 Found 133 hits with Last Name = 'rotter' and Initial = 'c'
Found 133 hits with Last Name = 'rotter' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50003407
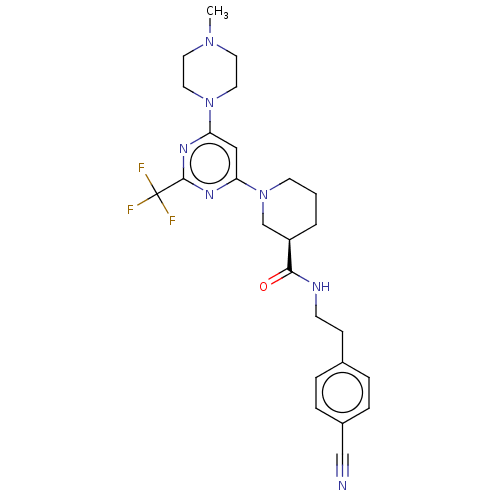
(CHEMBL3234568)Show SMILES CN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CCC[C@H](C1)C(=O)NCCc1ccc(cc1)C#N |r| Show InChI InChI=1S/C25H30F3N7O/c1-33-11-13-34(14-12-33)21-15-22(32-24(31-21)25(26,27)28)35-10-2-3-20(17-35)23(36)30-9-8-18-4-6-19(16-29)7-5-18/h4-7,15,20H,2-3,8-14,17H2,1H3,(H,30,36)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at TGR5 in human PBMC assessed as inhibition of LPS-induced TNFalpha production preincubated for 30 mins followed by LPS stimulation... |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50003477
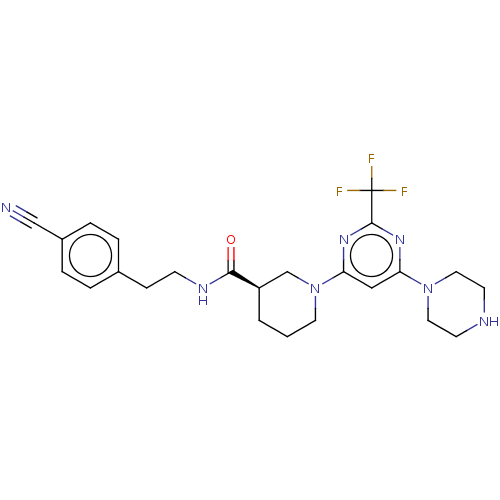
(CHEMBL3234569)Show SMILES FC(F)(F)c1nc(cc(n1)N1CCC[C@H](C1)C(=O)NCCc1ccc(cc1)C#N)N1CCNCC1 |r| Show InChI InChI=1S/C24H28F3N7O/c25-24(26,27)23-31-20(33-12-9-29-10-13-33)14-21(32-23)34-11-1-2-19(16-34)22(35)30-8-7-17-3-5-18(15-28)6-4-17/h3-6,14,19,29H,1-2,7-13,16H2,(H,30,35)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50003573
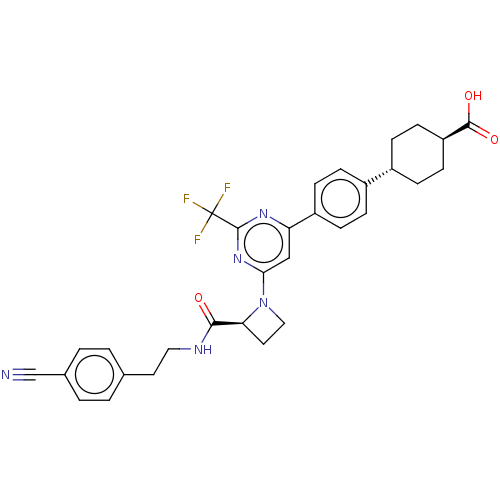
(CHEMBL3234871)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)c1ccc(cc1)-c1cc(nc(n1)C(F)(F)F)N1CC[C@H]1C(=O)NCCc1ccc(cc1)C#N |r,wU:6.9,28.32,wD:3.2,(43.64,-56.93,;44.97,-56.16,;46.31,-56.93,;44.97,-54.62,;46.31,-53.85,;46.3,-52.3,;44.97,-51.54,;43.65,-52.32,;43.64,-53.85,;44.96,-50,;43.62,-49.23,;43.62,-47.69,;44.96,-46.92,;46.29,-47.69,;46.29,-49.23,;44.96,-45.38,;46.29,-44.61,;46.29,-43.07,;44.96,-42.3,;43.63,-43.07,;43.62,-44.61,;42.29,-42.3,;40.95,-43.07,;42.29,-40.76,;40.95,-41.53,;47.63,-42.3,;48.02,-40.81,;49.51,-41.22,;49.11,-42.7,;49.87,-44.04,;49.1,-45.37,;51.41,-44.04,;52.19,-42.71,;53.73,-42.72,;54.5,-41.38,;56.04,-41.4,;56.81,-40.07,;56.04,-38.73,;54.49,-38.73,;53.73,-40.06,;56.81,-37.4,;57.58,-36.07,)| Show InChI InChI=1S/C31H30F3N5O3/c32-31(33,34)30-37-25(23-9-5-21(6-10-23)22-7-11-24(12-8-22)29(41)42)17-27(38-30)39-16-14-26(39)28(40)36-15-13-19-1-3-20(18-35)4-2-19/h1-6,9-10,17,22,24,26H,7-8,11-16H2,(H,36,40)(H,41,42)/t22-,24-,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at TGR5 in human PBMC assessed as inhibition of LPS-induced TNFalpha production preincubated for 30 mins followed by LPS stimulation... |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50003562
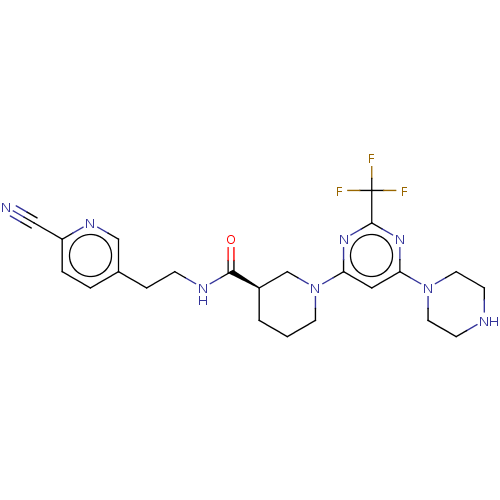
(CHEMBL3234572)Show SMILES FC(F)(F)c1nc(cc(n1)N1CCC[C@H](C1)C(=O)NCCc1ccc(nc1)C#N)N1CCNCC1 |r| Show InChI InChI=1S/C23H27F3N8O/c24-23(25,26)22-31-19(33-10-7-28-8-11-33)12-20(32-22)34-9-1-2-17(15-34)21(35)29-6-5-16-3-4-18(13-27)30-14-16/h3-4,12,14,17,28H,1-2,5-11,15H2,(H,29,35)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <300 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50003076
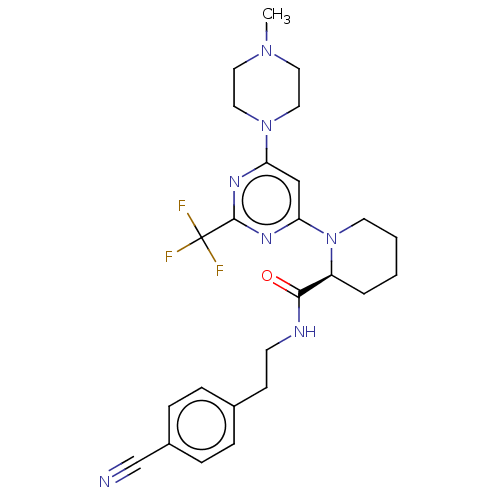
(CHEMBL3234574)Show SMILES CN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CCCC[C@H]1C(=O)NCCc1ccc(cc1)C#N |r| Show InChI InChI=1S/C25H30F3N7O/c1-33-12-14-34(15-13-33)21-16-22(32-24(31-21)25(26,27)28)35-11-3-2-4-20(35)23(36)30-10-9-18-5-7-19(17-29)8-6-18/h5-8,16,20H,2-4,9-15H2,1H3,(H,30,36)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50003286
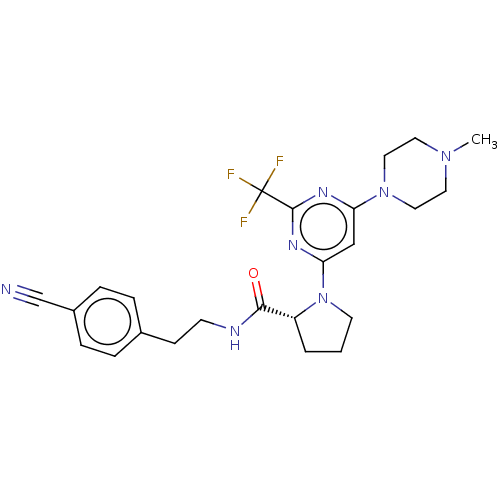
(CHEMBL3234576)Show SMILES CN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CCC[C@@H]1C(=O)NCCc1ccc(cc1)C#N |r| Show InChI InChI=1S/C24H28F3N7O/c1-32-11-13-33(14-12-32)20-15-21(31-23(30-20)24(25,26)27)34-10-2-3-19(34)22(35)29-9-8-17-4-6-18(16-28)7-5-17/h4-7,15,19H,2-3,8-14H2,1H3,(H,29,35)/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50003289
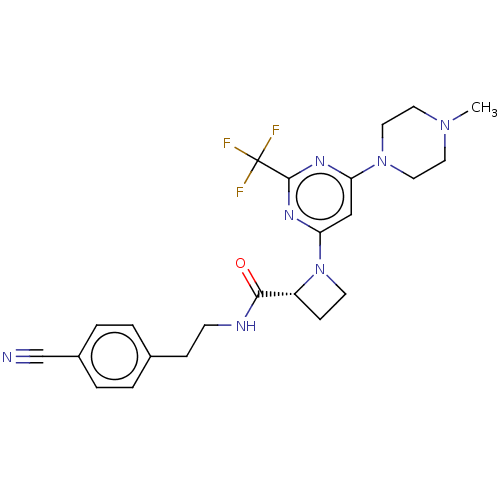
(CHEMBL3234578)Show SMILES CN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CC[C@@H]1C(=O)NCCc1ccc(cc1)C#N |r| Show InChI InChI=1S/C23H26F3N7O/c1-31-10-12-32(13-11-31)19-14-20(30-22(29-19)23(24,25)26)33-9-7-18(33)21(34)28-8-6-16-2-4-17(15-27)5-3-16/h2-5,14,18H,6-13H2,1H3,(H,28,34)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50003407

