 Found 680 hits with Last Name = 'rouse' and Initial = 'm'
Found 680 hits with Last Name = 'rouse' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278693
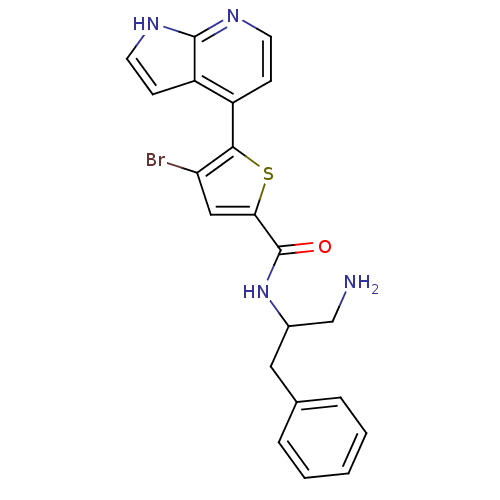
((+/-)-N-(1-amino-3-phenylpropan-2-yl)-4-bromo-5-(1...)Show SMILES NCC(Cc1ccccc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C21H19BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h1-9,11,14H,10,12,23H2,(H,24,25)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278098
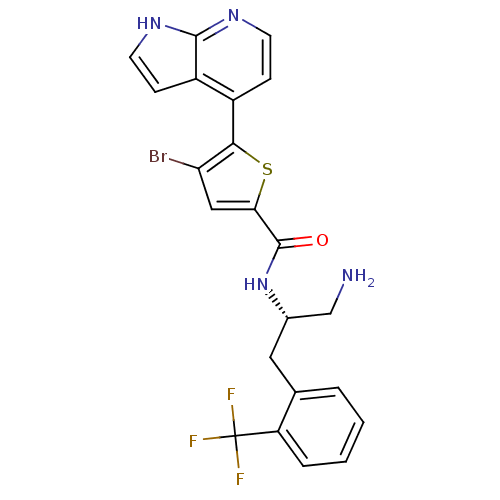
(CHEMBL520788 | N-((S)-1-amino-3-(2-(trifluoromethy...)Show SMILES NC[C@H](Cc1ccccc1C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)14-5-7-28-20-15(14)6-8-29-20)21(31)30-13(11-27)9-12-3-1-2-4-16(12)22(24,25)26/h1-8,10,13H,9,11,27H2,(H,28,29)(H,30,31)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278837
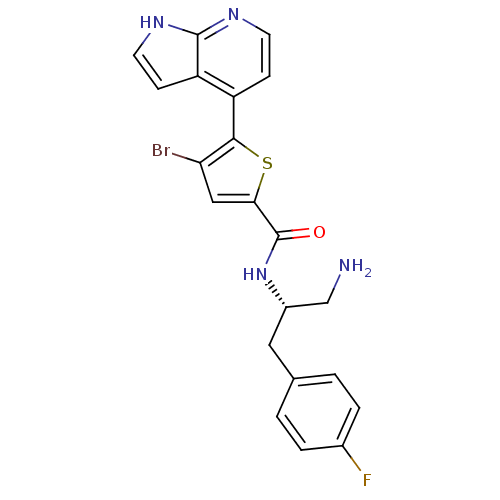
(CHEMBL496690 | N-((S)-1-amino-3-(4-fluorophenyl)pr...)Show SMILES NC[C@H](Cc1ccc(F)cc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H18BrFN4OS/c22-17-10-18(29-19(17)15-5-7-25-20-16(15)6-8-26-20)21(28)27-14(11-24)9-12-1-3-13(23)4-2-12/h1-8,10,14H,9,11,24H2,(H,25,26)(H,27,28)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278099
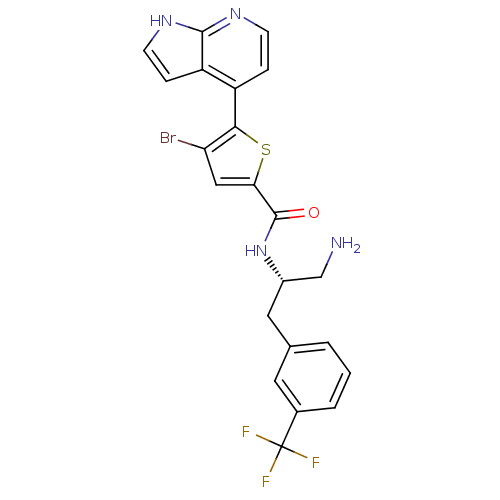
(CHEMBL482536 | N-((S)-1-amino-3-(3-(trifluoromethy...)Show SMILES NC[C@H](Cc1cccc(c1)C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)15-4-6-28-20-16(15)5-7-29-20)21(31)30-14(11-27)9-12-2-1-3-13(8-12)22(24,25)26/h1-8,10,14H,9,11,27H2,(H,28,29)(H,30,31)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278099

(CHEMBL482536 | N-((S)-1-amino-3-(3-(trifluoromethy...)Show SMILES NC[C@H](Cc1cccc(c1)C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)15-4-6-28-20-16(15)5-7-29-20)21(31)30-14(11-27)9-12-2-1-3-13(8-12)22(24,25)26/h1-8,10,14H,9,11,27H2,(H,28,29)(H,30,31)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316184

