

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM140012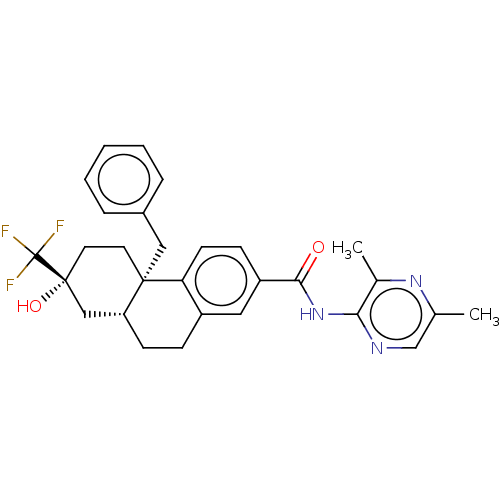 (US8901310, Comparator B) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The glucocorticoid receptor fluorescence polarization ligand binding (GRFP) assay is used to evaluate direct binding of testing compounds to full-len... | US Patent US8901310 (2014) BindingDB Entry DOI: 10.7270/Q2MC8XQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19190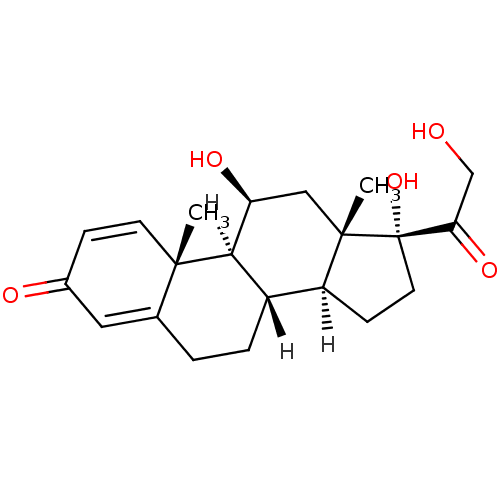 ((1S,2R,10S,11S,14R,15S,17S)-14,17-dihydroxy-14-(2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank US Patent | n/a | n/a | 0.526 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The glucocorticoid receptor fluorescence polarization ligand binding (GRFP) assay is used to evaluate direct binding of testing compounds to full-len... | US Patent US8901310 (2014) BindingDB Entry DOI: 10.7270/Q2MC8XQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50575344 (CHEMBL4854822) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of RET (658 to 1072) (unknown origin) by HTRF assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00450 BindingDB Entry DOI: 10.7270/Q23R0XP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50575348 (CHEMBL4853155) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of RET (658 to 1072) (unknown origin) by HTRF assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00450 BindingDB Entry DOI: 10.7270/Q23R0XP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50575350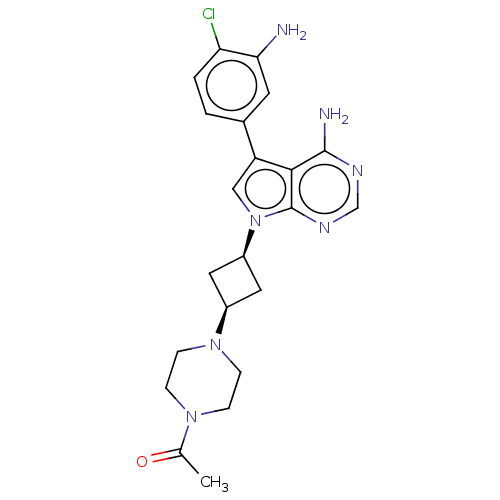 (CHEMBL4877264) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of RET (658 to 1072) (unknown origin) by HTRF assay | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00450 BindingDB Entry DOI: 10.7270/Q23R0XP8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50347099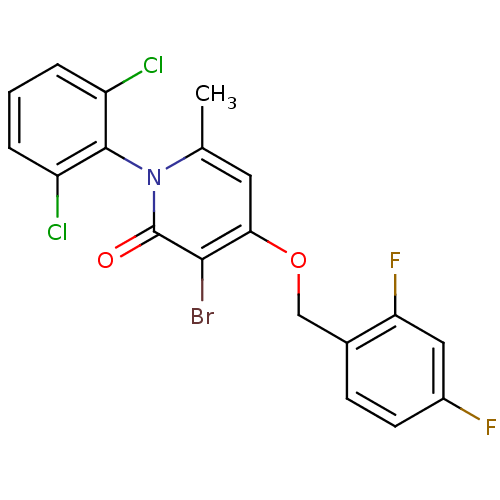 (CHEMBL1797202) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 21: 4059-65 (2011) Article DOI: 10.1016/j.bmcl.2011.04.120 BindingDB Entry DOI: 