(CHEMBL3234568)Show SMILES CN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CCC[C@H](C1)C(=O)NCCc1ccc(cc1)C#N |r| Show InChI InChI=1S/C25H30F3N7O/c1-33-11-13-34(14-12-33)21-15-22(32-24(31-21)25(26,27)28)35-10-2-3-20(17-35)23(36)30-9-8-18-4-6-19(16-29)7-5-18/h4-7,15,20H,2-3,8-14,17H2,1H3,(H,30,36)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50003559
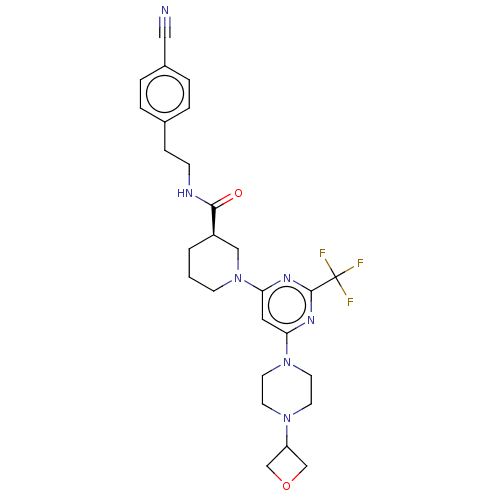
(CHEMBL3234570)Show SMILES FC(F)(F)c1nc(cc(n1)N1CCC[C@H](C1)C(=O)NCCc1ccc(cc1)C#N)N1CCN(CC1)C1COC1 |r| Show InChI InChI=1S/C27H32F3N7O2/c28-27(29,30)26-33-23(36-12-10-35(11-13-36)22-17-39-18-22)14-24(34-26)37-9-1-2-21(16-37)25(38)32-8-7-19-3-5-20(15-31)6-4-19/h3-6,14,21-22H,1-2,7-13,16-18H2,(H,32,38)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50444409
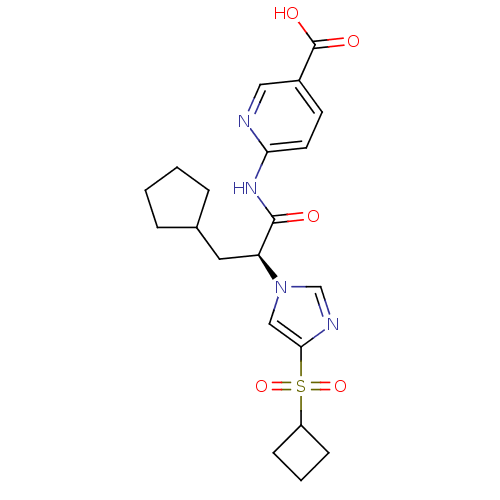
(CHEMBL3091581)Show SMILES OC(=O)c1ccc(NC(=O)[C@H](CC2CCCC2)n2cnc(c2)S(=O)(=O)C2CCC2)nc1 |r| Show InChI InChI=1S/C21H26N4O5S/c26-20(24-18-9-8-15(11-22-18)21(27)28)17(10-14-4-1-2-5-14)25-12-19(23-13-25)31(29,30)16-6-3-7-16/h8-9,11-14,16-17H,1-7,10H2,(H,27,28)(H,22,24,26)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Activation of glucokinase in Wistar rat hepatocytes assessed as nuclear to cytosolic translocation of protein by immunofluorescence assay |
Bioorg Med Chem Lett 23: 6588-92 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.057
BindingDB Entry DOI: 10.7270/Q2QZ2CFX |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50003336
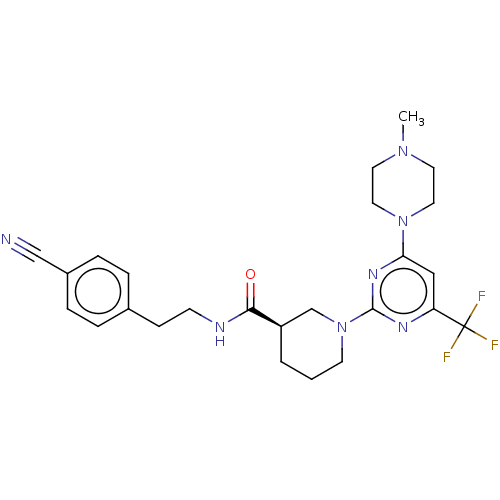
(CHEMBL3234565)Show SMILES CN1CCN(CC1)c1cc(nc(n1)N1CCC[C@H](C1)C(=O)NCCc1ccc(cc1)C#N)C(F)(F)F |r| Show InChI InChI=1S/C25H30F3N7O/c1-33-11-13-34(14-12-33)22-15-21(25(26,27)28)31-24(32-22)35-10-2-3-20(17-35)23(36)30-9-8-18-4-6-19(16-29)7-5-18/h4-7,15,20H,2-3,8-14,17H2,1H3,(H,30,36)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Transcriptional regulator ERG
(Homo sapiens (Human)) | BDBM50003336

