 Found 1509 hits with Last Name = 'ruan' and Initial = 'z'
Found 1509 hits with Last Name = 'ruan' and Initial = 'z' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358883

(CHEMBL1923468)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N[C@H]1CCCN(CC(=O)N2C[C@@H]3C[C@H](C2)c2cccc(=O)n2C3)C1=O |r| Show InChI InChI=1S/C28H29ClN4O5S/c29-22-8-6-20-13-23(9-7-19(20)12-22)39(37,38)30-24-3-2-10-31(28(24)36)17-27(35)32-14-18-11-21(16-32)25-4-1-5-26(34)33(25)15-18/h1,4-9,12-13,18,21,24,30H,2-3,10-11,14-17H2/t18-,21+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 4.71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Michaelis Menten equation analysis |
Bioorg Med Chem Lett 21: 7516-21 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.098
BindingDB Entry DOI: 10.7270/Q2MK6DBS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50358883

(CHEMBL1923468)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N[C@H]1CCCN(CC(=O)N2C[C@@H]3C[C@H](C2)c2cccc(=O)n2C3)C1=O |r| Show InChI InChI=1S/C28H29ClN4O5S/c29-22-8-6-20-13-23(9-7-19(20)12-22)39(37,38)30-24-3-2-10-31(28(24)36)17-27(35)32-14-18-11-21(16-32)25-4-1-5-26(34)33(25)15-18/h1,4-9,12-13,18,21,24,30H,2-3,10-11,14-17H2/t18-,21+,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human TPA by Michaelis Menten equation analysis |
Bioorg Med Chem Lett 21: 7516-21 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.098
BindingDB Entry DOI: 10.7270/Q2MK6DBS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50358883

(CHEMBL1923468)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N[C@H]1CCCN(CC(=O)N2C[C@@H]3C[C@H](C2)c2cccc(=O)n2C3)C1=O |r| Show InChI InChI=1S/C28H29ClN4O5S/c29-22-8-6-20-13-23(9-7-19(20)12-22)39(37,38)30-24-3-2-10-31(28(24)36)17-27(35)32-14-18-11-21(16-32)25-4-1-5-26(34)33(25)15-18/h1,4-9,12-13,18,21,24,30H,2-3,10-11,14-17H2/t18-,21+,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin by Michaelis Menten equation analysis |
Bioorg Med Chem Lett 21: 7516-21 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.098
BindingDB Entry DOI: 10.7270/Q2MK6DBS |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50358883

(CHEMBL1923468)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N[C@H]1CCCN(CC(=O)N2C[C@@H]3C[C@H](C2)c2cccc(=O)n2C3)C1=O |r| Show InChI InChI=1S/C28H29ClN4O5S/c29-22-8-6-20-13-23(9-7-19(20)12-22)39(37,38)30-24-3-2-10-31(28(24)36)17-27(35)32-14-18-11-21(16-32)25-4-1-5-26(34)33(25)15-18/h1,4-9,12-13,18,21,24,30H,2-3,10-11,14-17H2/t18-,21+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 7a by Michaelis Menten equation analysis |
Bioorg Med Chem Lett 21: 7516-21 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.098
BindingDB Entry DOI: 10.7270/Q2MK6DBS |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50358883

(CHEMBL1923468)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N[C@H]1CCCN(CC(=O)N2C[C@@H]3C[C@H](C2)c2cccc(=O)n2C3)C1=O |r| Show InChI InChI=1S/C28H29ClN4O5S/c29-22-8-6-20-13-23(9-7-19(20)12-22)39(37,38)30-24-3-2-10-31(28(24)36)17-27(35)32-14-18-11-21(16-32)25-4-1-5-26(34)33(25)15-18/h1,4-9,12-13,18,21,24,30H,2-3,10-11,14-17H2/t18-,21+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a by Michaelis Menten equation analysis |