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((S)-3-amino...)Show SMILES CCn1c(nc2c(nc(O[C@@H](CCN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-17-14-19(33-18(11-13-25)15-8-6-5-7-9-15)27-16(10-12-24(2,3)32)20(17)28-23(31)21-22(26)30-34-29-21/h5-9,14,18,32H,4,11,13,25H2,1-3H3,(H2,26,30)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316183
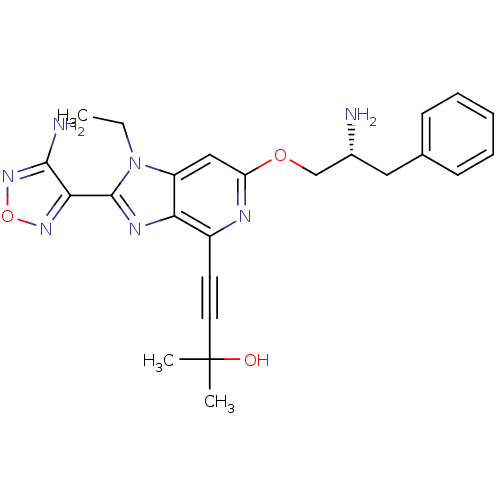
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-2-amino...)Show SMILES CCn1c(nc2c(nc(OC[C@H](N)Cc3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-18-13-19(33-14-16(25)12-15-8-6-5-7-9-15)27-17(10-11-24(2,3)32)20(18)28-23(31)21-22(26)30-34-29-21/h5-9,13,16,32H,4,12,14,25H2,1-3H3,(H2,26,30)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316192
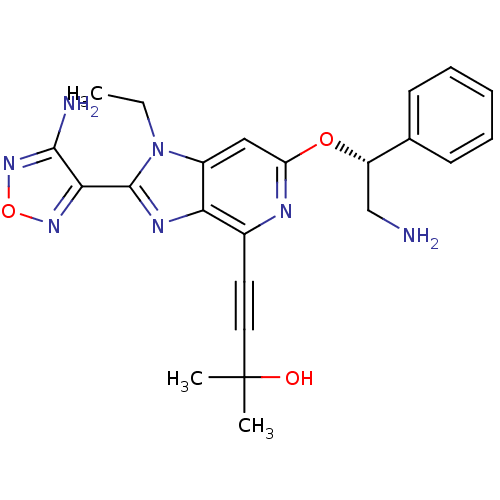
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-2-amino...)Show SMILES CCn1c(nc2c(nc(O[C@@H](CN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C23H25N7O3/c1-4-30-16-12-18(32-17(13-24)14-8-6-5-7-9-14)26-15(10-11-23(2,3)31)19(16)27-22(30)20-21(25)29-33-28-20/h5-9,12,17,31H,4,13,24H2,1-3H3,(H2,25,29)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278836
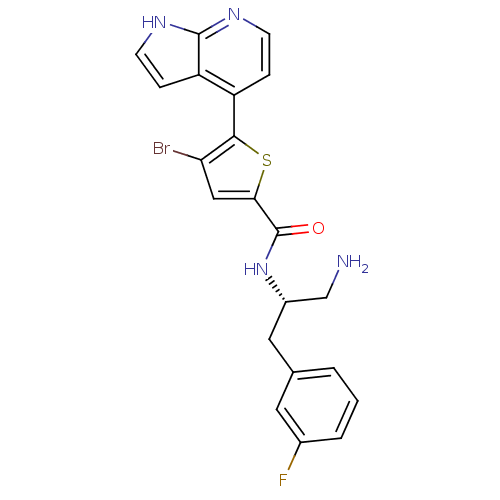
(CHEMBL523586 | N-((S)-1-amino-3-(3-fluorophenyl)pr...)Show SMILES NC[C@H](Cc1cccc(F)c1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H18BrFN4OS/c22-17-10-18(29-19(17)15-4-6-25-20-16(15)5-7-26-20)21(28)27-14(11-24)9-12-2-1-3-13(23)8-12/h1-8,10,14H,9,11,24H2,(H,25,26)(H,27,28)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316184