(CHEMBL3234565)Show SMILES CN1CCN(CC1)c1cc(nc(n1)N1CCC[C@H](C1)C(=O)NCCc1ccc(cc1)C#N)C(F)(F)F |r| Show InChI InChI=1S/C25H30F3N7O/c1-33-11-13-34(14-12-33)22-15-21(25(26,27)28)31-24(32-22)35-10-2-3-20(17-35)23(36)30-9-8-18-4-6-19(16-29)7-5-18/h4-7,15,20H,2-3,8-14,17H2,1H3,(H,30,36)/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp method |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50003283
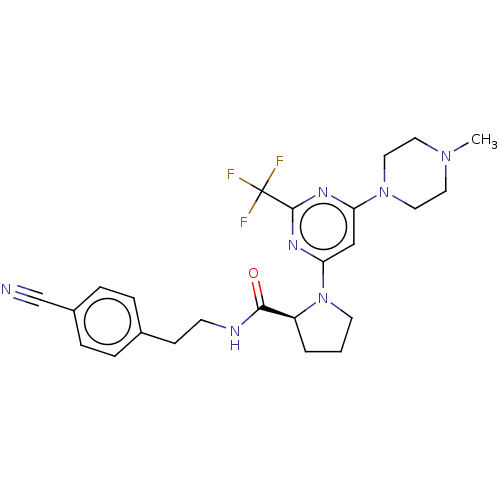
(CHEMBL3234575)Show SMILES CN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CCC[C@H]1C(=O)NCCc1ccc(cc1)C#N |r| Show InChI InChI=1S/C24H28F3N7O/c1-32-11-13-33(14-12-32)20-15-21(31-23(30-20)24(25,26)27)34-10-2-3-19(34)22(35)29-9-8-17-4-6-18(16-28)7-5-17/h4-7,15,19H,2-3,8-14H2,1H3,(H,29,35)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Transcriptional regulator ERG
(Homo sapiens (Human)) | BDBM50003076

