

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin K (Homo sapiens (Human)) | BDBM50098576 (5-(2-MORPHOLIN-4-YLETHOXY)BENZOFURAN-2-CARBOXYLIC ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.00480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Human cathepsin K | J Med Chem 44: 1380-95 (2001) BindingDB Entry DOI: 10.7270/Q2QR4WDC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM177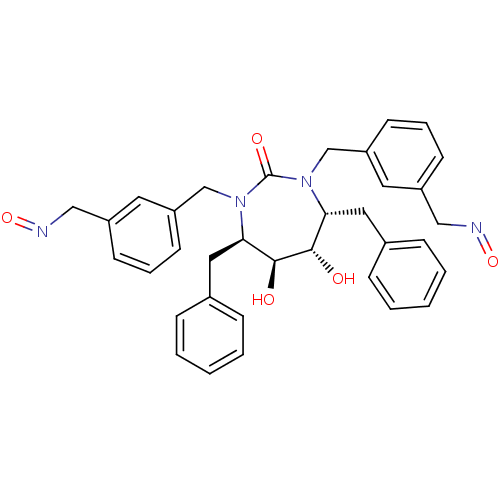 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | -65.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Ki values were determined with recombinant single-chain dimeric HIV protease and a fluorescent substrate. The use of single-chain dimeric protease a... | J Med Chem 39: 3514-25 (1996) Article DOI: 10.1021/jm9602571 BindingDB Entry DOI: 10.7270/Q2ZW1J3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054159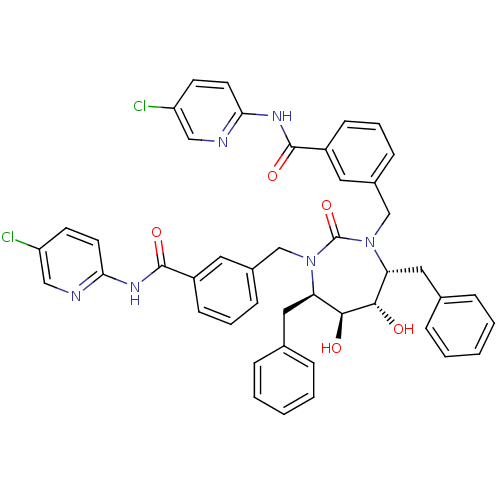 (5-chloro-2-{3-[4,7-dibenzyl-3-[3-(5-chloro-2-pyrid...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity for HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054156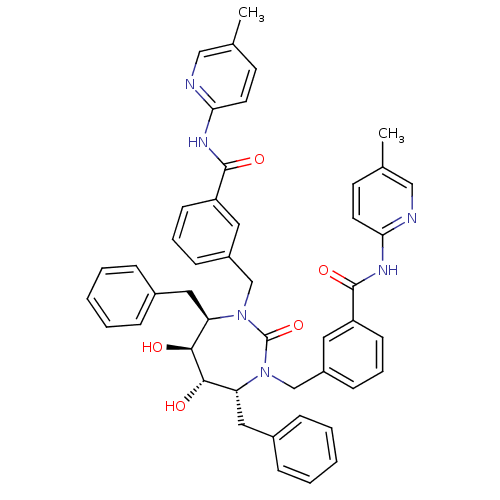 ((4r,5r,6,7)-3,3-[[Tetrahydro-5,6-dihydroxy-2-oxo-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity for HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM159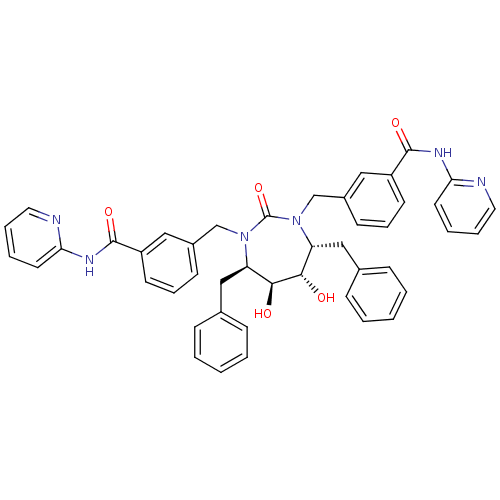 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-2-oxo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity for HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36648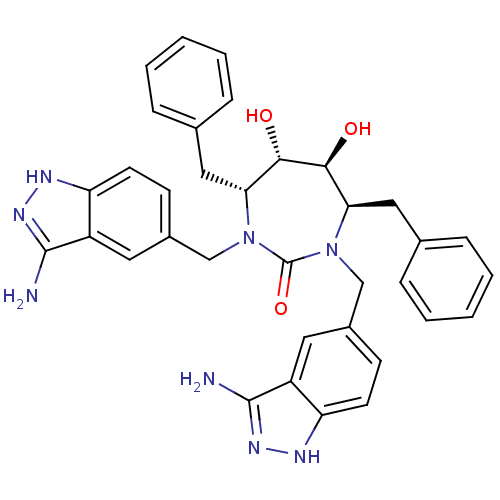 (3-alkylaminoindazole cyclic urea, (H)) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM162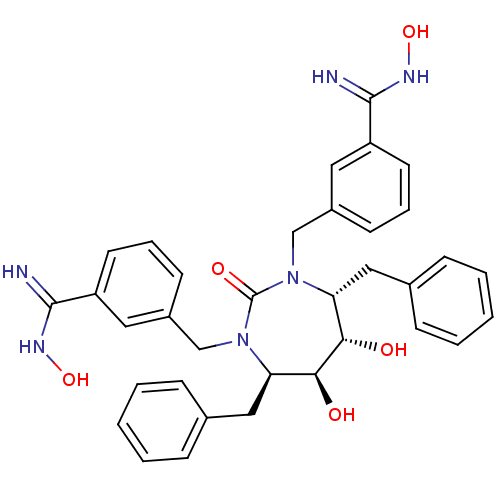 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-3-{[3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | -65.