4H3,(H,39,41)/t24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.2 | n/a |
HOFFMANN-LA INC.
US Patent
| Assay Description
The assay is a capture of radioactive 33P phosphorylated product through filtration. The interactions of BTK, biotinylated SH2 peptide substrate (Src... |
US Patent US9556147 (2017)
BindingDB Entry DOI: 10.7270/Q2HX1FP9 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50071839

(4-Benzoyl-1-(4-phenoxy-benzenesulfonyl)-[1,4]diaze...)Show SMILES ONC(=O)C1CN(CCCN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1)C(=O)c1ccccc1 Show InChI InChI=1S/C25H25N3O6S/c29-24(26-31)23-18-27(25(30)19-8-3-1-4-9-19)16-7-17-28(23)35(32,33)22-14-12-21(13-15-22)34-20-10-5-2-6-11-20/h1-6,8-15,23,31H,7,16-18H2,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-13 (MMP-13) |
Bioorg Med Chem Lett 8: 2657-62 (1999)
BindingDB Entry DOI: 10.7270/Q28K79KZ |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50118974

(1-Acetyl-4-(4-methoxy-benzenesulfonyl)-2,3,4,5-tet...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1Cc2ccccc2N(CC1C(=O)NO)C(C)=O Show InChI InChI=1S/C19H21N3O6S/c1-13(23)21-12-18(19(24)20-25)22(11-14-5-3-4-6-17(14)21)29(26,27)16-9-7-15(28-2)8-10-16/h3-10,18,25H,11-12H2,1-2H3,(H,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration required in vitro to inhibit Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50118970
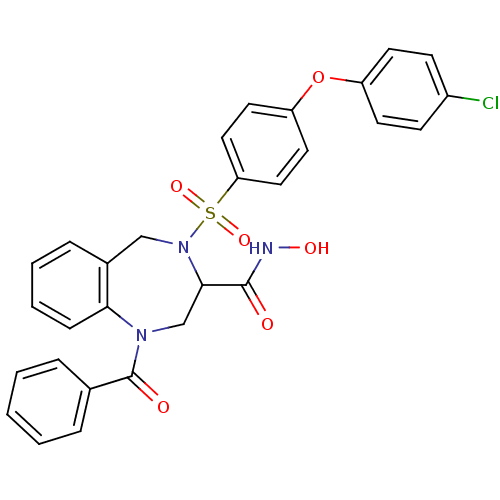
(1-Benzoyl-4-[4-(4-chloro-phenoxy)-benzenesulfonyl]...)Show SMILES ONC(=O)C1CN(C(=O)c2ccccc2)c2ccccc2CN1S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C29H24ClN3O6S/c30-22-10-12-23(13-11-22)39-24-14-16-25(17-15-24)40(37,38)33-18-21-8-4-5-9-26(21)32(19-27(33)28(34)31-36)29(35)20-6-2-1-3-7-20/h1-17,27,36H,18-19H2,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration required in vitro to inhibit Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50118976
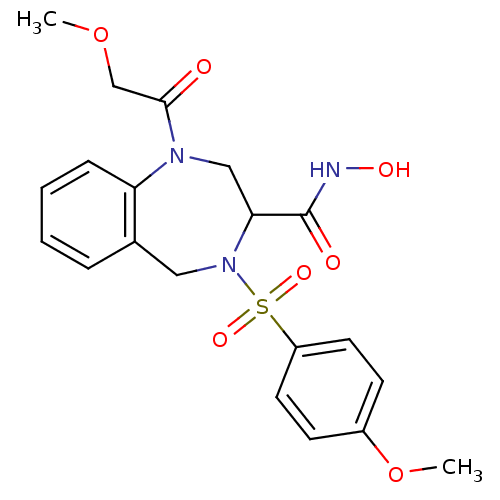