(CHEMBL3234574)Show SMILES CN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CCCC[C@H]1C(=O)NCCc1ccc(cc1)C#N |r| Show InChI InChI=1S/C25H30F3N7O/c1-33-12-14-34(15-13-33)21-16-22(32-24(31-21)25(26,27)28)35-11-3-2-4-20(35)23(36)30-10-9-18-5-7-19(17-29)8-6-18/h5-8,16,20H,2-4,9-15H2,1H3,(H,30,36)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp method |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50003287
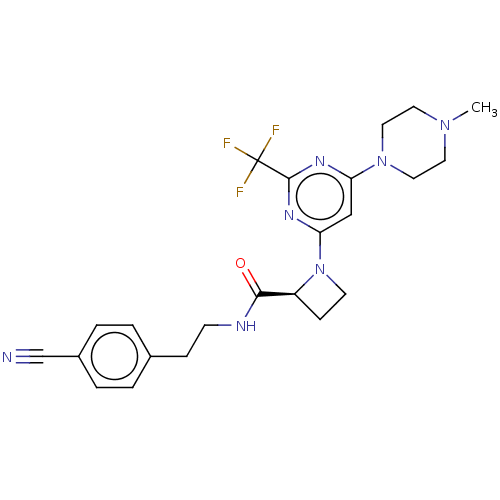
(CHEMBL3234577)Show SMILES CN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CC[C@H]1C(=O)NCCc1ccc(cc1)C#N |r| Show InChI InChI=1S/C23H26F3N7O/c1-31-10-12-32(13-11-31)19-14-20(30-22(29-19)23(24,25)26)33-9-7-18(33)21(34)28-8-6-16-2-4-17(15-27)5-3-16/h2-5,14,18H,6-13H2,1H3,(H,28,34)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Transcriptional regulator ERG
(Homo sapiens (Human)) | BDBM50003334
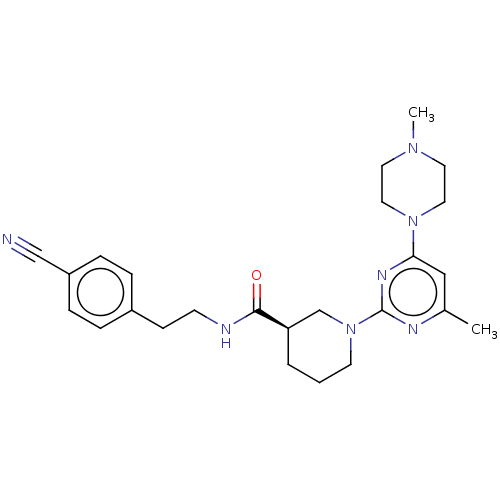
(CHEMBL3234564)Show SMILES CN1CCN(CC1)c1cc(C)nc(n1)N1CCC[C@H](C1)C(=O)NCCc1ccc(cc1)C#N |r| Show InChI InChI=1S/C25H33N7O/c1-19-16-23(31-14-12-30(2)13-15-31)29-25(28-19)32-11-3-4-22(18-32)24(33)27-10-9-20-5-7-21(17-26)8-6-20/h5-8,16,22H,3-4,9-15,18H2,1-2H3,(H,27,33)/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp method |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Transcriptional regulator ERG
(Homo sapiens (Human)) | BDBM50003407

(CHEMBL3234568)Show SMILES CN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CCC[C@H](C1)C(=O)NCCc1ccc(cc1)C#N |r| Show InChI InChI=1S/C25H30F3N7O/c1-33-11-13-34(14-12-33)21-15-22(32-24(31-21)25(26,27)28)35-10-2-3-20(17-35)23(36)30-9-8-18-4-6-19(16-29)7-5-18/h4-7,15,20H,2-3,8-14,17H2,1H3,(H,30,36)/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp method |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Transcriptional regulator ERG
(Homo sapiens (Human)) | BDBM50003477

(CHEMBL3234569)Show SMILES FC(F)(F)c1nc(cc(n1)N1CCC[C@H](C1)C(=O)NCCc1ccc(cc1)C#N)N1CCNCC1 |r| Show InChI InChI=1S/C24H28F3N7O/c25-24(26,27)23-31-20(33-12-9-29-10-13-33)14-21(32-23)34-11-1-2-19(16-34)22(35)30-8-7-17-3-5-18(15-28)6-4-17/h3-6,14,19,29H,1-2,7-13,16H2,(H,30,35)/t19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp method |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50003407

(CHEMBL3234568)Show SMILES CN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CCC[C@H](C1)C(=O)NCCc1ccc(cc1)C#N |r| Show InChI InChI=1S/C25H30F3N7O/c1-33-11-13-34(14-12-33)21-15-22(32-24(31-21)25(26,27)28)35-10-2-3-20(17-35)23(36)30-9-8-18-4-6-19(16-29)7-5-18/h4-7,15,20H,2-3,8-14,17H2,1H3,(H,30,36)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50003563
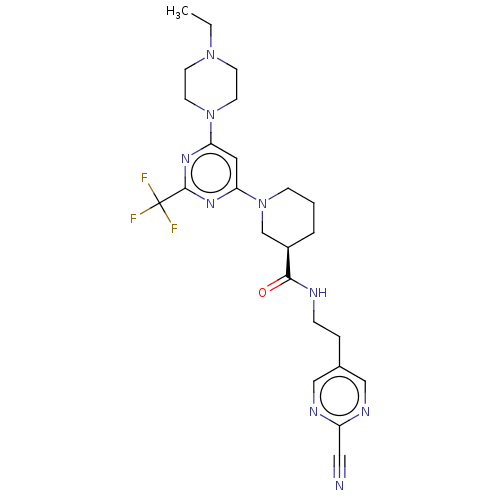
(CHEMBL3234573)Show SMILES CCN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CCC[C@H](C1)C(=O)NCCc1cnc(nc1)C#N |r| Show InChI InChI=1S/C24H30F3N9O/c1-2-34-8-10-35(11-9-34)20-12-21(33-23(32-20)24(25,26)27)36-7-3-4-18(16-36)22(37)29-6-5-17-14-30-19(13-28)31-15-17/h12,14-15,18H,2-11,16H2,1H3,(H,29,37)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Transcriptional regulator ERG
(Homo sapiens (Human)) | BDBM50003286

(CHEMBL3234576)Show SMILES CN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CCC[C@@H]1C(=O)NCCc1ccc(cc1)C#N |r| Show InChI InChI=1S/C24H28F3N7O/c1-32-11-13-33(14-12-32)20-15-21(31-23(30-20)24(25,26)27)34-10-2-3-19(34)22(35)29-9-8-17-4-6-18(16-28)7-5-17/h4-7,15,19H,2-3,8-14H2,1H3,(H,29,35)/t19-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp method |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Transcriptional regulator ERG
(Homo sapiens (Human)) | BDBM50003289

(CHEMBL3234578)Show SMILES CN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CC[C@@H]1C(=O)NCCc1ccc(cc1)C#N |r| Show InChI InChI=1S/C23H26F3N7O/c1-31-10-12-32(13-11-31)19-14-20(30-22(29-19)23(24,25)26)33-9-7-18(33)21(34)28-8-6-16-2-4-17(15-27)5-3-16/h2-5,14,18H,6-13H2,1H3,(H,28,34)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp method |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50003337