Bioorg Med Chem Lett 21: 7516-21 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.098
BindingDB Entry DOI: 10.7270/Q2MK6DBS |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50358883

(CHEMBL1923468)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N[C@H]1CCCN(CC(=O)N2C[C@@H]3C[C@H](C2)c2cccc(=O)n2C3)C1=O |r| Show InChI InChI=1S/C28H29ClN4O5S/c29-22-8-6-20-13-23(9-7-19(20)12-22)39(37,38)30-24-3-2-10-31(28(24)36)17-27(35)32-14-18-11-21(16-32)25-4-1-5-26(34)33(25)15-18/h1,4-9,12-13,18,21,24,30H,2-3,10-11,14-17H2/t18-,21+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human u-PA by Michaelis Menten equation analysis |
Bioorg Med Chem Lett 21: 7516-21 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.098
BindingDB Entry DOI: 10.7270/Q2MK6DBS |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50358883

(CHEMBL1923468)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N[C@H]1CCCN(CC(=O)N2C[C@@H]3C[C@H](C2)c2cccc(=O)n2C3)C1=O |r| Show InChI InChI=1S/C28H29ClN4O5S/c29-22-8-6-20-13-23(9-7-19(20)12-22)39(37,38)30-24-3-2-10-31(28(24)36)17-27(35)32-14-18-11-21(16-32)25-4-1-5-26(34)33(25)15-18/h1,4-9,12-13,18,21,24,30H,2-3,10-11,14-17H2/t18-,21+,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin by Michaelis Menten equation analysis |
Bioorg Med Chem Lett 21: 7516-21 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.098
BindingDB Entry DOI: 10.7270/Q2MK6DBS |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50358883

(CHEMBL1923468)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N[C@H]1CCCN(CC(=O)N2C[C@@H]3C[C@H](C2)c2cccc(=O)n2C3)C1=O |r| Show InChI InChI=1S/C28H29ClN4O5S/c29-22-8-6-20-13-23(9-7-19(20)12-22)39(37,38)30-24-3-2-10-31(28(24)36)17-27(35)32-14-18-11-21(16-32)25-4-1-5-26(34)33(25)15-18/h1,4-9,12-13,18,21,24,30H,2-3,10-11,14-17H2/t18-,21+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 2.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human activated protein C by Michaelis Menten equation analysis |
Bioorg Med Chem Lett 21: 7516-21 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.098
BindingDB Entry DOI: 10.7270/Q2MK6DBS |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50239718
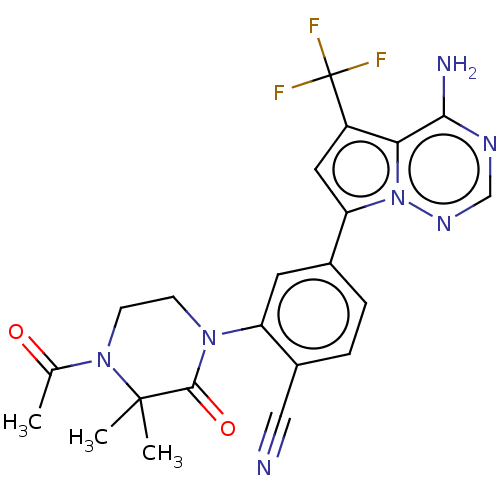
(CHEMBL4064666 | US10214537, Example 639)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F Show InChI InChI=1S/C22H20F3N7O2/c1-12(33)31-7-6-30(20(34)21(31,2)3)16-8-13(4-5-14(16)10-26)17-9-15(22(23,24)25)18-19(27)28-11-29-32(17)18/h4-5,8-9,11H,6-7H2,1-3H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine |
J Med Chem 60: 5193-5208 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00618
BindingDB Entry DOI: 10.7270/Q2WW7KVJ |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E]
(Homo sapiens (Human)) | BDBM32522
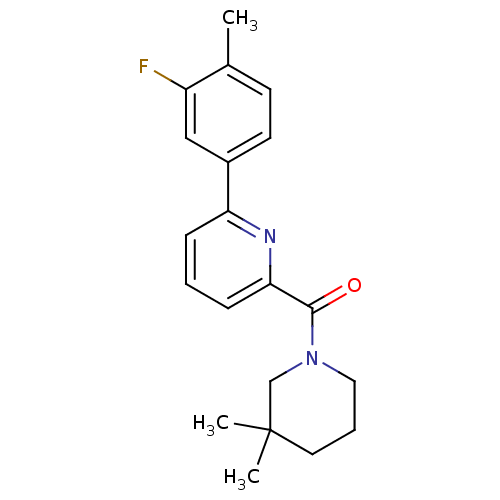