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((S)-3-amino...)Show SMILES CCn1c(nc2c(nc(O[C@@H](CCN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-17-14-19(33-18(11-13-25)15-8-6-5-7-9-15)27-16(10-12-24(2,3)32)20(17)28-23(31)21-22(26)30-34-29-21/h5-9,14,18,32H,4,11,13,25H2,1-3H3,(H2,26,30)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25013
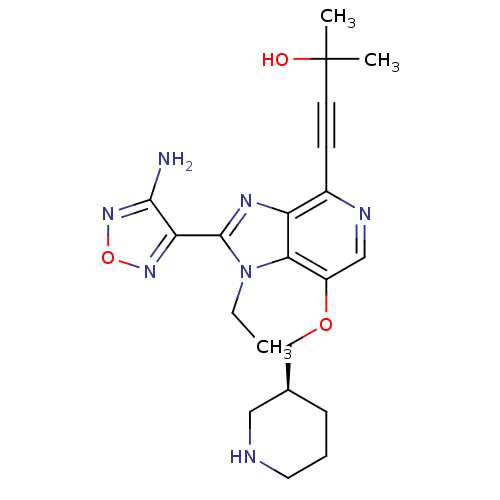
(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3S...)Show SMILES CCn1c(nc2c(ncc(OC[C@H]3CCCNC3)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C21H27N7O3/c1-4-28-18-15(30-12-13-6-5-9-23-10-13)11-24-14(7-8-21(2,3)29)16(18)25-20(28)17-19(22)27-31-26-17/h11,13,23,29H,4-6,9-10,12H2,1-3H3,(H2,22,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316185
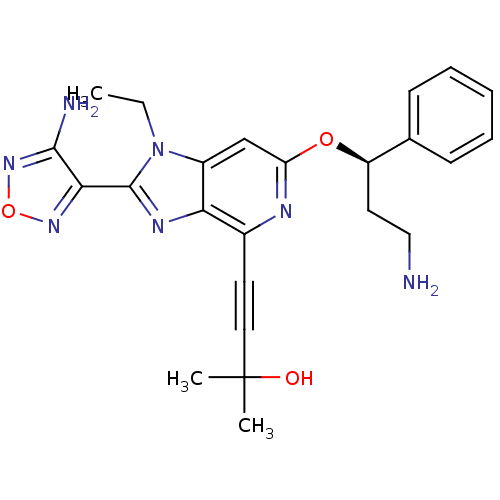
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-3-amino...)Show SMILES CCn1c(nc2c(nc(O[C@H](CCN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-17-14-19(33-18(11-13-25)15-8-6-5-7-9-15)27-16(10-12-24(2,3)32)20(17)28-23(31)21-22(26)30-34-29-21/h5-9,14,18,32H,4,11,13,25H2,1-3H3,(H2,26,30)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316183

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-2-amino...)Show SMILES CCn1c(nc2c(nc(OC[C@H](N)Cc3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-18-13-19(33-14-16(25)12-15-8-6-5-7-9-15)27-17(10-11-24(2,3)32)20(18)28-23(31)21-22(26)30-34-29-21/h5-9,13,16,32H,4,12,14,25H2,1-3H3,(H2,26,30)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278100
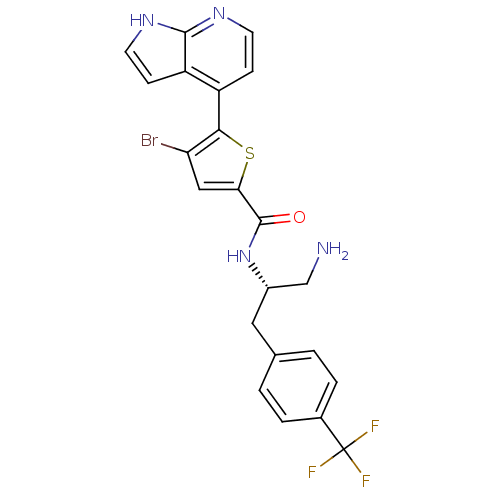
(CHEMBL482537 | N-((S)-1-amino-3-(4-(trifluoromethy...)Show SMILES NC[C@H](Cc1ccc(cc1)C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)15-5-7-28-20-16(15)6-8-29-20)21(31)30-14(11-27)9-12-1-3-13(4-2-12)22(24,25)26/h1-8,10,14H,9,11,27H2,(H,28,29)(H,30,31)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278837

(CHEMBL496690 | N-((S)-1-amino-3-(4-fluorophenyl)pr...)Show SMILES NC[C@H](Cc1ccc(F)cc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H18BrFN4OS/c22-17-10-18(29-19(17)15-5-7-25-20-16(15)6-8-26-20)21(28)27-14(11-24)9-12-1-3-13(23)4-2-12/h1-8,10,14H,9,11,24H2,(H,25,26)(H,27,28)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278833
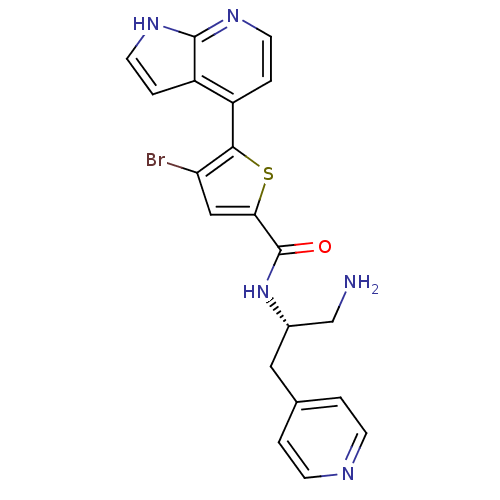
(CHEMBL498051 | N-((S)-1-amino-3-(pyridin-4-yl)prop...)Show SMILES NC[C@H](Cc1ccncc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C20H18BrN5OS/c21-16-10-17(20(27)26-13(11-22)9-12-1-5-23-6-2-12)28-18(16)14-3-7-24-19-15(14)4-8-25-19/h1-8,10,13H,9,11,22H2,(H,24,25)(H,26,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50131550
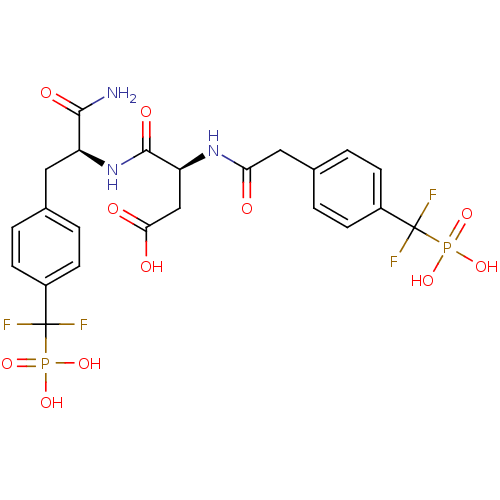
((3S)-3-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...)Show SMILES NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O |r| Show InChI InChI=1S/C23H25F4N3O11P2/c24-22(25,42(36,37)38)14-5-1-12(2-6-14)9-16(20(28)34)30-21(35)17(11-19(32)33)29-18(31)10-13-3-7-15(8-4-13)23(26,27)43(39,40)41/h1-8,16-17H,9-11H2,(H2,28,34)(H,29,31)(H,30,35)(H,32,33)(H2,36,37,38)(H2,39,40,41)/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein-tyrosine phosphatase 1B (PTP 1B) was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278770