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | J Med Chem 39: 3514-25 (1996) Article DOI: 10.1021/jm9602571 BindingDB Entry DOI: 10.7270/Q2ZW1J3M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM177 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36647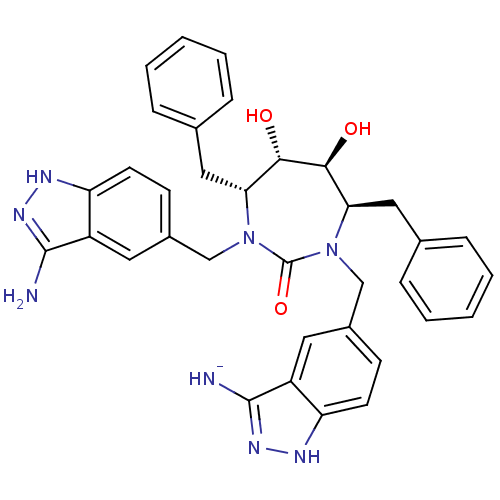 (3-Aminoindazole, 2) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054156 ((4r,5r,6,7)-3,3-[[Tetrahydro-5,6-dihydroxy-2-oxo-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054159 (5-chloro-2-{3-[4,7-dibenzyl-3-[3-(5-chloro-2-pyrid...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM160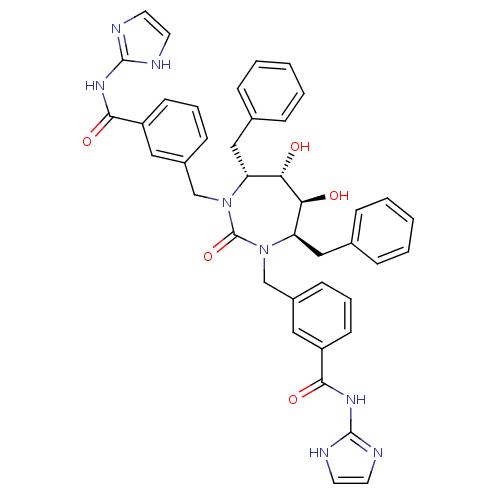 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-3-{[3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0140 | -64.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | J Med Chem 39: 3514-25 (1996) Article DOI: 10.1021/jm9602571 BindingDB Entry DOI: 10.7270/Q2ZW1J3M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM160 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-3-{[3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054184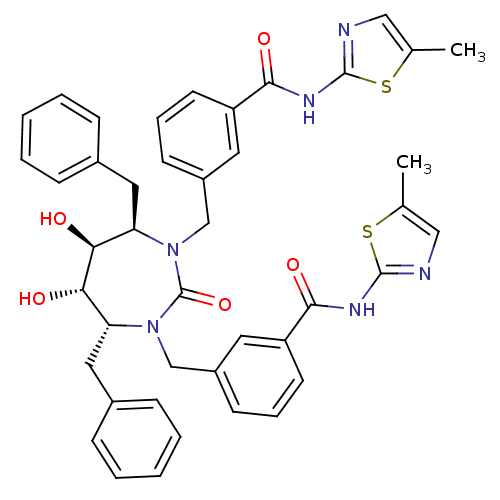 (2-{3-[4,7-dibenzyl-5,6-dihydroxy-3-[3-(5-methyl-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054178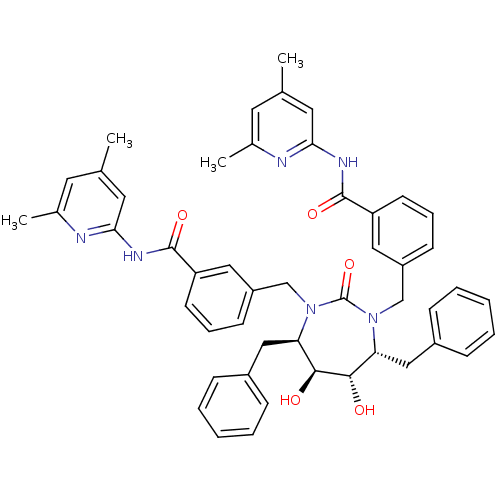 (2-{3-[4,7-dibenzyl-3-[3-(4,6-dimethyl-2-pyridylcar...