(pyridine amide, 30)Show InChI InChI=1S/C20H23FN2O/c1-14-8-9-15(12-16(14)21)17-6-4-7-18(22-17)19(24)23-11-5-10-20(2,3)13-23/h4,6-9,12H,5,10-11,13H2,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company
| Assay Description
11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... |
Bioorg Med Chem Lett 18: 3168-72 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.069
BindingDB Entry DOI: 10.7270/Q2P26WGT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358637
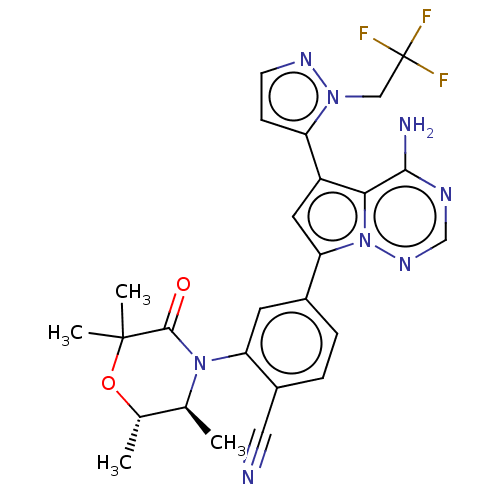
(4-(4-amino-5-(1-(2,2,2-trifluoroethyl)- 1H-pyrazol...)Show SMILES C[C@@H]1OC(C)(C)C(=O)N([C@H]1C)c1cc(ccc1C#N)-c1cc(-c2ccnn2CC(F)(F)F)c2c(N)ncnn12 |r| Show InChI InChI=1S/C26H25F3N8O2/c1-14-15(2)39-25(3,4)24(38)36(14)20-9-16(5-6-17(20)11-30)21-10-18(22-23(31)32-13-34-37(21)22)19-7-8-33-35(19)12-26(27,28)29/h5-10,13-15H,12H2,1-4H3,(H2,31,32,34)/t14-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358627
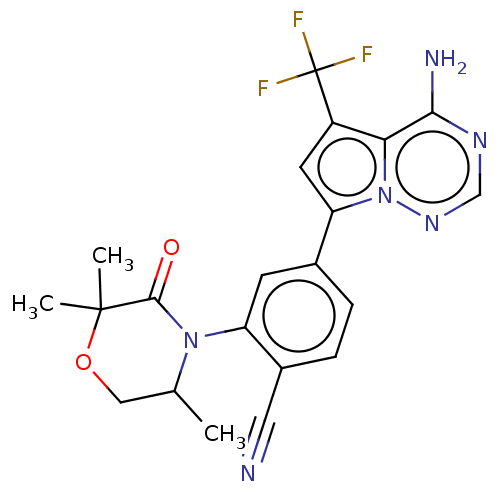
(4-(4-amino-5-(trifluoromethyl)pyrrolo [2,1-f][1,2,...)Show SMILES CC1COC(C)(C)C(=O)N1c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F Show InChI InChI=1S/C21H19F3N6O2/c1-11-9-32-20(2,3)19(31)29(11)15-6-12(4-5-13(15)8-25)16-7-14(21(22,23)24)17-18(26)27-10-28-30(16)17/h4-7,10-11H,9H2,1-3H3,(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50245571
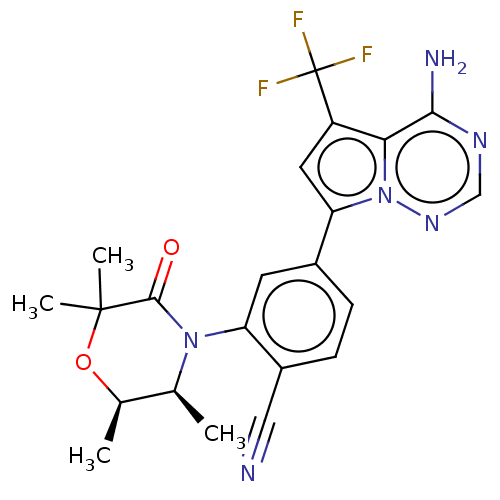
(CHEMBL4091875)Show SMILES C[C@H]1OC(C)(C)C(=O)N([C@H]1C)c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F |r| Show InChI InChI=1S/C22H21F3N6O2/c1-11-12(2)33-21(3,4)20(32)30(11)16-7-13(5-6-14(16)9-26)17-8-15(22(23,24)25)18-19(27)28-10-29-31(17)18/h5-8,10-12H,1-4H3,(H2,27,28,29)/t11-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358735
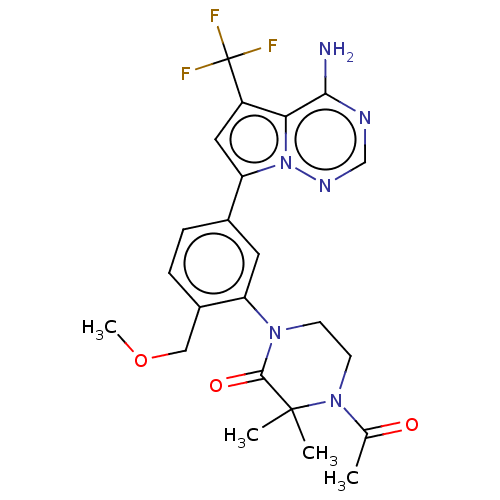
(4-acetyl-1-(5-(4-amino-5- (trifluoromethyl)pyrrolo...)Show SMILES COCc1ccc(cc1N1CCN(C(C)=O)C(C)(C)C1=O)-c1cc(c2c(N)ncnn12)C(F)(F)F Show InChI InChI=1S/C23H25F3N6O3/c1-13(33)31-8-7-30(21(34)22(31,2)3)17-9-14(5-6-15(17)11-35-4)18-10-16(23(24,25)26)19-20(27)28-12-29-32(18)19/h5-6,9-10,12H,7-8,11H2,1-4H3,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358679
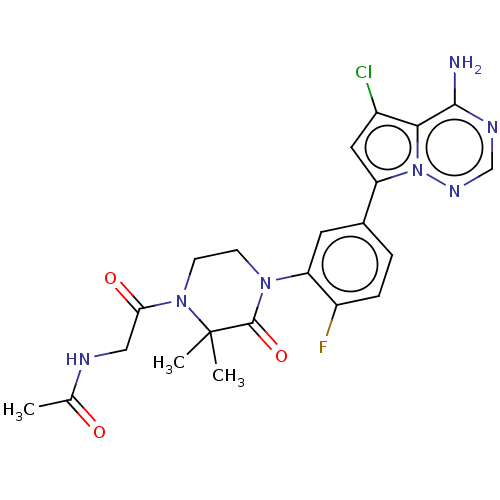
(N-(2-(4-(5-(4-amino-5-chloropyrrolo [2,1-f][1,2,4]...)Show SMILES CC(=O)NCC(=O)N1CCN(C(=O)C1(C)C)c1cc(ccc1F)-c1cc(Cl)c2c(N)ncnn12 Show InChI InChI=1S/C22H23ClFN7O3/c1-12(32)26-10-18(33)30-7-6-29(21(34)22(30,2)3)17-8-13(4-5-15(17)24)16-9-14(23)19-20(25)27-11-28-31(16)19/h4-5,8-9,11H,6-7,10H2,1-3H3,(H,26,32)(H2,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358684
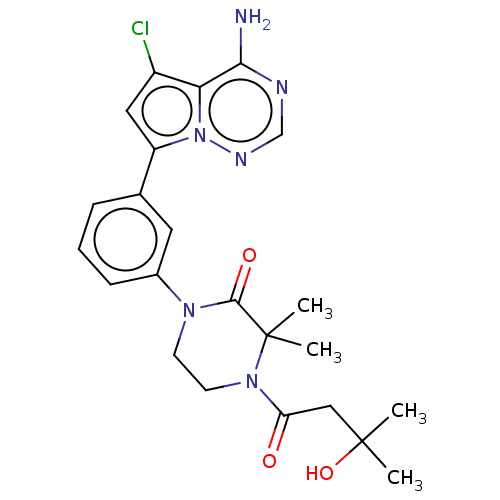
(1-(3-(4-amino-5-chloropyrrolo[2,1-f] [1,2,4]triazi...)Show SMILES CC(C)(O)CC(=O)N1CCN(C(=O)C1(C)C)c1cccc(c1)-c1cc(Cl)c2c(N)ncnn12 Show InChI InChI=1S/C23H27ClN6O3/c1-22(2,33)12-18(31)29-9-8-28(21(32)23(29,3)4)15-7-5-6-14(10-15)17-11-16(24)19-20(25)26-13-27-30(17)19/h5-7,10-11,13,33H,8-9,12H2,1-4H3,(H2,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358690
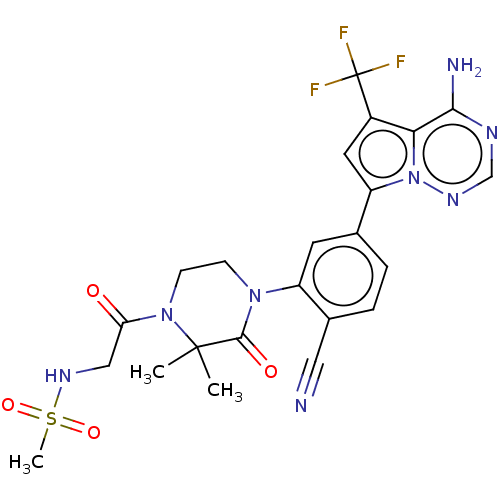