(1-(2-Methoxy-acetyl)-4-(4-methoxy-benzenesulfonyl)...)Show SMILES COCC(=O)N1CC(N(Cc2ccccc12)S(=O)(=O)c1ccc(OC)cc1)C(=O)NO Show InChI InChI=1S/C20H23N3O7S/c1-29-13-19(24)22-12-18(20(25)21-26)23(11-14-5-3-4-6-17(14)22)31(27,28)16-9-7-15(30-2)8-10-16/h3-10,18,26H,11-13H2,1-2H3,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration required in vitro to inhibit Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50118971
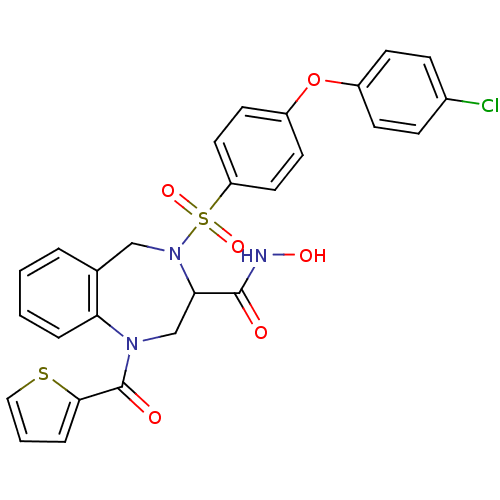
(4-[4-(4-Chloro-phenoxy)-benzenesulfonyl]-1-(thioph...)Show SMILES ONC(=O)C1CN(C(=O)c2cccs2)c2ccccc2CN1S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C27H22ClN3O6S2/c28-19-7-9-20(10-8-19)37-21-11-13-22(14-12-21)39(35,36)31-16-18-4-1-2-5-23(18)30(17-24(31)26(32)29-34)27(33)25-6-3-15-38-25/h1-15,24,34H,16-17H2,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration required in vitro to inhibit Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50118984
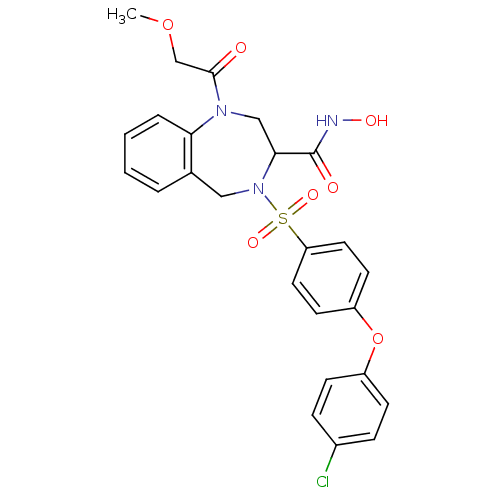
(4-[4-(4-Chloro-phenoxy)-benzenesulfonyl]-1-(2-meth...)Show SMILES COCC(=O)N1CC(N(Cc2ccccc12)S(=O)(=O)c1ccc(Oc2ccc(Cl)cc2)cc1)C(=O)NO Show InChI InChI=1S/C25H24ClN3O7S/c1-35-16-24(30)28-15-23(25(31)27-32)29(14-17-4-2-3-5-22(17)28)37(33,34)21-12-10-20(11-13-21)36-19-8-6-18(26)7-9-19/h2-13,23,32H,14-16H2,1H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration required in vitro to inhibit Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50118969
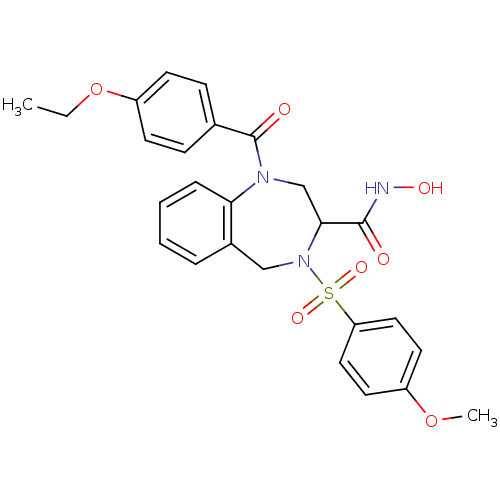
(1-(4-Ethoxy-benzoyl)-4-(4-methoxy-benzenesulfonyl)...)Show SMILES CCOc1ccc(cc1)C(=O)N1CC(N(Cc2ccccc12)S(=O)(=O)c1ccc(OC)cc1)C(=O)NO Show InChI InChI=1S/C26H27N3O7S/c1-3-36-21-10-8-18(9-11-21)26(31)28-17-24(25(30)27-32)29(16-19-6-4-5-7-23(19)28)37(33,34)22-14-12-20(35-2)13-15-22/h4-15,24,32H,3,16-17H2,1-2H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Concentration required in vitro to inhibit Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 12: 2867-70 (2002)
BindingDB Entry DOI: 10.7270/Q2JS9PSH |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50126623