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36656 (Cyclobutylmethyl cyclic urea) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM178 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054179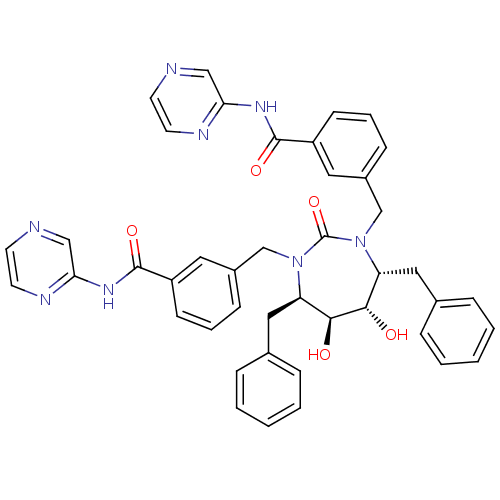 ((4r,5r,6,7)-3,3-[[Tetrahydro-5,6-dihydroxy-2-oxo-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054174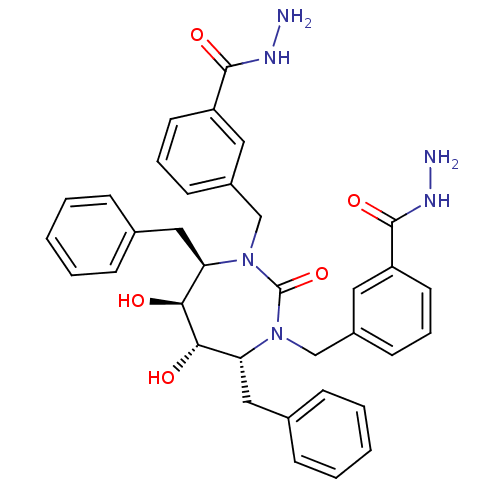 (3-[4,7-dibenzyl-3-(3-hydrazinocarbonylbenzyl)-5,6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity for HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054174 (3-[4,7-dibenzyl-3-(3-hydrazinocarbonylbenzyl)-5,6-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM161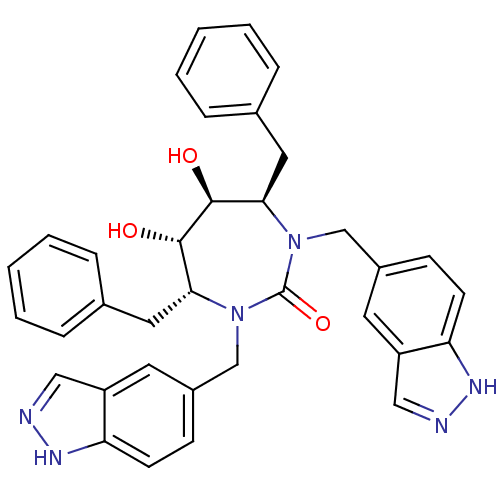 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | -63.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | J Med Chem 39: 3514-25 (1996) Article DOI: 10.1021/jm9602571 BindingDB Entry DOI: 10.7270/Q2ZW1J3M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM161 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36649 (3-alkylaminoindazole cyclic urea, (Me)) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM178 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | -63.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Ki values were determined with recombinant single-chain dimeric HIV protease and a fluorescent substrate. The use of single-chain dimeric protease a... | J Med Chem 39: 3514-25 (1996) Article DOI: 10.1021/jm9602571 BindingDB Entry DOI: 10.7270/Q2ZW1J3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50325534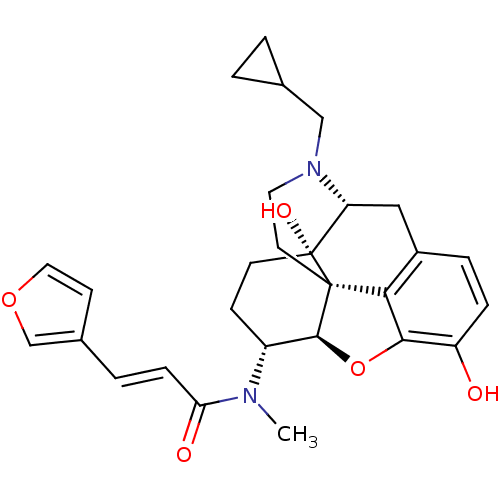 (CHEMBL267495 | nalfurafine) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128527 BindingDB Entry DOI: 10.7270/Q2HT2TDM | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM155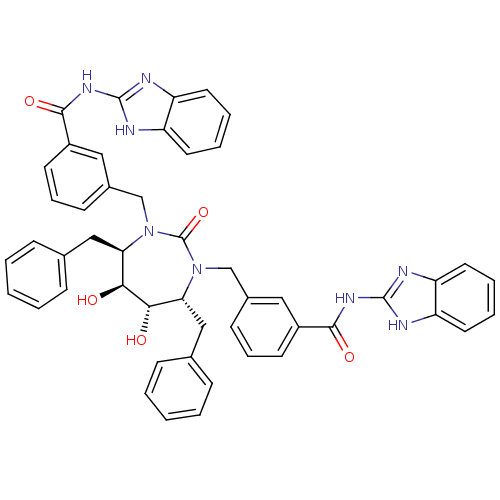 (CHEMBL11266 | N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0200 | -63.