(N-(2-(4-(5-(4-amino-5- (trifluoromethyl)pyrrolo[2,...)Show SMILES CC1(C)N(CCN(C1=O)c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F)C(=O)CNS(C)(=O)=O Show InChI InChI=1S/C23H23F3N8O4S/c1-22(2)21(36)32(6-7-33(22)18(35)11-31-39(3,37)38)16-8-13(4-5-14(16)10-27)17-9-15(23(24,25)26)19-20(28)29-12-30-34(17)19/h4-5,8-9,12,31H,6-7,11H2,1-3H3,(H2,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358691
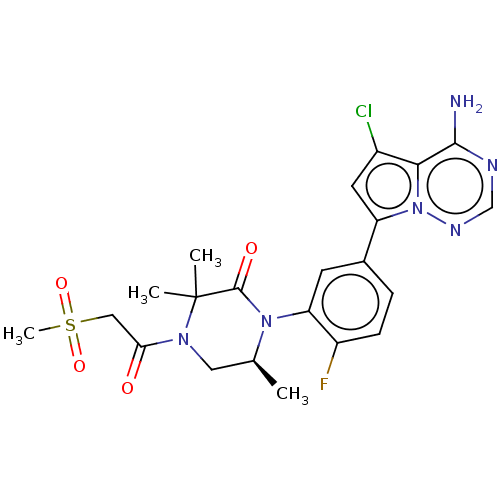
((S)-1-(5-(4-amino-5-chloropyrrolo [2,1-f][1,2,4]tr...)Show SMILES C[C@H]1CN(C(=O)CS(C)(=O)=O)C(C)(C)C(=O)N1c1cc(ccc1F)-c1cc(Cl)c2c(N)ncnn12 |r| Show InChI InChI=1S/C22H24ClFN6O4S/c1-12-9-28(18(31)10-35(4,33)34)22(2,3)21(32)29(12)17-7-13(5-6-15(17)24)16-8-14(23)19-20(25)26-11-27-30(16)19/h5-8,11-12H,9-10H2,1-4H3,(H2,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358600
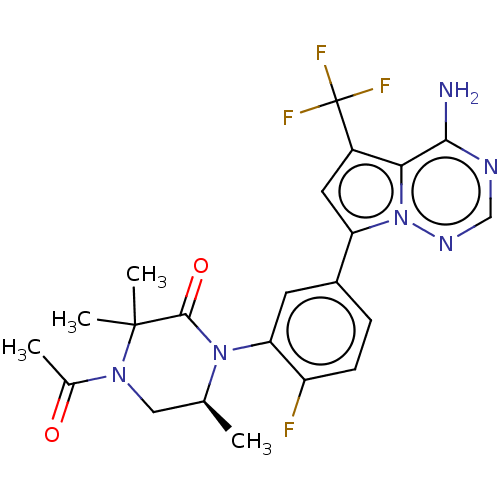
((S)-4-acetyl-1-(5-(4-amino-5- (trifluoromethyl)pyr...)Show SMILES C[C@H]1CN(C(C)=O)C(C)(C)C(=O)N1c1cc(ccc1F)-c1cc(c2c(N)ncnn12)C(F)(F)F |r| Show InChI InChI=1S/C22H22F4N6O2/c1-11-9-30(12(2)33)21(3,4)20(34)31(11)17-7-13(5-6-15(17)23)16-8-14(22(24,25)26)18-19(27)28-10-29-32(16)18/h5-8,10-11H,9H2,1-4H3,(H2,27,28,29)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358601
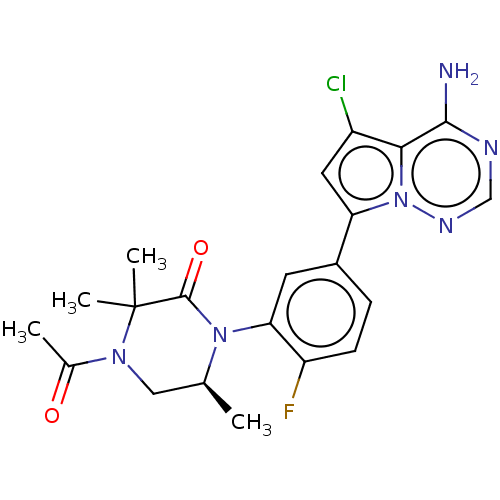
((S)-4-acetyl-1-(5-(4-amino-5- chloropyrrolo[2,1-f]...)Show SMILES C[C@H]1CN(C(C)=O)C(C)(C)C(=O)N1c1cc(ccc1F)-c1cc(Cl)c2c(N)ncnn12 |r| Show InChI InChI=1S/C21H22ClFN6O2/c1-11-9-27(12(2)30)21(3,4)20(31)28(11)17-7-13(5-6-15(17)23)16-8-14(22)18-19(24)25-10-26-29(16)18/h5-8,10-11H,9H2,1-4H3,(H2,24,25,26)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358604
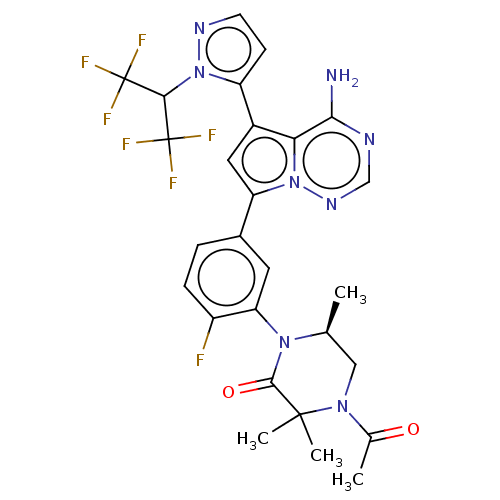
((S)-4-acetyl-1-(5-(4-amino-5-(1-((S)- 1,1,1-triflu...)Show SMILES C[C@H]1CN(C(C)=O)C(C)(C)C(=O)N1c1cc(ccc1F)-c1cc(-c2ccnn2C(C(F)(F)F)C(F)(F)F)c2c(N)ncnn12 |r| Show InChI InChI=1S/C27H25F7N8O2/c1-13-11-39(14(2)43)25(3,4)24(44)40(13)20-9-15(5-6-17(20)28)19-10-16(21-22(35)36-12-38-41(19)21)18-7-8-37-42(18)23(26(29,30)31)27(32,33)34/h5-10,12-13,23H,11H2,1-4H3,(H2,35,36,38)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358606
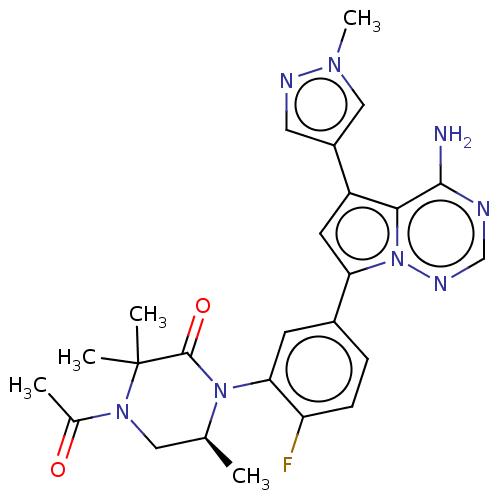
((S)-4-acetyl-1-(5-(4-amino-5-(1- methyl-1H-pyrazol...)Show SMILES C[C@H]1CN(C(C)=O)C(C)(C)C(=O)N1c1cc(ccc1F)-c1cc(-c2cnn(C)c2)c2c(N)ncnn12 |r| Show InChI InChI=1S/C25H27FN8O2/c1-14-11-32(15(2)35)25(3,4)24(36)33(14)21-8-16(6-7-19(21)26)20-9-18(17-10-29-31(5)12-17)22-23(27)28-13-30-34(20)22/h6-10,12-14H,11H2,1-5H3,(H2,27,28,30)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358608
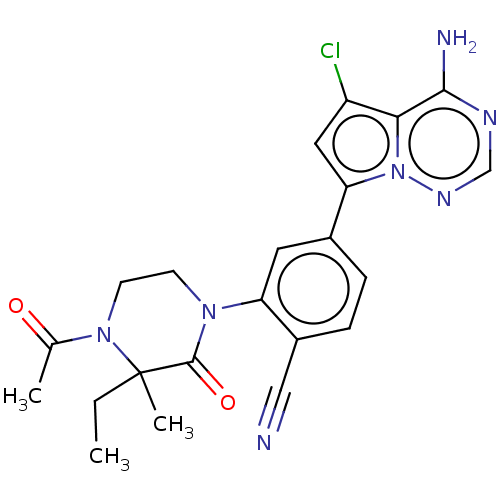
(2-(4-acetyl-3-ethyl-3-methyl-2- oxopiperazin-1-yl)...)Show SMILES CCC1(C)N(CCN(C1=O)c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12)C(C)=O Show InChI InChI=1S/C22H22ClN7O2/c1-4-22(3)21(32)28(7-8-29(22)13(2)31)17-9-14(5-6-15(17)11-24)18-10-16(23)19-20(25)26-12-27-30(18)19/h5-6,9-10,12H,4,7-8H2,1-3H3,(H2,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
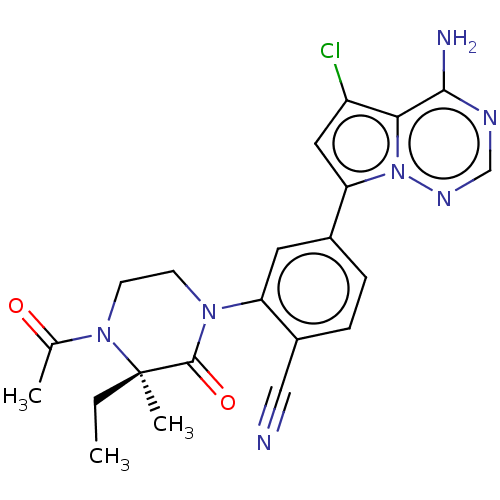
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358610