10.7270/Q25Q4WFH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM50092082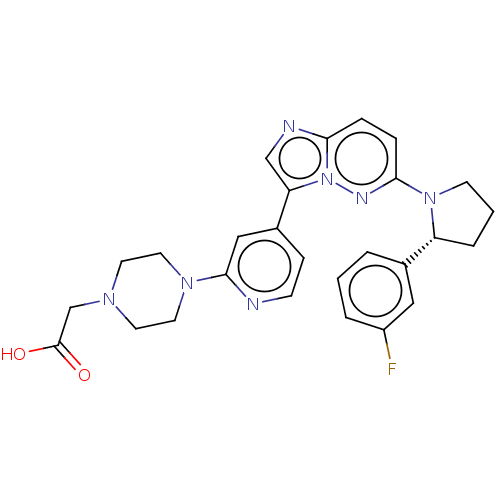 (CHEMBL3582441) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of Tel-fused TRKB (unknown origin) overexpressed in mouse BA/F3 cells assessed as inhibition of cell proliferation after 48 hrs by lucifer... | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50092082 (CHEMBL3582441) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of Tel-fused TRKA (unknown origin) overexpressed in mouse BA/F3 cells assessed as inhibition of cell proliferation after 48 hrs by lucifer... | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50092082 (CHEMBL3582441) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of NGF-activated full length TRKA (unknown origin) expressed in mouse BA/F3 cells assessed as inhibition of cell proliferation | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50092082 (CHEMBL3582441) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of TPM3-fused TRKA (unknown origin) expressed in human KM12 cells assessed as inhibition of cell proliferation after 48 hrs by luciferase ... | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50092082 (CHEMBL3582441) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of Tel-fused TRKC (unknown origin) overexpressed in mouse BA/F3 cells assessed as inhibition of cell proliferation after 48 hrs by lucifer... | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50092079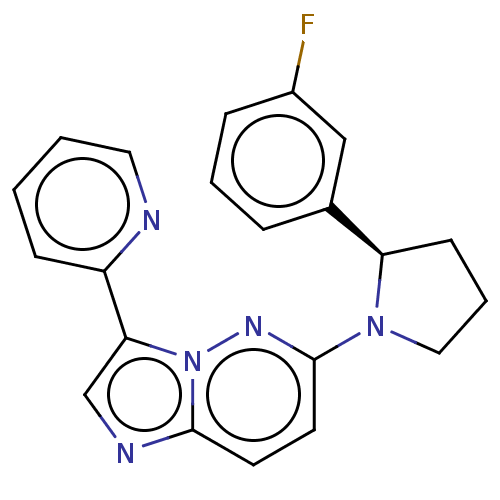 (CHEMBL3582439) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of Tel-fused TRKC (unknown origin) overexpressed in mouse BA/F3 cells assessed as inhibition of cell proliferation after 48 hrs by lucifer... | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM50092079 (CHEMBL3582439) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of Tel-fused TRKB (unknown origin) overexpressed in mouse BA/F3 cells assessed as inhibition of cell proliferation after 48 hrs by lucifer... | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-6 (Homo sapiens (Human)) | BDBM140015 (US8901310, Comparator E) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The glucocorticoid receptor fluorescence polarization ligand binding (GRFP) assay is used to evaluate direct binding of testing compounds to full-len... | US Patent US8901310 (2014) BindingDB Entry DOI: 10.7270/Q2MC8XQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-6 (Homo sapiens (Human)) | BDBM140013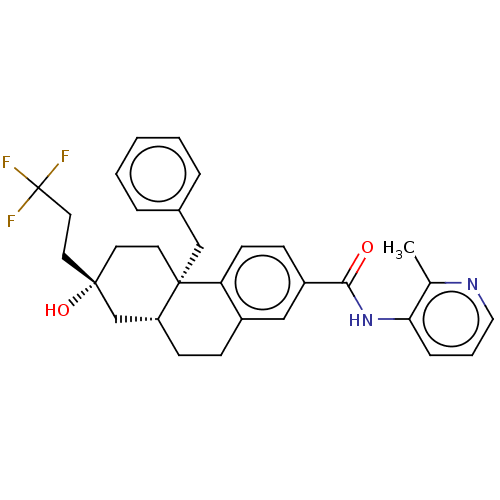 (US8901310, Comparator C) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The glucocorticoid receptor fluorescence polarization ligand binding (GRFP) assay is used to evaluate direct binding of testing compounds to full-len... | US Patent US8901310 (2014) BindingDB Entry DOI: 10.7270/Q2MC8XQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM140015 (US8901310, Comparator E) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.18 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The glucocorticoid receptor fluorescence polarization ligand binding (GRFP) assay is used to evaluate direct binding of testing compounds to full-len... | US Patent US8901310 (2014) BindingDB Entry DOI: 10.7270/Q2MC8XQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM140013 (US8901310, Comparator C) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The glucocorticoid receptor fluorescence polarization ligand binding (GRFP) assay is used to evaluate direct binding of testing compounds to full-len... | US Patent US8901310 (2014) BindingDB Entry DOI: 10.7270/Q2MC8XQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-6 (Homo sapiens (Human)) | BDBM140014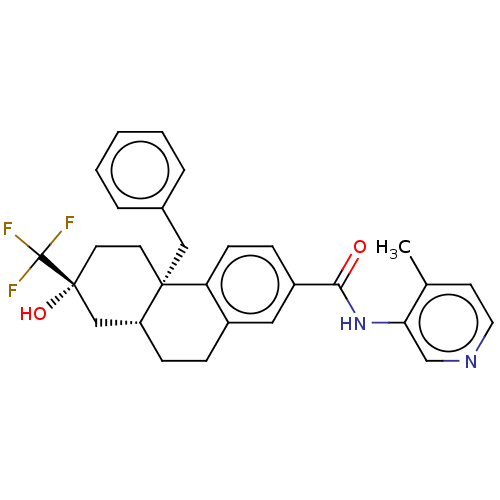 (US8901310, Comparator D) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The glucocorticoid receptor fluorescence polarization ligand binding (GRFP) assay is used to evaluate direct binding of testing compounds to full-len... | US Patent US8901310 (2014) BindingDB Entry DOI: 10.7270/Q2MC8XQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM140009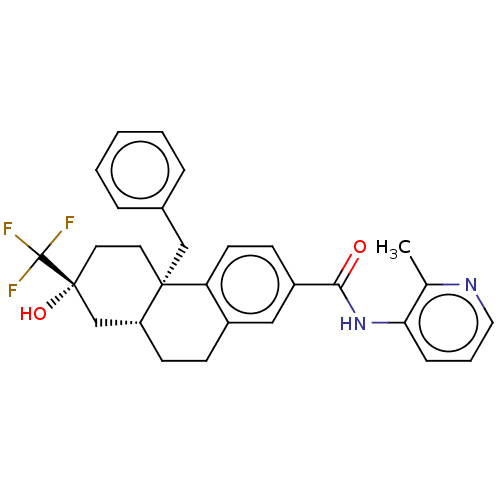 (US8901310, Example 