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Ki values were determined with recombinant single-chain dimeric HIV protease and a fluorescent substrate. The use of single-chain dimeric protease a... | J Med Chem 39: 3514-25 (1996) Article DOI: 10.1021/jm9602571 BindingDB Entry DOI: 10.7270/Q2ZW1J3M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054179 ((4r,5r,6,7)-3,3-[[Tetrahydro-5,6-dihydroxy-2-oxo-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity for HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054168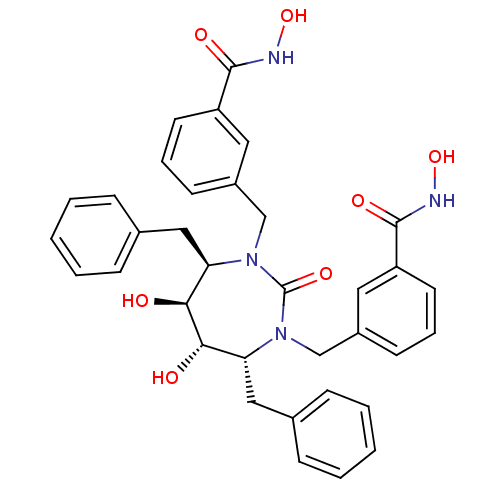 ((4alpha,5alpha,6beta,7beta)-3,3'-[Tetrahydro-5,6-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054180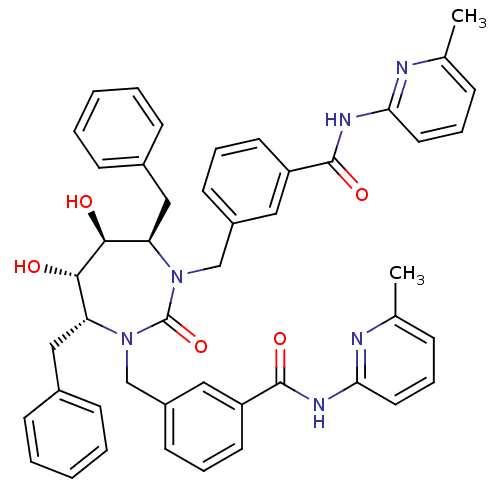 (2-{3-[4,7-dibenzyl-5,6-dihydroxy-3-[3-(6-methyl-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity for HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36655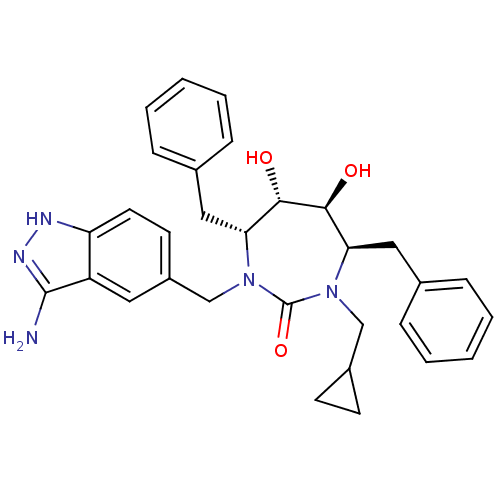 (Cyclopropylmethyl cyclic urea) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054168 ((4alpha,5alpha,6beta,7beta)-3,3'-[Tetrahydro-5,6-d...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity for HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054180 (2-{3-[4,7-dibenzyl-5,6-dihydroxy-3-[3-(6-methyl-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054178 (2-{3-[4,7-dibenzyl-3-[3-(4,6-dimethyl-2-pyridylcar...