((R)-2-(4-acetyl-3-ethyl-3-methyl-2- oxopiperazin-1...)Show SMILES CC[C@@]1(C)N(CCN(C1=O)c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12)C(C)=O |r| Show InChI InChI=1S/C22H22ClN7O2/c1-4-22(3)21(32)28(7-8-29(22)13(2)31)17-9-14(5-6-15(17)11-24)18-10-16(23)19-20(25)26-12-27-30(18)19/h5-6,9-10,12H,4,7-8H2,1-3H3,(H2,25,26,27)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358613
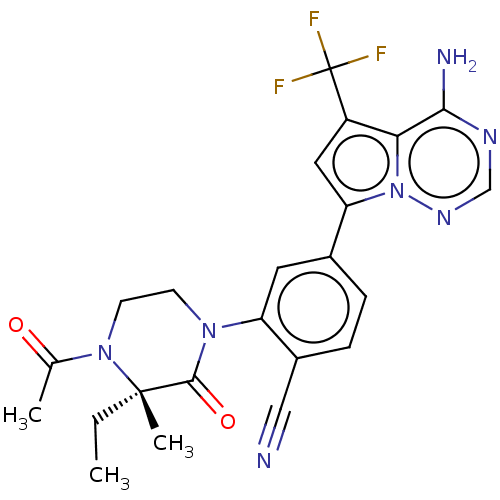
((S)-2-(4-acetyl-3-ethyl-3-methyl-2- oxopiperazin-1...)Show SMILES CC[C@]1(C)N(CCN(C1=O)c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F)C(C)=O |r| Show InChI InChI=1S/C23H22F3N7O2/c1-4-22(3)21(35)31(7-8-32(22)13(2)34)17-9-14(5-6-15(17)11-27)18-10-16(23(24,25)26)19-20(28)29-12-30-33(18)19/h5-6,9-10,12H,4,7-8H2,1-3H3,(H2,28,29,30)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358652
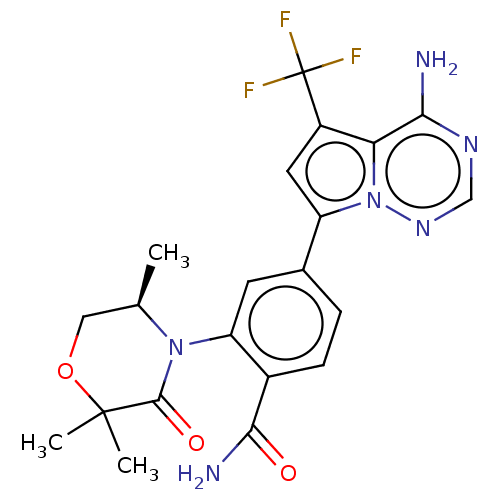
((R)-4-(4-amino-5-(trifluoromethyl) pyrrolo[2,1-f][...)Show SMILES C[C@@H]1COC(C)(C)C(=O)N1c1cc(ccc1C(N)=O)-c1cc(c2c(N)ncnn12)C(F)(F)F |r| Show InChI InChI=1S/C21H21F3N6O3/c1-10-8-33-20(2,3)19(32)29(10)15-6-11(4-5-12(15)18(26)31)14-7-13(21(22,23)24)16-17(25)27-9-28-30(14)16/h4-7,9-10H,8H2,1-3H3,(H2,26,31)(H2,25,27,28)/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50232433
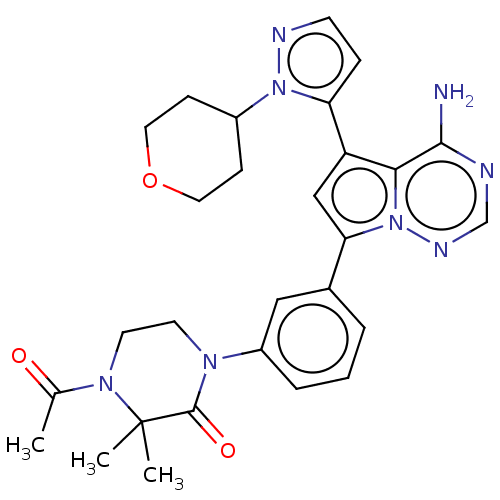
(CHEMBL4068514 | US10214537, Example 619)Show SMILES CC(=O)N1CCN(C(=O)C1(C)C)c1cccc(c1)-c1cc(-c2ccnn2C2CCOCC2)c2c(N)ncnn12 Show InChI InChI=1S/C28H32N8O3/c1-18(37)34-12-11-33(27(38)28(34,2)3)21-6-4-5-19(15-21)24-16-22(25-26(29)30-17-32-36(24)25)23-7-10-31-35(23)20-8-13-39-14-9-20/h4-7,10,15-17,20H,8-9,11-14H2,1-3H3,(H2,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2:PS as substrate incubated for 3 hrs by ADP-Glo assay |
Bioorg Med Chem Lett 27: 855-861 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.016
BindingDB Entry DOI: 10.7270/Q2BR8VFH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50245561
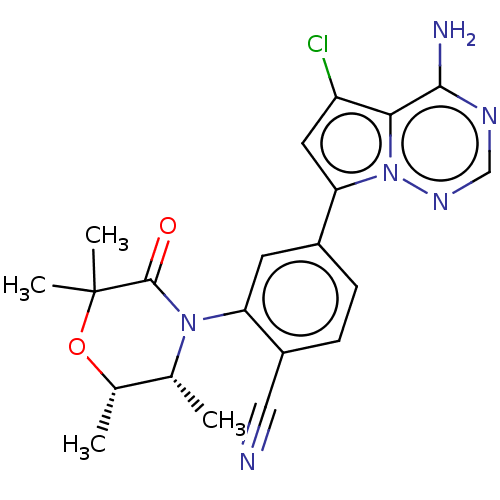
(CHEMBL4069213)Show SMILES C[C@@H]1OC(C)(C)C(=O)N([C@@H]1C)c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 |r| Show InChI InChI=1S/C21H21ClN6O2/c1-11-12(2)30-21(3,4)20(29)27(11)16-7-13(5-6-14(16)9-23)17-8-15(22)18-19(24)25-10-26-28(17)18/h5-8,10-12H,1-4H3,(H2,24,25,26)/t11-,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50245582
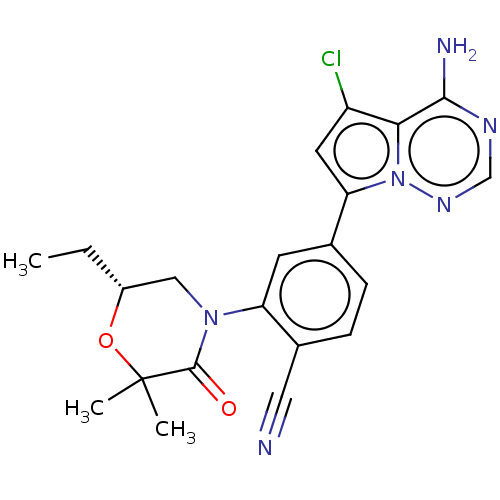
(CHEMBL4080090)Show SMILES CC[C@@H]1CN(C(=O)C(C)(C)O1)c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 |r| Show InChI InChI=1S/C21H21ClN6O2/c1-4-14-10-27(20(29)21(2,3)30-14)16-7-12(5-6-13(16)9-23)17-8-15(22)18-19(24)25-11-26-28(17)18/h5-8,11,14H,4,10H2,1-3H3,(H2,24,25,26)/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
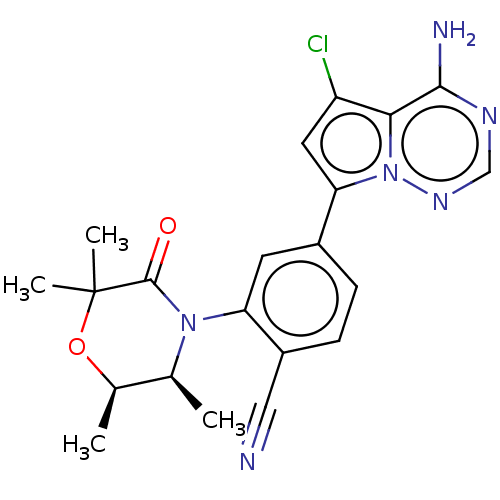
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50245550
(CHEMBL4096456)Show SMILES C[C@H]1OC(C)(C)C(=O)N([C@H]1C)c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 |r| Show InChI InChI=1S/C21H21ClN6O2/c1-11-12(2)30-21(3,4)20(29)27(11)16-7-13(5-6-14(16)9-23)17-8-15(22)18-19(24)25-10-26-28(17)18/h5-8,10-12H,1-4H3,(H2,24,25,26)/t11-,12+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
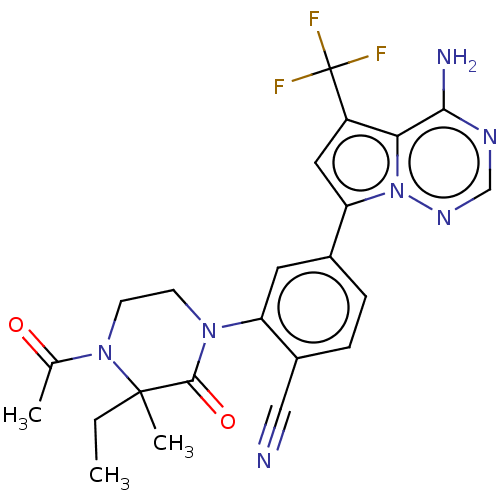