(8-Methoxy-4-[(4-methoxy-benzenesulfonyl)-pyridin-3...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1cccnc1)c1c(cnc2c(OC)cccc12)C(=O)NO Show InChI InChI=1S/C24H22N4O6S/c1-33-17-8-10-18(11-9-17)35(31,32)28(15-16-5-4-12-25-13-16)23-19-6-3-7-21(34-2)22(19)26-14-20(23)24(29)27-30/h3-14,30H,15H2,1-2H3,(H,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloproteinase 9 (MMP-9). |
Bioorg Med Chem Lett 13: 1487-90 (2003)
BindingDB Entry DOI: 10.7270/Q29W0DW0 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50106145
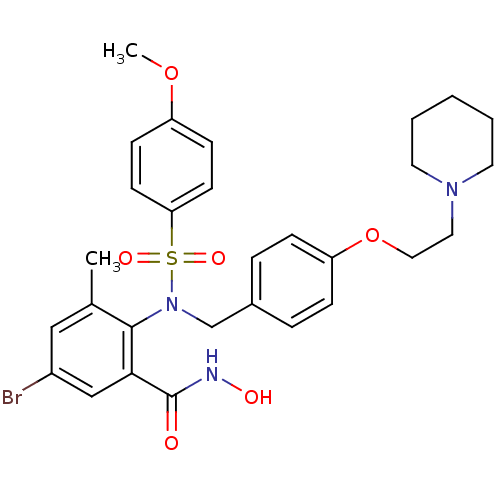
(5-Bromo-N-hydroxy-2-{(4-methoxy-benzenesulfonyl)-[...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccc(OCCN2CCCCC2)cc1)c1c(C)cc(Br)cc1C(=O)NO Show InChI InChI=1S/C29H34BrN3O6S/c1-21-18-23(30)19-27(29(34)31-35)28(21)33(40(36,37)26-12-10-24(38-2)11-13-26)20-22-6-8-25(9-7-22)39-17-16-32-14-4-3-5-15-32/h6-13,18-19,35H,3-5,14-17,20H2,1-2H3,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of Matrix metalloprotease-9. |
Bioorg Med Chem Lett 11: 2975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2125T64 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50106141

(4-[(4-Methoxy-benzenesulfonyl)-methyl-amino]-5-(4-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(C)c1c(CN2CCN(C)CC2)cc(cc1C(=O)NO)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C28H31F3N4O6S/c1-33-12-14-35(15-13-33)18-21-16-20(19-4-6-23(7-5-19)41-28(29,30)31)17-25(27(36)32-37)26(21)34(2)42(38,39)24-10-8-22(40-3)9-11-24/h4-11,16-17,37H,12-15,18H2,1-3H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of Matrix metalloprotease-9. |
Bioorg Med Chem Lett 11: 2975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2125T64 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50096472

(2-(N-benzyl-4-methoxyphenylsulfonamido)-N-hydroxy-...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccccc1)c1c(OCC(=O)NO)cccc1C(=O)NO Show InChI InChI=1S/C23H23N3O8S/c1-33-17-10-12-18(13-11-17)35(31,32)26(14-16-6-3-2-4-7-16)22-19(23(28)25-30)8-5-9-20(22)34-15-21(27)24-29/h2-13,29-30H,14-15H2,1H3,(H,24,27)(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Matrix metalloprotease-9 |
Bioorg Med Chem Lett 11: 235-8 (2001)
BindingDB Entry DOI: 10.7270/Q2TH8N78 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50106131
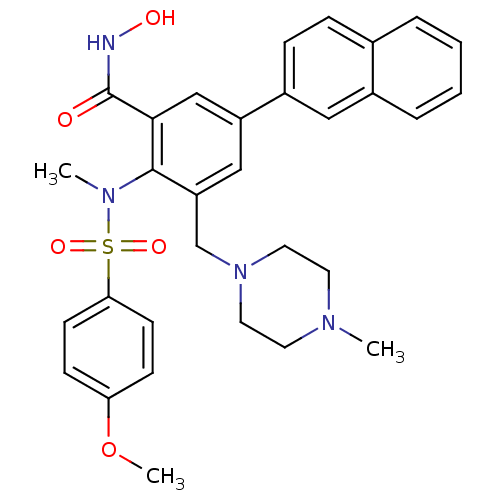
(CHEMBL100777 | N-Hydroxy-2-[(4-methoxy-benzenesulf...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(C)c1c(CN2CCN(C)CC2)cc(cc1C(=O)NO)-c1ccc2ccccc2c1 Show InChI InChI=1S/C31H34N4O5S/c1-33-14-16-35(17-15-33)21-26-19-25(24-9-8-22-6-4-5-7-23(22)18-24)20-29(31(36)32-37)30(26)34(2)41(38,39)28-12-10-27(40-3)11-13-28/h4-13,18-20,37H,14-17,21H2,1-3H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
In vitro inhibition of Matrix metalloprotease-9. |
Bioorg Med Chem Lett 11: 2975-8 (2001)
BindingDB Entry DOI: 10.7270/Q2125T64 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data