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity for HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM50124714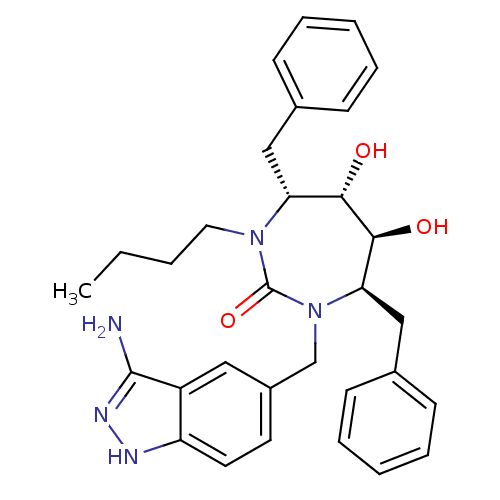 ((4R,5S,6S,7R)-1-(3-Amino-1H-indazol-5-ylmethyl)-4,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50064592 ((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1-(4-hydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50351401 (CHEMBL1819091) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes pre-incubated for 15 mins by LC/MS/MS analysis | Bioorg Med Chem 19: 5490-9 (2011) Article DOI: 10.1016/j.bmc.2011.07.042 BindingDB Entry DOI: 10.7270/Q2T72HT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM164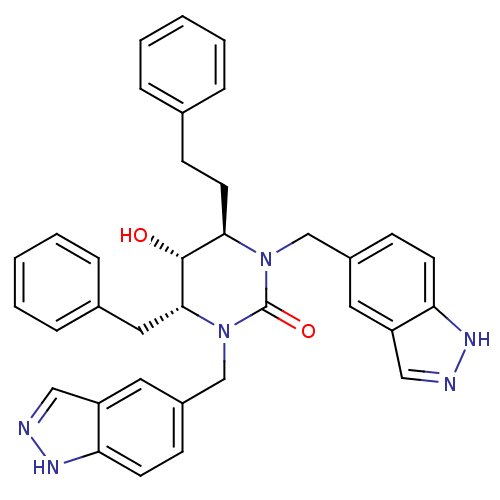 ((4R,5R,6R)-4-benzyl-5-hydroxy-1,3-bis(1H-indazol-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0230 | -63.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | J Med Chem 39: 3514-25 (1996) Article DOI: 10.1021/jm9602571 BindingDB Entry DOI: 10.7270/Q2ZW1J3M | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIV-1 protease (Human immunodeficiency virus) | BDBM36657 (2-Naphthylmethyl cyclic urea) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company | Assay Description Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. | Chem Biol 5: 597-608 (1998) Article DOI: 10.1016/s1074-5521(98)90117-x BindingDB Entry DOI: 10.7270/Q2R78CK2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM155 (CHEMBL11266 | N-(1H-1,3-benzodiazol-2-yl)-3-{[(4R,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054181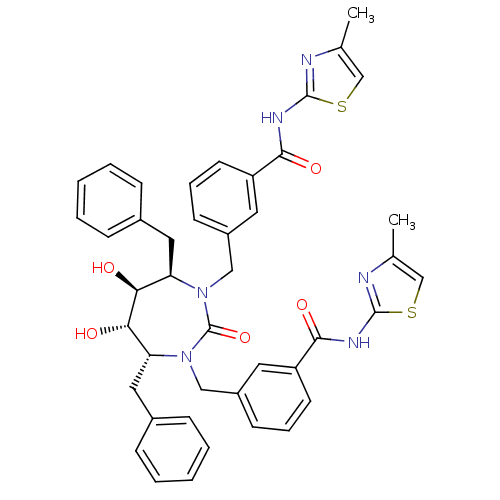 (2-{3-[4,7-dibenzyl-5,6-dihydroxy-3-[3-(4-methyl-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50351399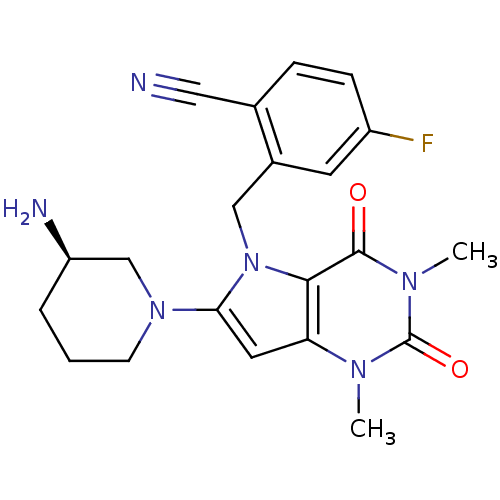 (CHEMBL1819089) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co. Ltd Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes pre-incubated for 15 mins by LC/MS/MS analysis | Bioorg Med Chem 19: 5490-9 (2011) Article DOI: 10.1016/j.bmc.2011.07.042 BindingDB Entry DOI: 10.7270/Q2T72HT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM182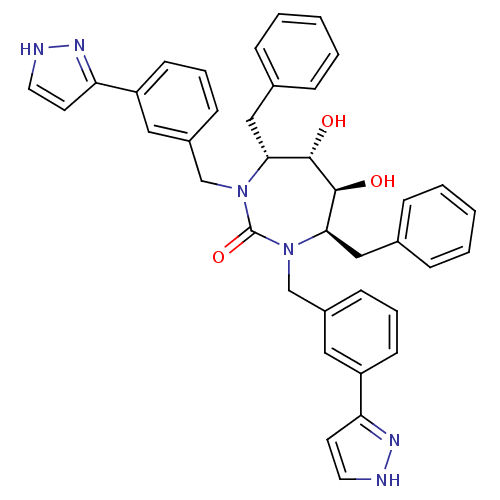 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM182 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0270 | -62.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Ki values were determined with recombinant single-chain dimeric HIV protease and a fluorescent substrate. The use of single-chain dimeric protease a... | J Med Chem 39: 3514-25 (1996) Article DOI: 10.1021/jm9602571 BindingDB Entry DOI: 10.7270/Q2ZW1J3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM154 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-2-oxo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054183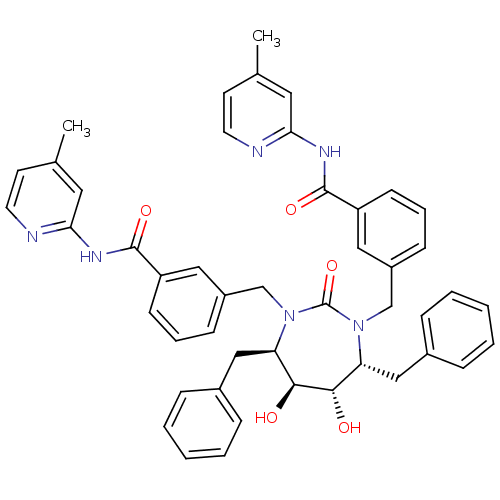 (2-{3-[4,7-dibenzyl-5,6-dihydroxy-3-[3-(4-methyl-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50596292 (CHEMBL5185211) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0272 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmcl.2022.128527 BindingDB Entry DOI: 10.7270/Q2HT2TDM | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM154 (3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-2-oxo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.0300 | -62.