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358609
(2-(4-acetyl-3-ethyl-3-methyl-2- oxopiperazin-1-yl)...)Show SMILES CCC1(C)N(CCN(C1=O)c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F)C(C)=O Show InChI InChI=1S/C23H22F3N7O2/c1-4-22(3)21(35)31(7-8-32(22)13(2)34)17-9-14(5-6-15(17)11-27)18-10-16(23(24,25)26)19-20(28)29-12-30-33(18)19/h5-6,9-10,12H,4,7-8H2,1-3H3,(H2,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
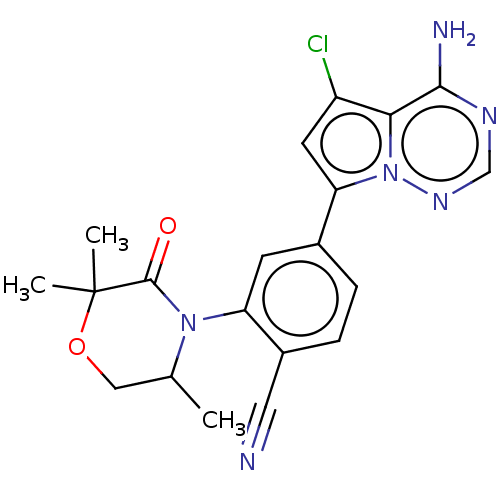
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358628
(4-(4-amino-5-chloropyrrolo[2,1-f] [1,2,4]triazin-7...)Show SMILES CC1COC(C)(C)C(=O)N1c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 Show InChI InChI=1S/C20H19ClN6O2/c1-11-9-29-20(2,3)19(28)26(11)15-6-12(4-5-13(15)8-22)16-7-14(21)17-18(23)24-10-25-27(16)17/h4-7,10-11H,9H2,1-3H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358664
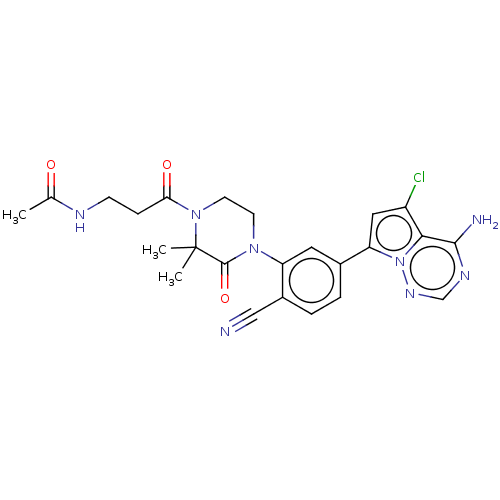
(N-(3-(4-(5-(4-amino-5-chloropyrrolo [2,1-f][1,2,4]...)Show SMILES CC(=O)NCCC(=O)N1CCN(C(=O)C1(C)C)c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 Show InChI InChI=1S/C24H25ClN8O3/c1-14(34)28-7-6-20(35)32-9-8-31(23(36)24(32,2)3)18-10-15(4-5-16(18)12-26)19-11-17(25)21-22(27)29-13-30-33(19)21/h4-5,10-11,13H,6-9H2,1-3H3,(H,28,34)(H2,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358607
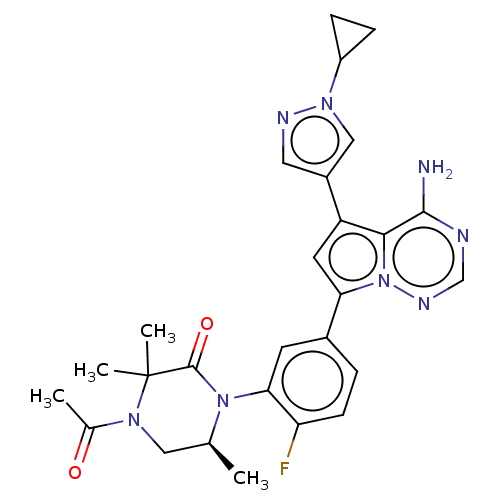
((S)-4-acetyl-1-(5-(4-amino-5-(1- cyclopropyl-1H-py...)Show SMILES C[C@H]1CN(C(C)=O)C(C)(C)C(=O)N1c1cc(ccc1F)-c1cc(-c2cnn(c2)C2CC2)c2c(N)ncnn12 |r| Show InChI InChI=1S/C27H29FN8O2/c1-15-12-33(16(2)37)27(3,4)26(38)35(15)23-9-17(5-8-21(23)28)22-10-20(24-25(29)30-14-32-36(22)24)18-11-31-34(13-18)19-6-7-19/h5,8-11,13-15,19H,6-7,12H2,1-4H3,(H2,29,30,32)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358638
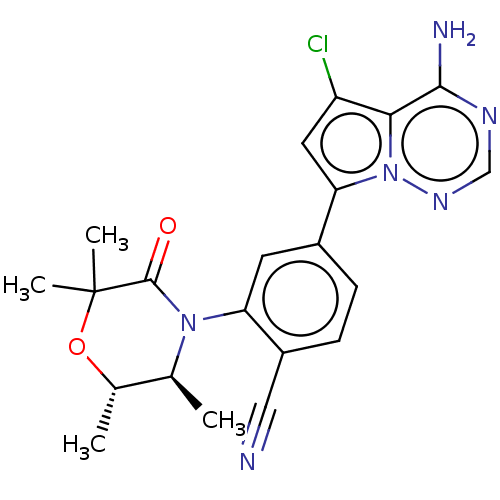
(4-(4-amino-5-chloropyrrolo[2,1-f] [1,2,4]triazin-7...)Show SMILES C[C@@H]1OC(C)(C)C(=O)N([C@H]1C)c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 |r| Show InChI InChI=1S/C21H21ClN6O2/c1-11-12(2)30-21(3,4)20(29)27(11)16-7-13(5-6-14(16)9-23)17-8-15(22)18-19(24)25-10-26-28(17)18/h5-8,10-12H,1-4H3,(H2,24,25,26)/t11-,12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358653
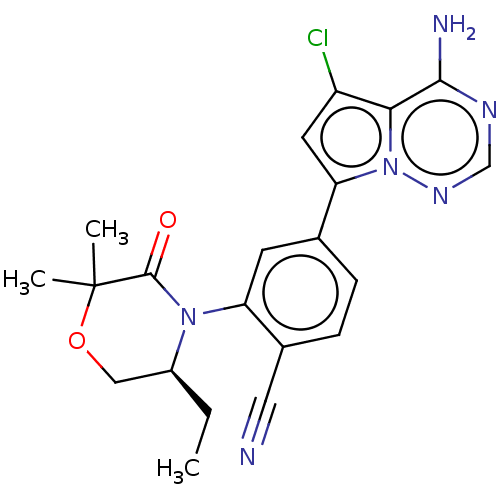
((S)-4-(4-amino-5-chloropyrrolo[2,1-f] [1,2,4]triaz...)Show SMILES CC[C@H]1COC(C)(C)C(=O)N1c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 |r| Show InChI InChI=1S/C21H21ClN6O2/c1-4-14-10-30-21(2,3)20(29)27(14)16-7-12(5-6-13(16)9-23)17-8-15(22)18-19(24)25-11-26-28(17)18/h5-8,11,14H,4,10H2,1-3H3,(H2,24,25,26)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358605
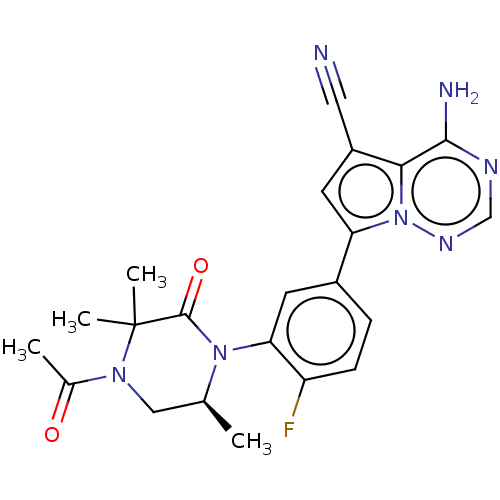
((S)-7-(3-(4-acetyl-3,3,6-trimethyl-2- oxopiperazin...)Show SMILES C[C@H]1CN(C(C)=O)C(C)(C)C(=O)N1c1cc(ccc1F)-c1cc(C#N)c2c(N)ncnn12 |r| Show InChI InChI=1S/C22H22FN7O2/c1-12-10-28(13(2)31)22(3,4)21(32)29(12)18-7-14(5-6-16(18)23)17-8-15(9-24)19-20(25)26-11-27-30(17)19/h5-8,11-12H,10H2,1-4H3,(H2,25,26,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358363
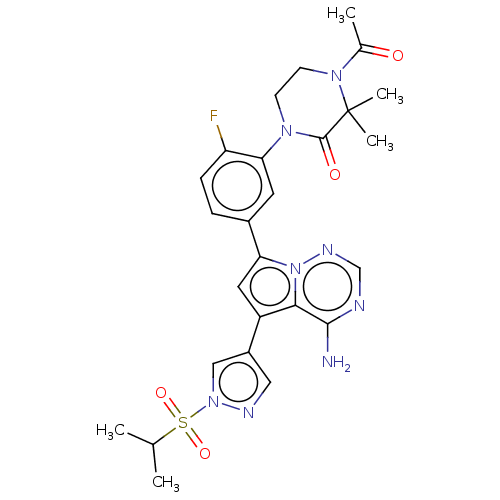