(CHEMBL3234566)Show SMILES CN1CCN(CC1)c1nc(nc(C)c1F)N1CCC[C@H](C1)C(=O)NCCc1ccc(cc1)C#N |r| Show InChI InChI=1S/C25H32FN7O/c1-18-22(26)23(32-14-12-31(2)13-15-32)30-25(29-18)33-11-3-4-21(17-33)24(34)28-10-9-19-5-7-20(16-27)8-6-19/h5-8,21H,3-4,9-15,17H2,1-2H3,(H,28,34)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50003291
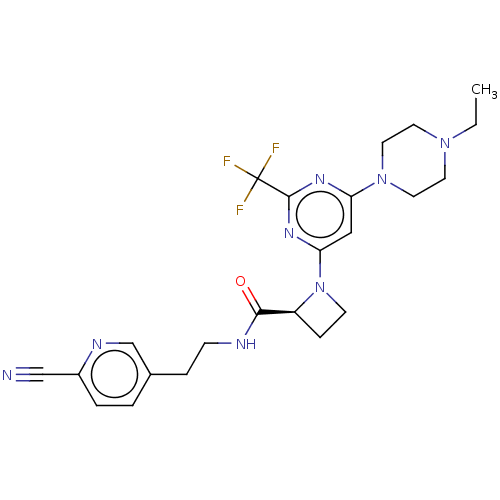
(CHEMBL3234579)Show SMILES CCN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CC[C@H]1C(=O)NCCc1ccc(nc1)C#N |r| Show InChI InChI=1S/C23H27F3N8O/c1-2-32-9-11-33(12-10-32)19-13-20(31-22(30-19)23(24,25)26)34-8-6-18(34)21(35)28-7-5-16-3-4-17(14-27)29-15-16/h3-4,13,15,18H,2,5-12H2,1H3,(H,28,35)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Transcriptional regulator ERG
(Homo sapiens (Human)) | BDBM50003287

(CHEMBL3234577)Show SMILES CN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CC[C@H]1C(=O)NCCc1ccc(cc1)C#N |r| Show InChI InChI=1S/C23H26F3N7O/c1-31-10-12-32(13-11-31)19-14-20(30-22(29-19)23(24,25)26)33-9-7-18(33)21(34)28-8-6-16-2-4-17(15-27)5-3-16/h2-5,14,18H,6-13H2,1H3,(H,28,34)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp method |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50003561
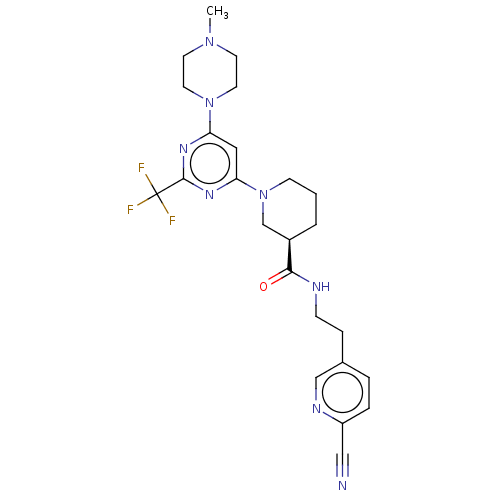
(CHEMBL3234571)Show SMILES CN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CCC[C@H](C1)C(=O)NCCc1ccc(nc1)C#N |r| Show InChI InChI=1S/C24H29F3N8O/c1-33-9-11-34(12-10-33)20-13-21(32-23(31-20)24(25,26)27)35-8-2-3-18(16-35)22(36)29-7-6-17-4-5-19(14-28)30-15-17/h4-5,13,15,18H,2-3,6-12,16H2,1H3,(H,29,36)/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50003407

(CHEMBL3234568)Show SMILES CN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CCC[C@H](C1)C(=O)NCCc1ccc(cc1)C#N |r| Show InChI InChI=1S/C25H30F3N7O/c1-33-11-13-34(14-12-33)21-15-22(32-24(31-21)25(26,27)28)35-10-2-3-20(17-35)23(36)30-9-8-18-4-6-19(16-29)7-5-18/h4-7,15,20H,2-3,8-14,17H2,1H3,(H,30,36)/t20-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Transcriptional regulator ERG
(Homo sapiens (Human)) | BDBM50003283

(CHEMBL3234575)Show SMILES CN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CCC[C@H]1C(=O)NCCc1ccc(cc1)C#N |r| Show InChI InChI=1S/C24H28F3N7O/c1-32-11-13-33(14-12-32)20-15-21(31-23(30-20)24(25,26)27)34-10-2-3-19(34)22(35)29-9-8-17-4-6-18(16-28)7-5-17/h4-7,15,19H,2-3,8-14H2,1H3,(H,29,35)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp method |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Transcriptional regulator ERG
(Homo sapiens (Human)) | BDBM50003563

(CHEMBL3234573)Show SMILES CCN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CCC[C@H](C1)C(=O)NCCc1cnc(nc1)C#N |r| Show InChI InChI=1S/C24H30F3N9O/c1-2-34-8-10-35(11-9-34)20-12-21(33-23(32-20)24(25,26)27)36-7-3-4-18(16-36)22(37)29-6-5-17-14-30-19(13-28)31-15-17/h12,14-15,18H,2-11,16H2,1H3,(H,29,37)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp method |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Transcriptional regulator ERG
(Homo sapiens (Human)) | BDBM50003562

(CHEMBL3234572)Show SMILES FC(F)(F)c1nc(cc(n1)N1CCC[C@H](C1)C(=O)NCCc1ccc(nc1)C#N)N1CCNCC1 |r| Show InChI InChI=1S/C23H27F3N8O/c24-23(25,26)22-31-19(33-10-7-28-8-11-33)12-20(32-22)34-9-1-2-17(15-34)21(35)29-6-5-16-3-4-18(13-27)30-14-16/h3-4,12,14,17,28H,1-2,5-11,15H2,(H,29,35)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp method |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Transcriptional regulator ERG
(Homo sapiens (Human)) | BDBM50003561

(CHEMBL3234571)Show SMILES CN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CCC[C@H](C1)C(=O)NCCc1ccc(nc1)C#N |r| Show InChI InChI=1S/C24H29F3N8O/c1-33-9-11-34(12-10-33)20-13-21(32-23(31-20)24(25,26)27)35-8-2-3-18(16-35)22(36)29-7-6-17-4-5-19(14-28)30-15-17/h4-5,13,15,18H,2-3,6-12,16H2,1H3,(H,29,36)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp method |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50003573