(CHEMBL470597 | N-((S)-1-amino-3-phenylpropan-2-yl)...)Show SMILES NC[C@H](Cc1ccccc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H19BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h1-9,11,14H,10,12,23H2,(H,24,25)(H,26,27)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316182
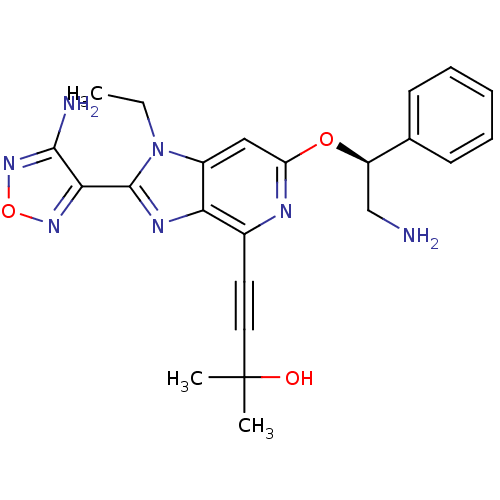
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((S)-2-amino...)Show SMILES CCn1c(nc2c(nc(O[C@H](CN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C23H25N7O3/c1-4-30-16-12-18(32-17(13-24)14-8-6-5-7-9-14)26-15(10-11-23(2,3)31)19(16)27-22(30)20-21(25)29-33-28-20/h5-9,12,17,31H,4,13,24H2,1-3H3,(H2,25,29)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278770

(CHEMBL470597 | N-((S)-1-amino-3-phenylpropan-2-yl)...)Show SMILES NC[C@H](Cc1ccccc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H19BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h1-9,11,14H,10,12,23H2,(H,24,25)(H,26,27)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50282079
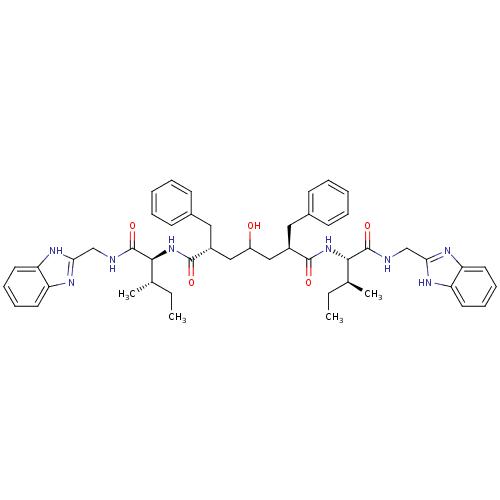
((2R,6R)-2,6-Dibenzyl-4-hydroxy-heptanedioic acid b...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](CC(O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)NCc1nc2ccccc2[nH]1)Cc1ccccc1)C(=O)NCc1nc2ccccc2[nH]1 Show InChI InChI=1S/C49H60N8O5/c1-5-31(3)44(48(61)50-29-42-52-38-21-13-14-22-39(38)53-42)56-46(59)35(25-33-17-9-7-10-18-33)27-37(58)28-36(26-34-19-11-8-12-20-34)47(60)57-45(32(4)6-2)49(62)51-30-43-54-40-23-15-16-24-41(40)55-43/h7-24,31-32,35-37,44-45,58H,5-6,25-30H2,1-4H3,(H,50,61)(H,51,62)(H,52,53)(H,54,55)(H,56,59)(H,57,60)/t31-,32-,35+,36+,44-,45-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity against HIV-1 protease using [125I]-SPA (scintillation proximity assay) |
Bioorg Med Chem Lett 3: 1589-1594 (1993)
Article DOI: 10.1016/S0960-894X(00)80023-2
BindingDB Entry DOI: 10.7270/Q24X57QC |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278098

(CHEMBL520788 | N-((S)-1-amino-3-(2-(trifluoromethy...)Show SMILES NC[C@H](Cc1ccccc1C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)14-5-7-28-20-15(14)6-8-29-20)21(31)30-13(11-27)9-12-3-1-2-4-16(12)22(24,25)26/h1-8,10,13H,9,11,27H2,(H,28,29)(H,30,31)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25013