1 ) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The glucocorticoid receptor fluorescence polarization ligand binding (GRFP) assay is used to evaluate direct binding of testing compounds to full-len... | US Patent US8901310 (2014) BindingDB Entry DOI: 10.7270/Q2MC8XQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kinesin-1 heavy chain/Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50538514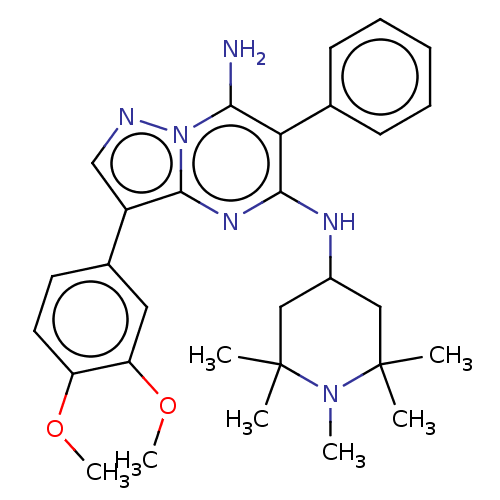 (CHEMBL4644274) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of KIF5B-RET (unknown origin) transfected in mouse Ba/F3 cells assessed as reduction in cell viability incubated for 48 hrs by brightglo-l... | ACS Med Chem Lett 11: 558-565 (2020) Article DOI: 10.1021/acsmedchemlett.0c00015 BindingDB Entry DOI: 10.7270/Q29S1VJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM140016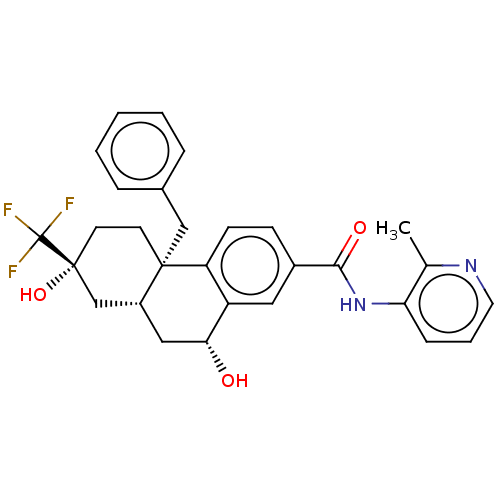 (US8901310, Comparator F) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The glucocorticoid receptor fluorescence polarization ligand binding (GRFP) assay is used to evaluate direct binding of testing compounds to full-len... | US Patent US8901310 (2014) BindingDB Entry DOI: 10.7270/Q2MC8XQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50347100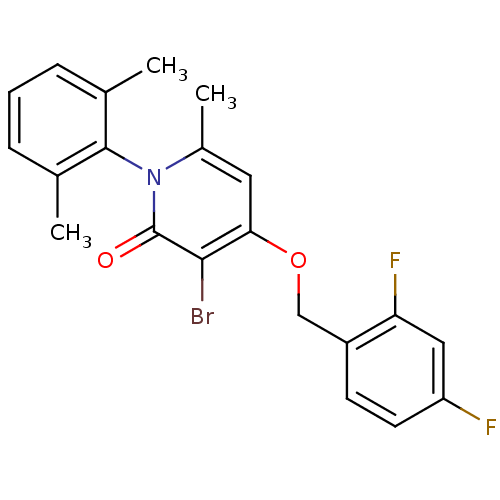 (CHEMBL1797203) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 21: 4059-65 (2011) Article DOI: 10.1016/j.bmcl.2011.04.120 BindingDB Entry DOI: 10.7270/Q25Q4WFH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50092132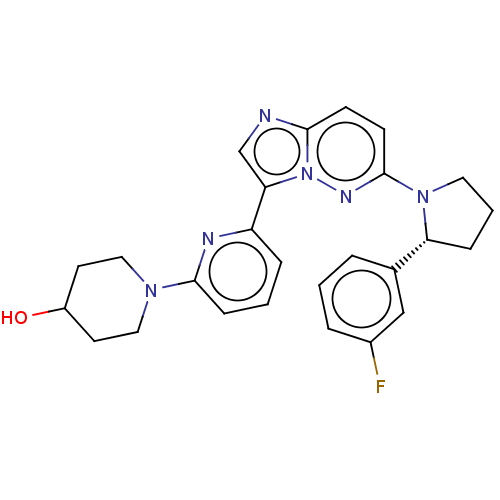 (CHEMBL3582440) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of Tel-fused TRKC (unknown origin) overexpressed in mouse BA/F3 cells assessed as inhibition of cell proliferation after 48 hrs by lucifer... | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50092081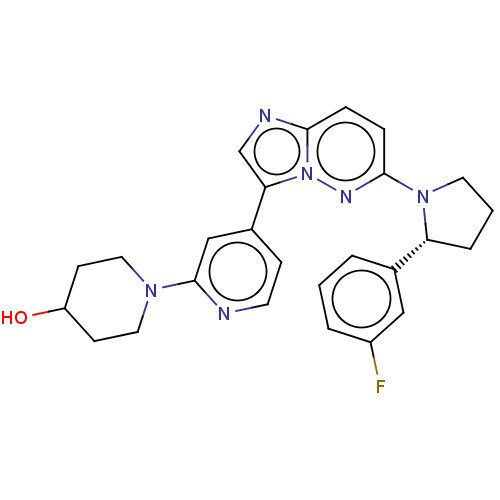 (CHEMBL3582442) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of Tel-fused TRKC (unknown origin) overexpressed in mouse BA/F3 cells assessed as inhibition of cell proliferation after 48 