(CHEMBL3234871)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)c1ccc(cc1)-c1cc(nc(n1)C(F)(F)F)N1CC[C@H]1C(=O)NCCc1ccc(cc1)C#N |r,wU:6.9,28.32,wD:3.2,(43.64,-56.93,;44.97,-56.16,;46.31,-56.93,;44.97,-54.62,;46.31,-53.85,;46.3,-52.3,;44.97,-51.54,;43.65,-52.32,;43.64,-53.85,;44.96,-50,;43.62,-49.23,;43.62,-47.69,;44.96,-46.92,;46.29,-47.69,;46.29,-49.23,;44.96,-45.38,;46.29,-44.61,;46.29,-43.07,;44.96,-42.3,;43.63,-43.07,;43.62,-44.61,;42.29,-42.3,;40.95,-43.07,;42.29,-40.76,;40.95,-41.53,;47.63,-42.3,;48.02,-40.81,;49.51,-41.22,;49.11,-42.7,;49.87,-44.04,;49.1,-45.37,;51.41,-44.04,;52.19,-42.71,;53.73,-42.72,;54.5,-41.38,;56.04,-41.4,;56.81,-40.07,;56.04,-38.73,;54.49,-38.73,;53.73,-40.06,;56.81,-37.4,;57.58,-36.07,)| Show InChI InChI=1S/C31H30F3N5O3/c32-31(33,34)30-37-25(23-9-5-21(6-10-23)22-7-11-24(12-8-22)29(41)42)17-27(38-30)39-16-14-26(39)28(40)36-15-13-19-1-3-20(18-35)4-2-19/h1-6,9-10,17,22,24,26H,7-8,11-16H2,(H,36,40)(H,41,42)/t22-,24-,26-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50003338

(CHEMBL3234567)Show SMILES CN1CCN(CC1)c1cc(nc(C)n1)N1CCC[C@H](C1)C(=O)NCCc1ccc(cc1)C#N |r| Show InChI InChI=1S/C25H33N7O/c1-19-28-23(31-14-12-30(2)13-15-31)16-24(29-19)32-11-3-4-22(18-32)25(33)27-10-9-20-5-7-21(17-26)8-6-20/h5-8,16,22H,3-4,9-15,18H2,1-2H3,(H,27,33)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50003573

(CHEMBL3234871)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)c1ccc(cc1)-c1cc(nc(n1)C(F)(F)F)N1CC[C@H]1C(=O)NCCc1ccc(cc1)C#N |r,wU:6.9,28.32,wD:3.2,(43.64,-56.93,;44.97,-56.16,;46.31,-56.93,;44.97,-54.62,;46.31,-53.85,;46.3,-52.3,;44.97,-51.54,;43.65,-52.32,;43.64,-53.85,;44.96,-50,;43.62,-49.23,;43.62,-47.69,;44.96,-46.92,;46.29,-47.69,;46.29,-49.23,;44.96,-45.38,;46.29,-44.61,;46.29,-43.07,;44.96,-42.3,;43.63,-43.07,;43.62,-44.61,;42.29,-42.3,;40.95,-43.07,;42.29,-40.76,;40.95,-41.53,;47.63,-42.3,;48.02,-40.81,;49.51,-41.22,;49.11,-42.7,;49.87,-44.04,;49.1,-45.37,;51.41,-44.04,;52.19,-42.71,;53.73,-42.72,;54.5,-41.38,;56.04,-41.4,;56.81,-40.07,;56.04,-38.73,;54.49,-38.73,;53.73,-40.06,;56.81,-37.4,;57.58,-36.07,)| Show InChI InChI=1S/C31H30F3N5O3/c32-31(33,34)30-37-25(23-9-5-21(6-10-23)22-7-11-24(12-8-22)29(41)42)17-27(38-30)39-16-14-26(39)28(40)36-15-13-19-1-3-20(18-35)4-2-19/h1-6,9-10,17,22,24,26H,7-8,11-16H2,(H,36,40)(H,41,42)/t22-,24-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50003573

(CHEMBL3234871)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)c1ccc(cc1)-c1cc(nc(n1)C(F)(F)F)N1CC[C@H]1C(=O)NCCc1ccc(cc1)C#N |r,wU:6.9,28.32,wD:3.2,(43.64,-56.93,;44.97,-56.16,;46.31,-56.93,;44.97,-54.62,;46.31,-53.85,;46.3,-52.3,;44.97,-51.54,;43.65,-52.32,;43.64,-53.85,;44.96,-50,;43.62,-49.23,;43.62,-47.69,;44.96,-46.92,;46.29,-47.69,;46.29,-49.23,;44.96,-45.38,;46.29,-44.61,;46.29,-43.07,;44.96,-42.3,;43.63,-43.07,;43.62,-44.61,;42.29,-42.3,;40.95,-43.07,;42.29,-40.76,;40.95,-41.53,;47.63,-42.3,;48.02,-40.81,;49.51,-41.22,;49.11,-42.7,;49.87,-44.04,;49.1,-45.37,;51.41,-44.04,;52.19,-42.71,;53.73,-42.72,;54.5,-41.38,;56.04,-41.4,;56.81,-40.07,;56.04,-38.73,;54.49,-38.73,;53.73,-40.06,;56.81,-37.4,;57.58,-36.07,)| Show InChI InChI=1S/C31H30F3N5O3/c32-31(33,34)30-37-25(23-9-5-21(6-10-23)22-7-11-24(12-8-22)29(41)42)17-27(38-30)39-16-14-26(39)28(40)36-15-13-19-1-3-20(18-35)4-2-19/h1-6,9-10,17,22,24,26H,7-8,11-16H2,(H,36,40)(H,41,42)/t22-,24-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50003334

(CHEMBL3234564)Show SMILES CN1CCN(CC1)c1cc(C)nc(n1)N1CCC[C@H](C1)C(=O)NCCc1ccc(cc1)C#N |r| Show InChI InChI=1S/C25H33N7O/c1-19-16-23(31-14-12-30(2)13-15-31)29-25(28-19)32-11-3-4-22(18-32)24(33)27-10-9-20-5-7-21(17-26)8-6-20/h5-8,16,22H,3-4,9-15,18H2,1-2H3,(H,27,33)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Transcriptional regulator ERG
(Homo sapiens (Human)) | BDBM50003573