(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3S...)Show SMILES CCn1c(nc2c(ncc(OC[C@H]3CCCNC3)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C21H27N7O3/c1-4-28-18-15(30-12-13-6-5-9-23-10-13)11-24-14(7-8-21(2,3)29)16(18)25-20(28)17-19(22)27-31-26-17/h11,13,23,29H,4-6,9-10,12H2,1-3H3,(H2,22,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278099

(CHEMBL482536 | N-((S)-1-amino-3-(3-(trifluoromethy...)Show SMILES NC[C@H](Cc1cccc(c1)C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)15-4-6-28-20-16(15)5-7-29-20)21(31)30-14(11-27)9-12-2-1-3-13(8-12)22(24,25)26/h1-8,10,14H,9,11,27H2,(H,28,29)(H,30,31)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278098

(CHEMBL520788 | N-((S)-1-amino-3-(2-(trifluoromethy...)Show SMILES NC[C@H](Cc1ccccc1C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)14-5-7-28-20-15(14)6-8-29-20)21(31)30-13(11-27)9-12-3-1-2-4-16(12)22(24,25)26/h1-8,10,13H,9,11,27H2,(H,28,29)(H,30,31)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316192

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-2-amino...)Show SMILES CCn1c(nc2c(nc(O[C@@H](CN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C23H25N7O3/c1-4-30-16-12-18(32-17(13-24)14-8-6-5-7-9-14)26-15(10-11-23(2,3)31)19(16)27-22(30)20-21(25)29-33-28-20/h5-9,12,17,31H,4,13,24H2,1-3H3,(H2,25,29)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278771
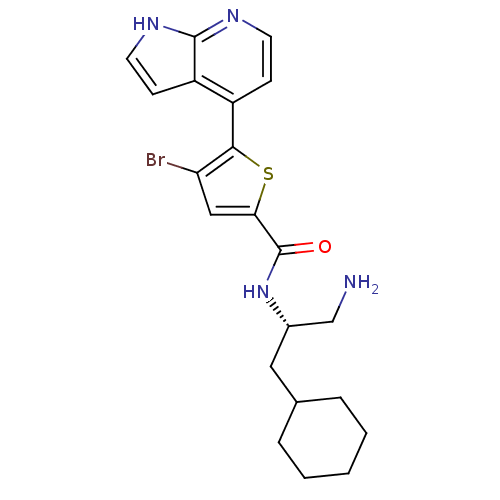
(CHEMBL470598 | N-((S)-1-amino-3-cyclohexylpropan-2...)Show SMILES NC[C@H](CC1CCCCC1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H25BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h6-9,11,13-14H,1-5,10,12,23H2,(H,24,25)(H,26,27)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316189
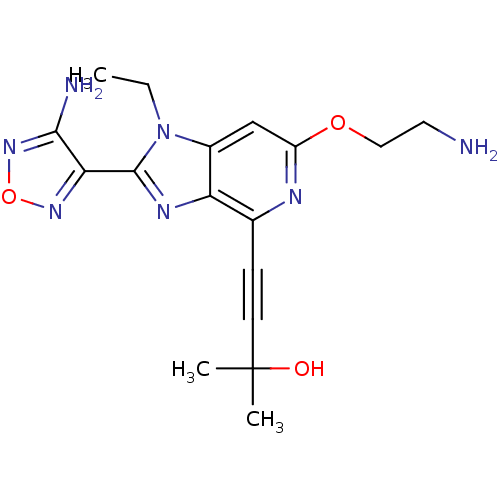
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-(2-aminoetho...)Show SMILES CCn1c(nc2c(nc(OCCN)cc12)C#CC(C)(C)O)-c1nonc1N Show InChI InChI=1S/C17H21N7O3/c1-4-24-11-9-12(26-8-7-18)20-10(5-6-17(2,3)25)13(11)21-16(24)14-15(19)23-27-22-14/h9,25H,4,7-8,18H2,1-3H3,(H2,19,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278771

(CHEMBL470598 | N-((S)-1-amino-3-cyclohexylpropan-2...)Show SMILES NC[C@H](CC1CCCCC1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H25BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h6-9,11,13-14H,1-5,10,12,23H2,(H,24,25)(H,26,27)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278693

((+/-)-N-(1-amino-3-phenylpropan-2-yl)-4-bromo-5-(1...)Show SMILES NCC(Cc1ccccc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C21H19BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h1-9,11,14H,10,12,23H2,(H,24,25)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278100

(CHEMBL482537 | N-((S)-1-amino-3-(4-(trifluoromethy...)Show SMILES NC[C@H](Cc1ccc(cc1)C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)15-5-7-28-20-16(15)6-8-29-20)21(31)30-14(11-27)9-12-1-3-13(4-2-12)22(24,25)26/h1-8,10,14H,9,11,27H2,(H,28,29)(H,30,31)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278100

(CHEMBL482537 | N-((S)-1-amino-3-(4-(trifluoromethy...)Show SMILES NC[C@H](Cc1ccc(cc1)C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)15-5-7-28-20-16(15)6-8-29-20)21(31)30-14(11-27)9-12-1-3-13(4-2-12)22(24,25)26/h1-8,10,14H,9,11,27H2,(H,28,29)(H,30,31)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316182