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Ki values were determined with recombinant single-chain dimeric HIV protease and a fluorescent substrate. The use of single-chain dimeric protease a... | J Med Chem 39: 3514-25 (1996) Article DOI: 10.1021/jm9602571 BindingDB Entry DOI: 10.7270/Q2ZW1J3M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50054183 (2-{3-[4,7-dibenzyl-5,6-dihydroxy-3-[3-(4-methyl-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Binding affinity for HIV-1 protease | J Med Chem 39: 4299-312 (1996) Article DOI: 10.1021/jm9602773 BindingDB Entry DOI: 10.7270/Q2T152QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM180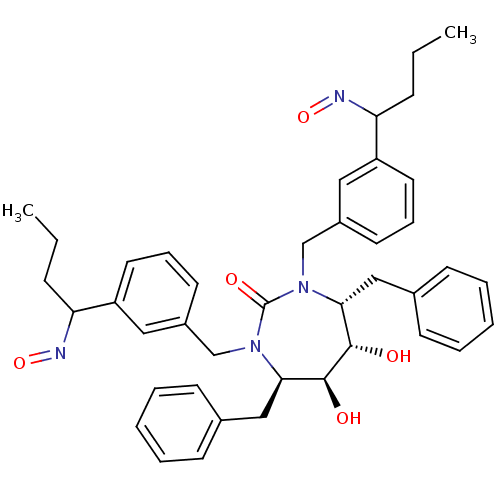 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company Curated by ChEMBL | Assay Description Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC | J Med Chem 41: 2019-28 (1998) Article DOI: 10.1021/jm9704199 BindingDB Entry DOI: 10.7270/Q29K49B0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [501-599] (Human immunodeficiency virus type 1) | BDBM179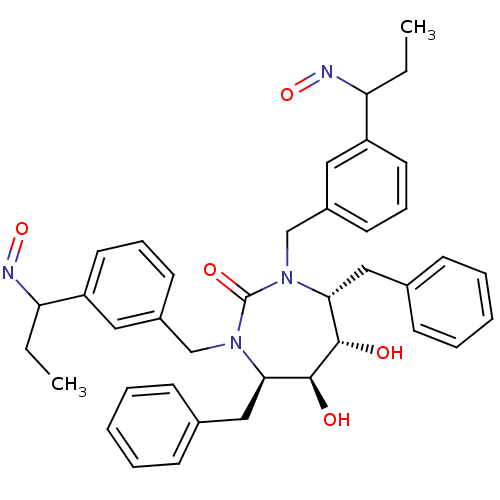 ((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0310 | -62.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company | Assay Description Ki values were determined with recombinant single-chain dimeric HIV protease and a fluorescent substrate. The use of single-chain dimeric protease a... | J Med Chem 39: 3514-25 (1996) Article DOI: 10.1021/jm9602571 BindingDB Entry DOI: 10.7270/Q2ZW1J3M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4550 total ) | Next | Last >> |