hrs by lucifer... | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50347929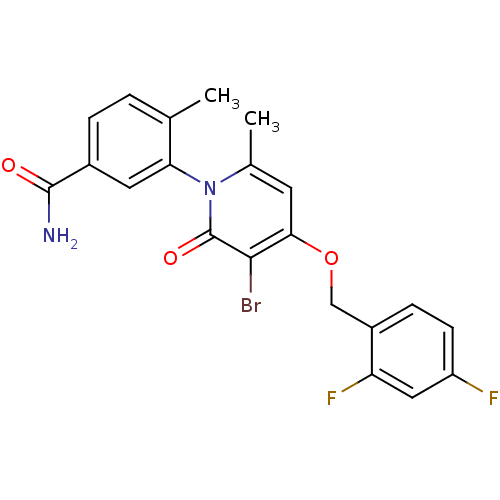 (CHEMBL1802632) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of p38alpha assessed as phosphorylation FITC-labeled Hsp27 after 60 mins by fluorescence based cascade assay | Bioorg Med Chem Lett 21: 4066-71 (2011) Article DOI: 10.1016/j.bmcl.2011.04.121 BindingDB Entry DOI: 10.7270/Q2SB46R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM140014 (US8901310, Comparator D) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.06 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The glucocorticoid receptor fluorescence polarization ligand binding (GRFP) assay is used to evaluate direct binding of testing compounds to full-len... | US Patent US8901310 (2014) BindingDB Entry DOI: 10.7270/Q2MC8XQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-6 (Homo sapiens (Human)) | BDBM140017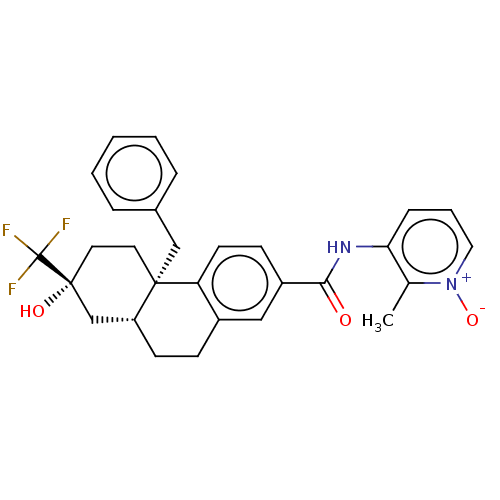 (US8901310, Comparator G) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The glucocorticoid receptor fluorescence polarization ligand binding (GRFP) assay is used to evaluate direct binding of testing compounds to full-len... | US Patent US8901310 (2014) BindingDB Entry DOI: 10.7270/Q2MC8XQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM140017 (US8901310, Comparator G) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The glucocorticoid receptor fluorescence polarization ligand binding (GRFP) assay is used to evaluate direct binding of testing compounds to full-len... | US Patent US8901310 (2014) BindingDB Entry DOI: 10.7270/Q2MC8XQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50314073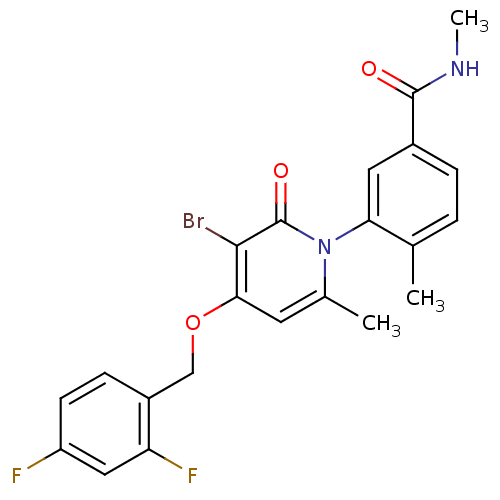 (3-(3-bromo-4-(2,4-difluorobenzyloxy)-6-methyl-2-ox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of p38alpha assessed as phosphorylation FITC-labeled Hsp27 after 60 mins by fluorescence based cascade assay | Bioorg Med Chem Lett 21: 4066-71 (2011) Article DOI: 10.1016/j.bmcl.2011.04.121 BindingDB Entry DOI: 