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((S)-2-amino...)Show SMILES CCn1c(nc2c(nc(O[C@H](CN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C23H25N7O3/c1-4-30-16-12-18(32-17(13-24)14-8-6-5-7-9-14)26-15(10-11-23(2,3)31)19(16)27-22(30)20-21(25)29-33-28-20/h5-9,12,17,31H,4,13,24H2,1-3H3,(H2,25,29)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13976

(Aminobenzoic acid analog 5 | CHEMBL116605)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cccc(O)c2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C34H37N3O11/c1-4-22-18-21(14-15-25(22)37(31(41)33(44)45)26-11-6-5-10-23(26)32(42)43)19-24(36-20(2)38)30(40)35-16-7-8-17-48-28-13-9-12-27(39)29(28)34(46)47-3/h5-6,9-15,18,24,39H,4,7-8,16-17,19H2,1-3H3,(H,35,40)(H,36,38)(H,42,43)(H,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant of the compound was determined against Protein-tyrosine phosphatase 1B (PTB1B) |
J Med Chem 46: 4232-5 (2003)
Article DOI: 10.1021/jm034122o
BindingDB Entry DOI: 10.7270/Q2BP0264 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13976

(Aminobenzoic acid analog 5 | CHEMBL116605)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cccc(O)c2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C34H37N3O11/c1-4-22-18-21(14-15-25(22)37(31(41)33(44)45)26-11-6-5-10-23(26)32(42)43)19-24(36-20(2)38)30(40)35-16-7-8-17-48-28-13-9-12-27(39)29(28)34(46)47-3/h5-6,9-15,18,24,39H,4,7-8,16-17,19H2,1-3H3,(H,35,40)(H,36,38)(H,42,43)(H,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein-tyrosine phosphatase 1B (PTP 1B) was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278603
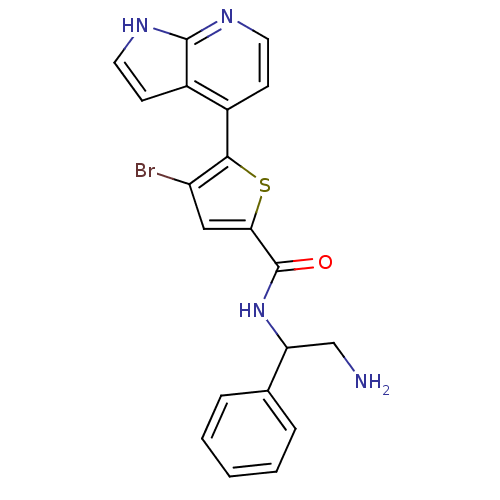
((+/-)-N-(2-amino-1-phenylethyl)-4-bromo-5-(1H-pyrr...)Show SMILES NCC(NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12)c1ccccc1 Show InChI InChI=1S/C20H17BrN4OS/c21-15-10-17(20(26)25-16(11-22)12-4-2-1-3-5-12)27-18(15)13-6-8-23-19-14(13)7-9-24-19/h1-10,16H,11,22H2,(H,23,24)(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1 [1-298]
(Homo sapiens (Human)) | BDBM13954

(3-({5-[(2S)-3-{4-[(2-carboxyphenyl)amidoformic aci...)Show SMILES CC(=O)N[C@@H](Cc1ccc(N(C(=O)C(O)=O)c2ccccc2C(O)=O)c2ccccc12)C(=O)NCCCCCOc1cc2ccccc2cc1C(O)=O |r| Show InChI InChI=1S/C40H37N3O10/c1-24(44)42-32(36(45)41-19-9-2-10-20-53-35-23-26-12-4-3-11-25(26)21-31(35)39(49)50)22-27-17-18-34(29-14-6-5-13-28(27)29)43(37(46)40(51)52)33-16-8-7-15-30(33)38(47)48/h3-8,11-18,21,23,32H,2,9-10,19-20,22H2,1H3,(H,41,45)(H,42,44)(H,47,48)(H,49,50)(H,51,52)/t32-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 22 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... |
J Am Chem Soc 125: 4087-96 (2003)
Article DOI: 10.1021/ja0296733
BindingDB Entry DOI: 10.7270/Q2B856CS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein phosphatase alpha
(Homo sapiens (Human)) | BDBM50131550