(4-acetyl-1-(5-{4-amino-5-[1-(propane- 2-sulfonyl)-...)Show SMILES CC(C)S(=O)(=O)n1cc(cn1)-c1cc(-c2ccc(F)c(c2)N2CCN(C(C)=O)C(C)(C)C2=O)n2ncnc(N)c12 Show InChI InChI=1S/C26H29FN8O4S/c1-15(2)40(38,39)34-13-18(12-30-34)19-11-21(35-23(19)24(28)29-14-31-35)17-6-7-20(27)22(10-17)32-8-9-33(16(3)36)26(4,5)25(32)37/h6-7,10-15H,8-9H2,1-5H3,(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358188
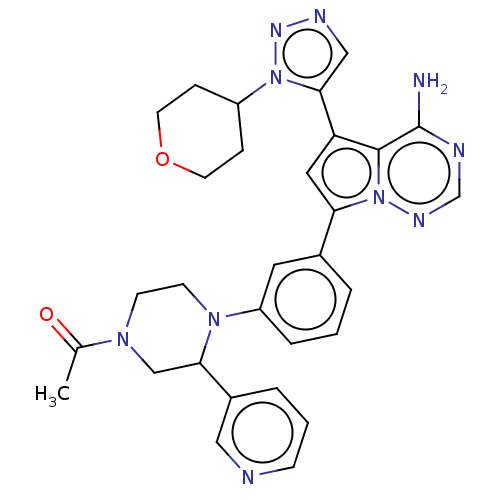
(1-(4-(3-(4-amino-5-(1-(tetrahydro-2H- pyran-4-yl)-...)Show SMILES CC(=O)N1CCN(C(C1)c1cccnc1)c1cccc(c1)-c1cc(-c2cnnn2C2CCOCC2)c2c(N)ncnn12 Show InChI InChI=1S/C30H32N10O2/c1-20(41)37-10-11-38(28(18-37)22-5-3-9-32-16-22)24-6-2-4-21(14-24)26-15-25(29-30(31)33-19-35-40(26)29)27-17-34-36-39(27)23-7-12-42-13-8-23/h2-6,9,14-17,19,23,28H,7-8,10-13,18H2,1H3,(H2,31,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358159
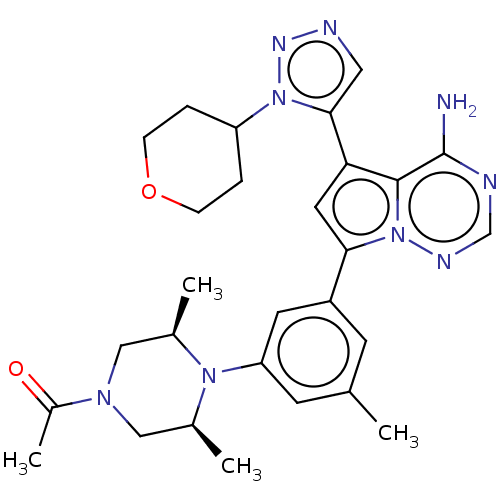
(1-((cis)-4-(3-(4-amino-5-(1-(tetrahydro-2H-pyran-4...)Show SMILES C[C@H]1CN(C[C@@H](C)N1c1cc(C)cc(c1)-c1cc(-c2cnnn2C2CCOCC2)c2c(N)ncnn12)C(C)=O Show InChI InChI=1S/C28H35N9O2/c1-17-9-21(11-23(10-17)35-18(2)14-34(20(4)38)15-19(35)3)25-12-24(27-28(29)30-16-32-37(25)27)26-13-31-33-36(26)22-5-7-39-8-6-22/h9-13,16,18-19,22H,5-8,14-15H2,1-4H3,(H2,29,30,32)/t18-,19+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM284822
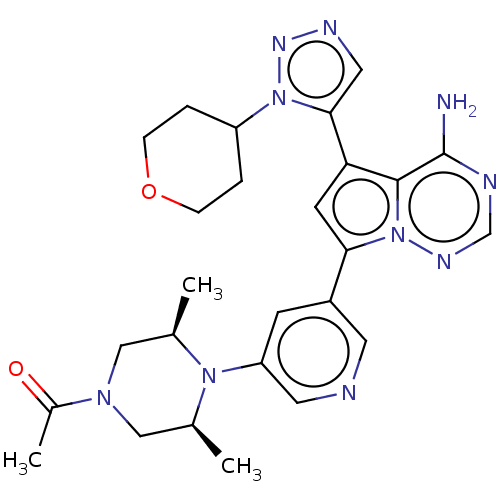
(1-((cis)-4-(5-(4-amino-5-(1-(tetrahydro-2H-pyran-4...)Show SMILES C[C@H]1CN(C[C@@H](C)N1c1cncc(c1)-c1cc(-c2cnnn2C2CCOCC2)c2c(N)ncnn12)C(C)=O |r| Show InChI InChI=1S/C26H32N10O2/c1-16-13-33(18(3)37)14-17(2)34(16)21-8-19(10-28-11-21)23-9-22(25-26(27)29-15-31-36(23)25)24-12-30-32-35(24)20-4-6-38-7-5-20/h8-12,15-17,20H,4-7,13-14H2,1-3H3,(H2,27,29,31)/t16-,17+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10023576 (2018)
BindingDB Entry DOI: 10.7270/Q2FQ9ZND |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358653

((S)-4-(4-amino-5-chloropyrrolo[2,1-f] [1,2,4]triaz...)Show SMILES CC[C@H]1COC(C)(C)C(=O)N1c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12 |r| Show InChI InChI=1S/C21H21ClN6O2/c1-4-14-10-30-21(2,3)20(29)27(14)16-7-12(5-6-13(16)9-23)17-8-15(22)18-19(24)25-11-26-28(17)18/h5-8,11,14H,4,10H2,1-3H3,(H2,24,25,26)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50245562

(CHEMBL4097187)Show SMILES [2H]C1([2H])OC(C)(C)C(=O)N(c2cc(ccc2C#N)-c2cc(Cl)c3c(N)ncnn23)C1([2H])[2H] Show InChI InChI=1S/C19H17ClN6O2/c1-19(2)18(27)25(5-6-28-19)14-7-11(3-4-12(14)9-21)15-8-13(20)16-17(22)23-10-24-26(15)16/h3-4,7-8,10H,5-6H2,1-2H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358657
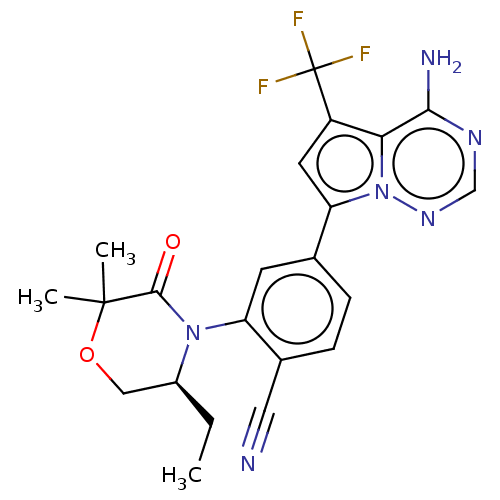
((S)-4-(4-amino-5-(trifluoromethyl) pyrrolo[2,1-f][...)Show SMILES CC[C@H]1COC(C)(C)C(=O)N1c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F |r| Show InChI InChI=1S/C22H21F3N6O2/c1-4-14-10-33-21(2,3)20(32)30(14)16-7-12(5-6-13(16)9-26)17-8-15(22(23,24)25)18-19(27)28-11-29-31(17)18/h5-8,11,14H,4,10H2,1-3H3,(H2,27,28,29)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) after 60 mins using fluorescein-labeled kinase tracer by HTRF assay |