10.7270/Q2SB46R2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50314073 (3-(3-bromo-4-(2,4-difluorobenzyloxy)-6-methyl-2-ox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of p38alpha assessed as phosphorylation FITC-labeled Hsp27 after 60 mins by fluorescence based cascade assay | Bioorg Med Chem Lett 21: 4066-71 (2011) Article DOI: 10.1016/j.bmcl.2011.04.121 BindingDB Entry DOI: 10.7270/Q2SB46R2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50314073 (3-(3-bromo-4-(2,4-difluorobenzyloxy)-6-methyl-2-ox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of p38alpha assessed as phosphorylation FITC-labeled Hsp27 after 60 mins by fluorescence based cascade assay | Bioorg Med Chem Lett 21: 4066-71 (2011) Article DOI: 10.1016/j.bmcl.2011.04.121 BindingDB Entry DOI: 10.7270/Q2SB46R2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Interleukin-6 (Homo sapiens (Human)) | BDBM140019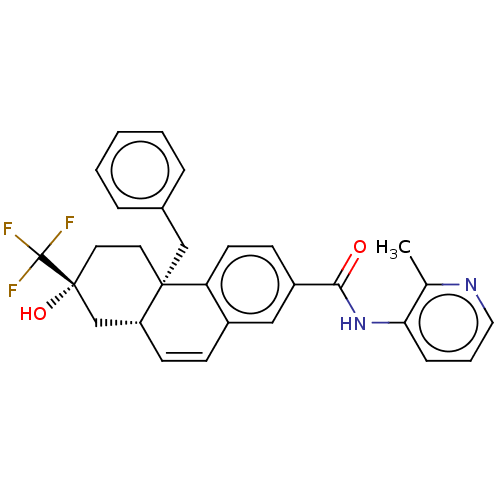 (US8901310, Comparator I) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The glucocorticoid receptor fluorescence polarization ligand binding (GRFP) assay is used to evaluate direct binding of testing compounds to full-len... | US Patent US8901310 (2014) BindingDB Entry DOI: 10.7270/Q2MC8XQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50347938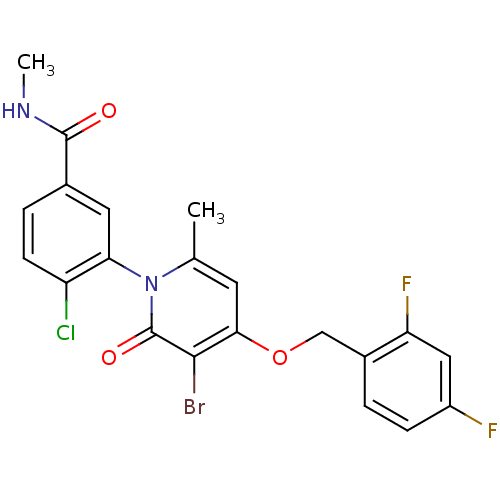 (CHEMBL1802637) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of p38alpha assessed as phosphorylation FITC-labeled Hsp27 after 60 mins by fluorescence based cascade assay | Bioorg Med Chem Lett 21: 4066-71 (2011) Article DOI: 10.1016/j.bmcl.2011.04.121 BindingDB Entry DOI: 10.7270/Q2SB46R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50347096 (CHEMBL1797123) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 21: 4059-65 (2011) Article DOI: 10.1016/j.bmcl.2011.04.120 BindingDB Entry DOI: 10.7270/Q25Q4WFH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50347106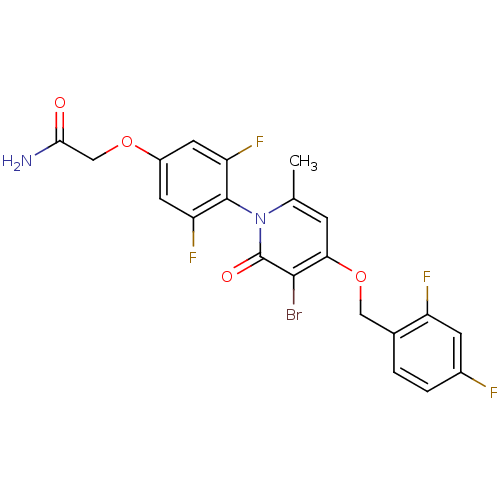 (CHEMBL1797209) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 21: 4059-65 (2011) Article DOI: 