((3S)-3-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...)Show SMILES NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O |r| Show InChI InChI=1S/C23H25F4N3O11P2/c24-22(25,42(36,37)38)14-5-1-12(2-6-14)9-16(20(28)34)30-21(35)17(11-19(32)33)29-18(31)10-13-3-7-15(8-4-13)23(26,27)43(39,40)41/h1-8,16-17H,9-11H2,(H2,28,34)(H,29,31)(H,30,35)(H,32,33)(H2,36,37,38)(H2,39,40,41)/t16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against T cell protein tyrosine phosphatase |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50133425
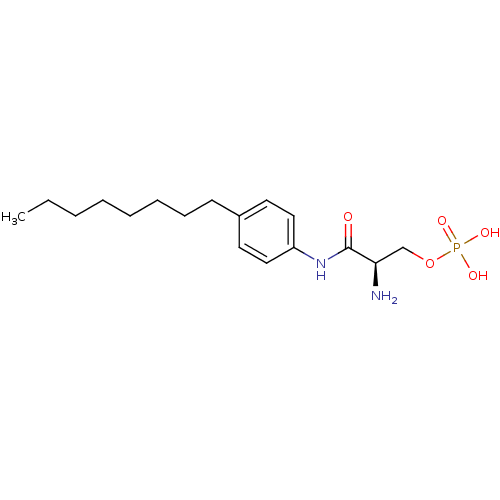
(CHEMBL227371 | CHEMBL422074 | Phosphoric acid mono...)Show SMILES CCCCCCCCc1ccc(NC(=O)[C@H](N)COP(O)(O)=O)cc1 Show InChI InChI=1S/C17H29N2O5P/c1-2-3-4-5-6-7-8-14-9-11-15(12-10-14)19-17(20)16(18)13-24-25(21,22)23/h9-12,16H,2-8,13,18H2,1H3,(H,19,20)(H2,21,22,23)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Displacement of [32P]S1P from human S1P1 receptor expressed in HEK293T cells |
Bioorg Med Chem 15: 663-77 (2006)
Article DOI: 10.1016/j.bmc.2006.10.060
BindingDB Entry DOI: 10.7270/Q2FN15VV |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50198836
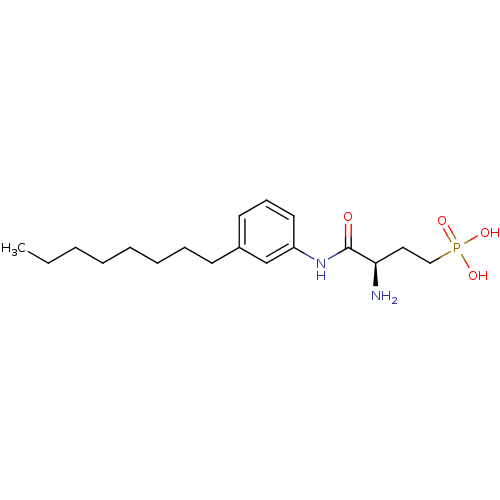
(CHEMBL389033 | [3-amino-3-(3-octylphenylcarbamoyl)...)Show SMILES CCCCCCCCc1cccc(NC(=O)[C@H](N)CCP(O)(O)=O)c1 Show InChI InChI=1S/C18H31N2O4P/c1-2-3-4-5-6-7-9-15-10-8-11-16(14-15)20-18(21)17(19)12-13-25(22,23)24/h8,10-11,14,17H,2-7,9,12-13,19H2,1H3,(H,20,21)(H2,22,23,24)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Displacement of [32P]S1P from human S1P1 receptor expressed in HEK293T cells |
Bioorg Med Chem 15: 663-77 (2006)
Article DOI: 10.1016/j.bmc.2006.10.060
BindingDB Entry DOI: 10.7270/Q2FN15VV |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316189

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-(2-aminoetho...)Show SMILES CCn1c(nc2c(nc(OCCN)cc12)C#CC(C)(C)O)-c1nonc1N Show InChI InChI=1S/C17H21N7O3/c1-4-24-11-9-12(26-8-7-18)20-10(5-6-17(2,3)25)13(11)21-16(24)14-15(19)23-27-22-14/h9,25H,4,7-8,18H2,1-3H3,(H2,19,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278833

(CHEMBL498051 | N-((S)-1-amino-3-(pyridin-4-yl)prop...)Show SMILES NC[C@H](Cc1ccncc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C20H18BrN5OS/c21-16-10-17(20(27)26-13(11-22)9-12-1-5-23-6-2-12)28-18(16)14-3-7-24-19-15(14)4-8-25-19/h1-8,10,13H,9,11,22H2,(H,24,25)(H,26,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50131547

(2-(N-(4-(2-acetamido-3-(4-(5-chloro-3-hydroxy-2-(m...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cc(Cl)cc(O)c2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C34H36ClN3O11/c1-4-21-15-20(11-12-25(21)38(31(42)33(45)46)26-10-6-5-9-23(26)32(43)44)16-24(37-19(2)39)30(41)36-13-7-8-14-49-28-18-22(35)17-27(40)29(28)34(47)48-3/h5-6,9-12,15,17-18,24,40H,4,7-8,13-14,16H2,1-3H3,(H,36,41)(H,37,39)(H,43,44)(H,45,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein-tyrosine phosphatase 1B (PTP 1B) was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278834
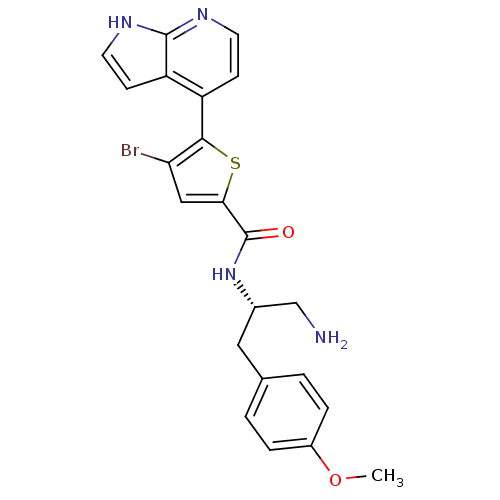
(CHEMBL524998 | N-((S)-1-amino-3-(4-methoxyphenyl)p...)Show SMILES COc1ccc(C[C@@H](CN)NC(=O)c2cc(Br)c(s2)-c2ccnc3[nH]ccc23)cc1 |r| Show InChI InChI=1S/C22H21BrN4O2S/c1-29-15-4-2-13(3-5-15)10-14(12-24)27-22(28)19-11-18(23)20(30-19)16-6-8-25-21-17(16)7-9-26-21/h2-9,11,14H,10,12,24H2,1H3,(H,25,26)(H,27,28)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50131555