(CHEMBL3234871)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)c1ccc(cc1)-c1cc(nc(n1)C(F)(F)F)N1CC[C@H]1C(=O)NCCc1ccc(cc1)C#N |r,wU:6.9,28.32,wD:3.2,(43.64,-56.93,;44.97,-56.16,;46.31,-56.93,;44.97,-54.62,;46.31,-53.85,;46.3,-52.3,;44.97,-51.54,;43.65,-52.32,;43.64,-53.85,;44.96,-50,;43.62,-49.23,;43.62,-47.69,;44.96,-46.92,;46.29,-47.69,;46.29,-49.23,;44.96,-45.38,;46.29,-44.61,;46.29,-43.07,;44.96,-42.3,;43.63,-43.07,;43.62,-44.61,;42.29,-42.3,;40.95,-43.07,;42.29,-40.76,;40.95,-41.53,;47.63,-42.3,;48.02,-40.81,;49.51,-41.22,;49.11,-42.7,;49.87,-44.04,;49.1,-45.37,;51.41,-44.04,;52.19,-42.71,;53.73,-42.72,;54.5,-41.38,;56.04,-41.4,;56.81,-40.07,;56.04,-38.73,;54.49,-38.73,;53.73,-40.06,;56.81,-37.4,;57.58,-36.07,)| Show InChI InChI=1S/C31H30F3N5O3/c32-31(33,34)30-37-25(23-9-5-21(6-10-23)22-7-11-24(12-8-22)29(41)42)17-27(38-30)39-16-14-26(39)28(40)36-15-13-19-1-3-20(18-35)4-2-19/h1-6,9-10,17,22,24,26H,7-8,11-16H2,(H,36,40)(H,41,42)/t22-,24-,26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp method |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Transcriptional regulator ERG
(Homo sapiens (Human)) | BDBM50003291

(CHEMBL3234579)Show SMILES CCN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CC[C@H]1C(=O)NCCc1ccc(nc1)C#N |r| Show InChI InChI=1S/C23H27F3N8O/c1-2-32-9-11-33(12-10-32)19-13-20(31-22(30-19)23(24,25)26)34-8-6-18(34)21(35)28-7-5-16-3-4-17(14-27)29-15-16/h3-4,13,15,18H,2,5-12H2,1H3,(H,28,35)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp method |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
Transcriptional regulator ERG
(Homo sapiens (Human)) | BDBM50003559

(CHEMBL3234570)Show SMILES FC(F)(F)c1nc(cc(n1)N1CCC[C@H](C1)C(=O)NCCc1ccc(cc1)C#N)N1CCN(CC1)C1COC1 |r| Show InChI InChI=1S/C27H32F3N7O2/c28-27(29,30)26-33-23(36-12-10-35(11-13-36)22-17-39-18-22)14-24(34-26)37-9-1-2-21(16-37)25(38)32-8-7-19-3-5-20(15-31)6-4-19/h3-6,14,21-22H,1-2,7-13,16-18H2,(H,32,38)/t21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp method |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50003076

(CHEMBL3234574)Show SMILES CN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CCCC[C@H]1C(=O)NCCc1ccc(cc1)C#N |r| Show InChI InChI=1S/C25H30F3N7O/c1-33-12-14-34(15-13-33)21-16-22(32-24(31-21)25(26,27)28)35-11-3-2-4-20(35)23(36)30-10-9-18-5-7-19(17-29)8-6-18/h5-8,16,20H,2-4,9-15H2,1H3,(H,30,36)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in Jurkat cells assessed as intracellular cAMP level after 1 hr by HTRF assay |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50003283

(CHEMBL3234575)Show SMILES CN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CCC[C@H]1C(=O)NCCc1ccc(cc1)C#N |r| Show InChI InChI=1S/C24H28F3N7O/c1-32-11-13-33(14-12-32)20-15-21(31-23(30-20)24(25,26)27)34-10-2-3-19(34)22(35)29-9-8-17-4-6-18(16-28)7-5-17/h4-7,15,19H,2-3,8-14H2,1H3,(H,29,35)/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in Jurkat cells assessed as intracellular cAMP level after 1 hr by HTRF assay |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50003286

(CHEMBL3234576)Show SMILES CN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CCC[C@@H]1C(=O)NCCc1ccc(cc1)C#N |r| Show InChI InChI=1S/C24H28F3N7O/c1-32-11-13-33(14-12-32)20-15-21(31-23(30-20)24(25,26)27)34-10-2-3-19(34)22(35)29-9-8-17-4-6-18(16-28)7-5-17/h4-7,15,19H,2-3,8-14H2,1H3,(H,29,35)/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 274 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in Jurkat cells assessed as intracellular cAMP level after 1 hr by HTRF assay |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50003287

(CHEMBL3234577)Show SMILES CN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CC[C@H]1C(=O)NCCc1ccc(cc1)C#N |r| Show InChI InChI=1S/C23H26F3N7O/c1-31-10-12-32(13-11-31)19-14-20(30-22(29-19)23(24,25)26)33-9-7-18(33)21(34)28-8-6-16-2-4-17(15-27)5-3-16/h2-5,14,18H,6-13H2,1H3,(H,28,34)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in Jurkat cells assessed as intracellular cAMP level after 1 hr by HTRF assay |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50003289

(CHEMBL3234578)Show SMILES CN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CC[C@@H]1C(=O)NCCc1ccc(cc1)C#N |r| Show InChI InChI=1S/C23H26F3N7O/c1-31-10-12-32(13-11-31)19-14-20(30-22(29-19)23(24,25)26)33-9-7-18(33)21(34)28-8-6-16-2-4-17(15-27)5-3-16/h2-5,14,18H,6-13H2,1H3,(H,28,34)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in Jurkat cells assessed as intracellular cAMP level after 1 hr by HTRF assay |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Homo sapiens (Human)) | BDBM50003291