Bioorg Med Chem Lett 27: 2849-2853 (2017)
Article DOI: 10.1016/j.bmcl.2017.01.077
BindingDB Entry DOI: 10.7270/Q25M684R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358666
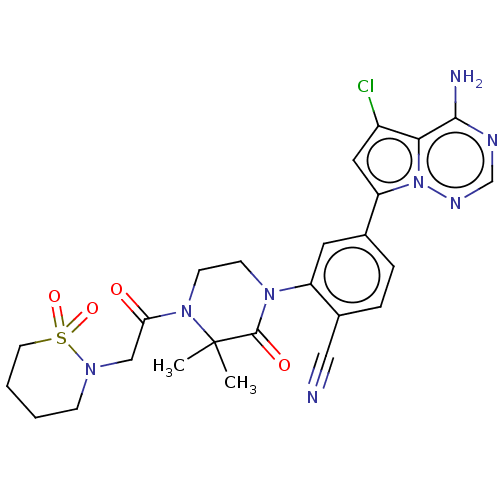
(4-(4-amino-5-chloropyrrolo[2,1-f] [1,2,4]triazin-7...)Show SMILES CC1(C)N(CCN(C1=O)c1cc(ccc1C#N)-c1cc(Cl)c2c(N)ncnn12)C(=O)CN1CCCCS1(=O)=O Show InChI InChI=1S/C25H27ClN8O4S/c1-25(2)24(36)32(8-9-33(25)21(35)14-31-7-3-4-10-39(31,37)38)19-11-16(5-6-17(19)13-27)20-12-18(26)22-23(28)29-15-30-34(20)22/h5-6,11-12,15H,3-4,7-10,14H2,1-2H3,(H2,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358669
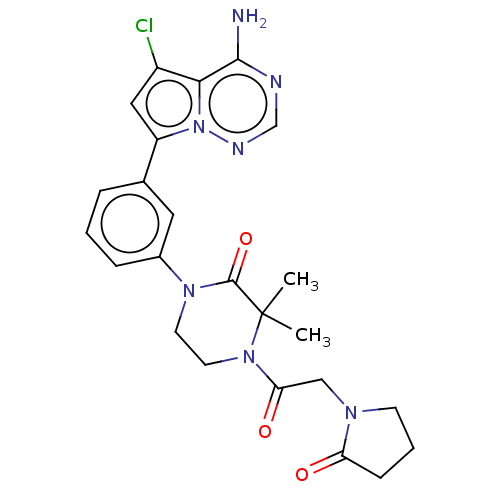
(1-(3-(4-amino-5-chloropyrrolo[2,1-f] [1,2,4]triazi...)Show SMILES CC1(C)N(CCN(C1=O)c1cccc(c1)-c1cc(Cl)c2c(N)ncnn12)C(=O)CN1CCCC1=O Show InChI InChI=1S/C24H26ClN7O3/c1-24(2)23(35)30(9-10-31(24)20(34)13-29-8-4-7-19(29)33)16-6-3-5-15(11-16)18-12-17(25)21-22(26)27-14-28-32(18)21/h3,5-6,11-12,14H,4,7-10,13H2,1-2H3,(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358027
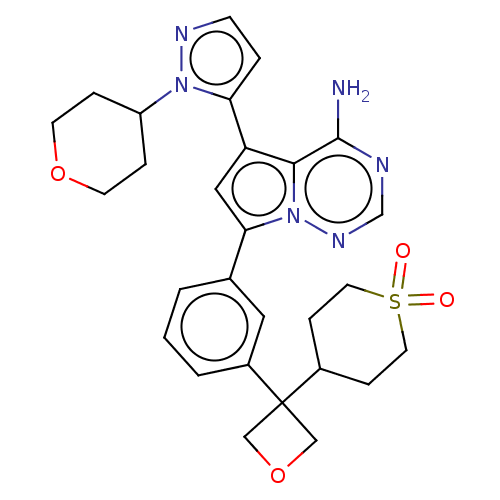
(4-(3-(3-(4-amino-5-(1-(tetrahydro-2H- pyran-4-yl)-...)Show SMILES Nc1ncnn2c(cc(-c3ccnn3C3CCOCC3)c12)-c1cccc(c1)C1(COC1)C1CCS(=O)(=O)CC1 Show InChI InChI=1S/C28H32N6O4S/c29-27-26-23(24-4-9-31-33(24)22-5-10-37-11-6-22)15-25(34(26)32-18-30-27)19-2-1-3-21(14-19)28(16-38-17-28)20-7-12-39(35,36)13-8-20/h1-4,9,14-15,18,20,22H,5-8,10-13,16-17H2,(H2,29,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358687
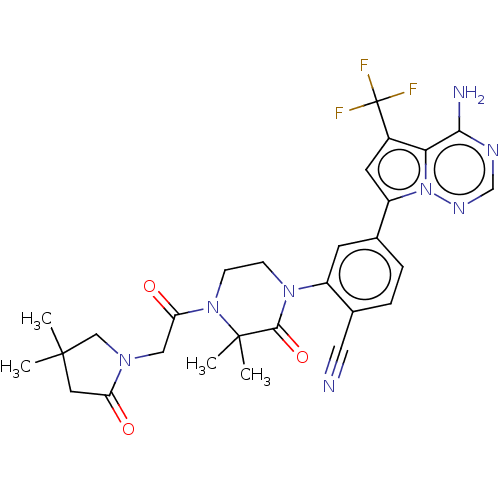
(4-(4-amino-5-(trifluoromethyl)pyrrolo [2,1-f][1,2,...)Show SMILES CC1(C)CN(CC(=O)N2CCN(C(=O)C2(C)C)c2cc(ccc2C#N)-c2cc(c3c(N)ncnn23)C(F)(F)F)C(=O)C1 Show InChI InChI=1S/C28H29F3N8O3/c1-26(2)11-21(40)36(14-26)13-22(41)38-8-7-37(25(42)27(38,3)4)19-9-16(5-6-17(19)12-32)20-10-18(28(29,30)31)23-24(33)34-15-35-39(20)23/h5-6,9-10,15H,7-8,11,13-14H2,1-4H3,(H2,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358694
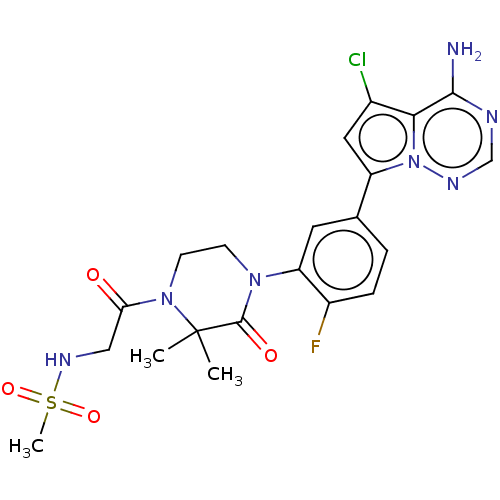
(N-(4-(4-(5-(4-amino-5-chloropyrrolo [2,1-f][1,2,4]...)Show SMILES CC1(C)N(CCN(C1=O)c1cc(ccc1F)-c1cc(Cl)c2c(N)ncnn12)C(=O)CNS(C)(=O)=O Show InChI InChI=1S/C21H23ClFN7O4S/c1-21(2)20(32)28(6-7-29(21)17(31)10-27-35(3,33)34)16-8-12(4-5-14(16)23)15-9-13(22)18-19(24)25-11-26-30(15)18/h4-5,8-9,11,27H,6-7,10H2,1-3H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM358657

((S)-4-(4-amino-5-(trifluoromethyl) pyrrolo[2,1-f][...)Show SMILES CC[C@H]1COC(C)(C)C(=O)N1c1cc(ccc1C#N)-c1cc(c2c(N)ncnn12)C(F)(F)F |r| Show InChI InChI=1S/C22H21F3N6O2/c1-4-14-10-33-21(2,3)20(32)30(14)16-7-12(5-6-13(16)9-26)17-8-15(22(23,24)25)18-19(27)28-11-29-31(17)18/h5-8,11,14H,4,10H2,1-3H3,(H2,27,28,29)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
US Patent
| Assay Description
The ADP-Glo format PI3K assays were performed in Proxiplate 384-well plates (Perkin Elmer #6008280). The final assay volume was 2 μl prepared fr... |
US Patent US10214537 (2019)
BindingDB Entry DOI: 10.7270/Q2HH6NB2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data