(2-(4-(2-acetamido-3-(4-(1-carboxy-N-(2-carboxyphen...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cccc(O)c2C(O)=O)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C33H35N3O11/c1-3-21-17-20(13-14-24(21)36(30(40)33(45)46)25-10-5-4-9-22(25)31(41)42)18-23(35-19(2)37)29(39)34-15-6-7-16-47-27-12-8-11-26(38)28(27)32(43)44/h4-5,8-14,17,23,38H,3,6-7,15-16,18H2,1-2H3,(H,34,39)(H,35,37)(H,41,42)(H,43,44)(H,45,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein-tyrosine phosphatase 1B (PTP 1B) was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 2
(Homo sapiens (Human)) | BDBM13954

(3-({5-[(2S)-3-{4-[(2-carboxyphenyl)amidoformic aci...)Show SMILES CC(=O)N[C@@H](Cc1ccc(N(C(=O)C(O)=O)c2ccccc2C(O)=O)c2ccccc12)C(=O)NCCCCCOc1cc2ccccc2cc1C(O)=O |r| Show InChI InChI=1S/C40H37N3O10/c1-24(44)42-32(36(45)41-19-9-2-10-20-53-35-23-26-12-4-3-11-25(26)21-31(35)39(49)50)22-27-17-18-34(29-14-6-5-13-28(27)29)43(37(46)40(51)52)33-16-8-7-15-30(33)38(47)48/h3-8,11-18,21,23,32H,2,9-10,19-20,22H2,1H3,(H,41,45)(H,42,44)(H,47,48)(H,49,50)(H,51,52)/t32-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 49 | -41.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... |
J Am Chem Soc 125: 4087-96 (2003)
Article DOI: 10.1021/ja0296733
BindingDB Entry DOI: 10.7270/Q2B856CS |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase alpha
(Homo sapiens (Human)) | BDBM13976

(Aminobenzoic acid analog 5 | CHEMBL116605)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cccc(O)c2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C34H37N3O11/c1-4-22-18-21(14-15-25(22)37(31(41)33(44)45)26-11-6-5-10-23(26)32(42)43)19-24(36-20(2)38)30(40)35-16-7-8-17-48-28-13-9-12-27(39)29(28)34(46)47-3/h5-6,9-15,18,24,39H,4,7-8,16-17,19H2,1-3H3,(H,35,40)(H,36,38)(H,42,43)(H,44,45) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant of compound against T cell protein tyrosine phosphatase was determined |
J Med Chem 46: 4232-5 (2003)
Article DOI: 10.1021/jm034122o
BindingDB Entry DOI: 10.7270/Q2BP0264 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase alpha
(Homo sapiens (Human)) | BDBM13976

(Aminobenzoic acid analog 5 | CHEMBL116605)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cccc(O)c2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C34H37N3O11/c1-4-22-18-21(14-15-25(22)37(31(41)33(44)45)26-11-6-5-10-23(26)32(42)43)19-24(36-20(2)38)30(40)35-16-7-8-17-48-28-13-9-12-27(39)29(28)34(46)47-3/h5-6,9-15,18,24,39H,4,7-8,16-17,19H2,1-3H3,(H,35,40)(H,36,38)(H,42,43)(H,44,45) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against T cell protein tyrosine phosphatase |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase alpha
(Homo sapiens (Human)) | BDBM50131555

(2-(4-(2-acetamido-3-(4-(1-carboxy-N-(2-carboxyphen...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cccc(O)c2C(O)=O)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C33H35N3O11/c1-3-21-17-20(13-14-24(21)36(30(40)33(45)46)25-10-5-4-9-22(25)31(41)42)18-23(35-19(2)37)29(39)34-15-6-7-16-47-27-12-8-11-26(38)28(27)32(43)44/h4-5,8-14,17,23,38H,3,6-7,15-16,18H2,1-2H3,(H,34,39)(H,35,37)(H,41,42)(H,43,44)(H,45,46) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against T cell protein tyrosine phosphatase |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM15819

(1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCC(=O)N[C@@H](CCSC)C(O)=O)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O |r| Show InChI InChI=1S/C32H40N4O10S/c1-4-21-17-20(12-13-25(21)36(29(40)32(45)46)26-10-6-5-9-22(26)30(41)42)18-24(34-19(2)37)28(39)33-15-8-7-11-27(38)35-23(31(43)44)14-16-47-3/h5-6,9-10,12-13,17,23-24H,4,7-8,11,14-16,18H2,1-3H3,(H,33,39)(H,34,37)(H,35,38)(H,41,42)(H,43,44)(H,45,46)/t23-,24?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein-tyrosine phosphatase 1B (PTP 1B) was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data