(CHEMBL3234579)Show SMILES CCN1CCN(CC1)c1cc(nc(n1)C(F)(F)F)N1CC[C@H]1C(=O)NCCc1ccc(nc1)C#N |r| Show InChI InChI=1S/C23H27F3N8O/c1-2-32-9-11-33(12-10-32)19-13-20(31-22(30-19)23(24,25)26)34-8-6-18(34)21(35)28-7-5-16-3-4-17(14-27)29-15-16/h3-4,13,15,18H,2,5-12H2,1H3,(H,28,35)/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at human TGR5 expressed in Jurkat cells assessed as intracellular cAMP level after 1 hr by HTRF assay |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Mus musculus) | BDBM50003293
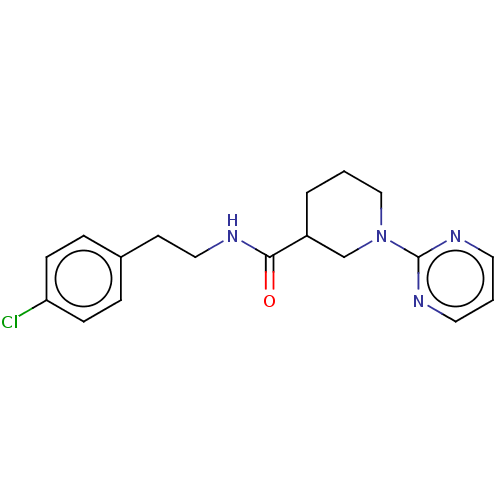
(CHEMBL3234546)Show InChI InChI=1S/C18H21ClN4O/c19-16-6-4-14(5-7-16)8-11-20-17(24)15-3-1-12-23(13-15)18-21-9-2-10-22-18/h2,4-7,9-10,15H,1,3,8,11-13H2,(H,20,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at mouse TGR5 expressed in Jurkat cells assessed as intracellular cAMP level after 1 hr by HTRF assay |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Mus musculus) | BDBM50003294
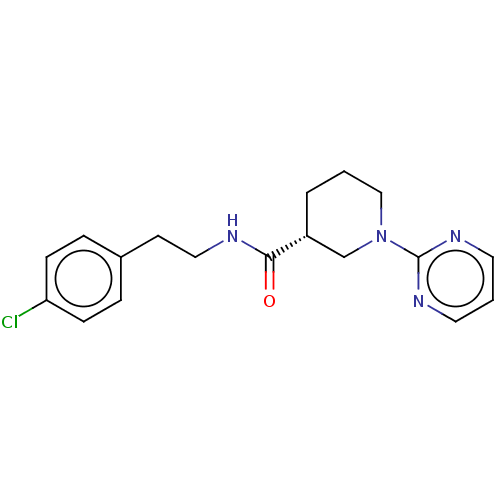
(CHEMBL3234547)Show SMILES Clc1ccc(CCNC(=O)[C@@H]2CCCN(C2)c2ncccn2)cc1 |r| Show InChI InChI=1S/C18H21ClN4O/c19-16-6-4-14(5-7-16)8-11-20-17(24)15-3-1-12-23(13-15)18-21-9-2-10-22-18/h2,4-7,9-10,15H,1,3,8,11-13H2,(H,20,24)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 280 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at mouse TGR5 expressed in Jurkat cells assessed as intracellular cAMP level after 1 hr by HTRF assay |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Mus musculus) | BDBM50003295
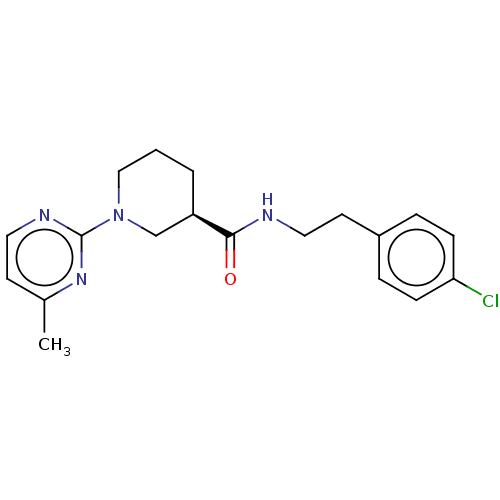
(CHEMBL3234549)Show SMILES Cc1ccnc(n1)N1CCC[C@H](C1)C(=O)NCCc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C19H23ClN4O/c1-14-8-10-22-19(23-14)24-12-2-3-16(13-24)18(25)21-11-9-15-4-6-17(20)7-5-15/h4-8,10,16H,2-3,9,11-13H2,1H3,(H,21,25)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at mouse TGR5 expressed in Jurkat cells assessed as intracellular cAMP level after 1 hr by HTRF assay |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Mus musculus) | BDBM50003302
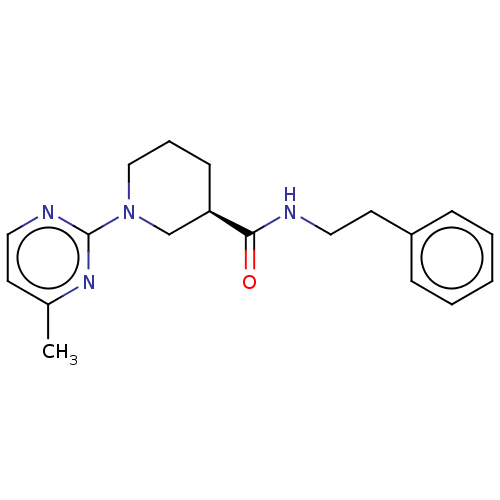
(CHEMBL3234550)Show SMILES Cc1ccnc(n1)N1CCC[C@H](C1)C(=O)NCCc1ccccc1 |r| Show InChI InChI=1S/C19H24N4O/c1-15-9-11-21-19(22-15)23-13-5-8-17(14-23)18(24)20-12-10-16-6-3-2-4-7-16/h2-4,6-7,9,11,17H,5,8,10,12-14H2,1H3,(H,20,24)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.64E+4 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at mouse TGR5 expressed in Jurkat cells assessed as intracellular cAMP level after 1 hr by HTRF assay |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |
G-protein coupled bile acid receptor 1
(Mus musculus) | BDBM50003303
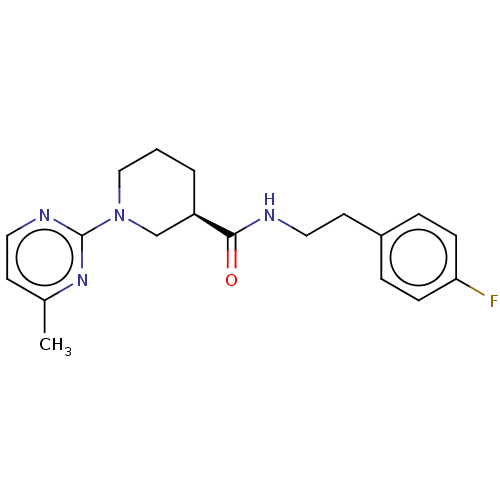
(CHEMBL3234551)Show SMILES Cc1ccnc(n1)N1CCC[C@H](C1)C(=O)NCCc1ccc(F)cc1 |r| Show InChI InChI=1S/C19H23FN4O/c1-14-8-10-22-19(23-14)24-12-2-3-16(13-24)18(25)21-11-9-15-4-6-17(20)7-5-15/h4-8,10,16H,2-3,9,11-13H2,1H3,(H,21,25)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 750 | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation
Curated by ChEMBL
| Assay Description
Agonist activity at mouse TGR5 expressed in Jurkat cells assessed as intracellular cAMP level after 1 hr by HTRF assay |
J Med Chem 57: 3263-82 (2014)
Article DOI: 10.1021/jm401731q
BindingDB Entry DOI: 10.7270/Q2H41SZ3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data