10.1016/j.bmcl.2011.04.120 BindingDB Entry DOI: 10.7270/Q25Q4WFH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50092104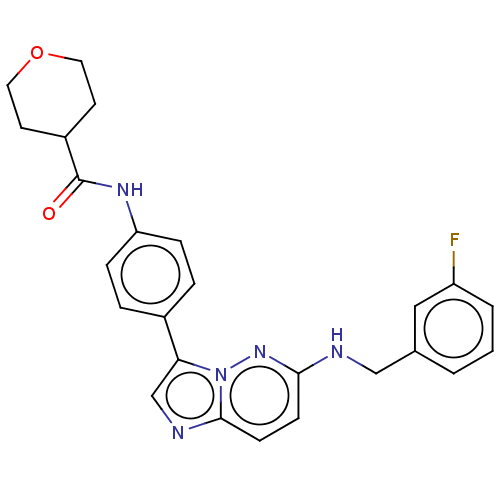 (CHEMBL3582432) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of Tel-fused TRKC (unknown origin) overexpressed in mouse BA/F3 cells assessed as inhibition of cell proliferation after 48 hrs by lucifer... | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50092085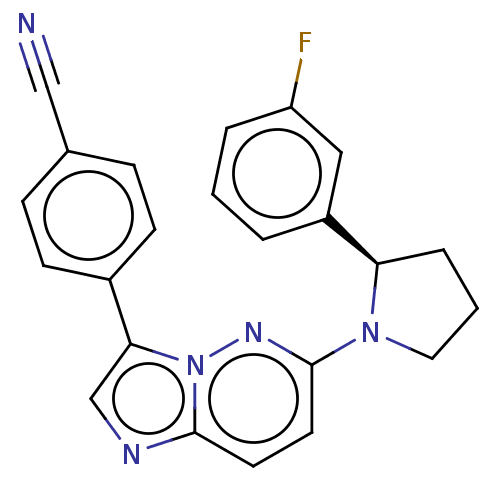 (CHEMBL3582438) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of Tel-fused TRKC (unknown origin) overexpressed in mouse BA/F3 cells assessed as inhibition of cell proliferation after 48 hrs by lucifer... | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50092081 (CHEMBL3582442) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of NGF-activated full length TRKA (unknown origin) expressed in mouse BA/F3 cells assessed as inhibition of cell proliferation | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50092079 (CHEMBL3582439) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of Tel-fused TRKA (unknown origin) overexpressed in mouse BA/F3 cells assessed as inhibition of cell proliferation after 48 hrs by lucifer... | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kinesin-1 heavy chain/Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50538516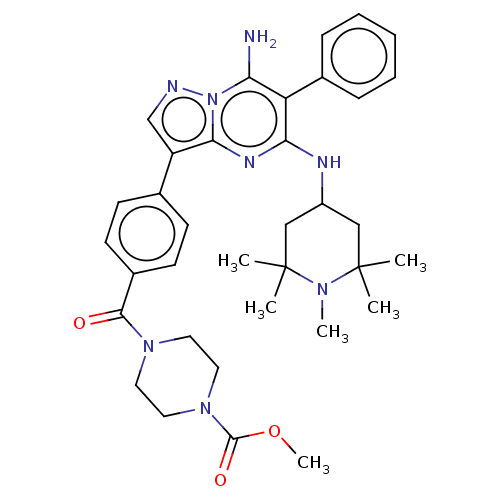 (CHEMBL4643578) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of KIF5B-RET (unknown origin) transfected in mouse Ba/F3 cells assessed as reduction in cell viability incubated for 48 hrs by brightglo-l... | ACS Med Chem Lett 11: 558-565 (2020) Article DOI: 10.1021/acsmedchemlett.0c00015 BindingDB Entry DOI: 10.7270/Q29S1VJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50097956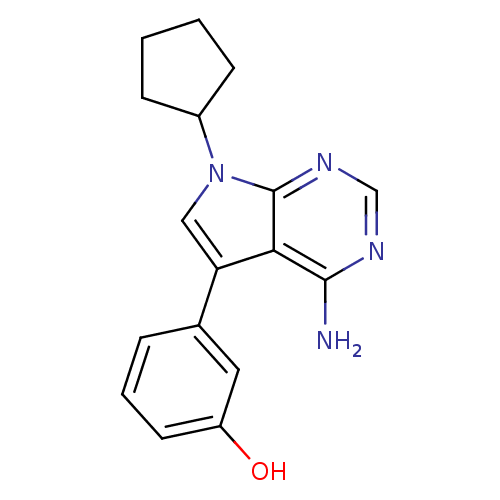 (3-(4-Amino-7-cyclopentyl-7H-pyrrolo[2,3-d]pyrimidi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of RET (unknown origin) expressed in mouse BaF3 cells assessed as reduction in cell proliferation incubated for 48 hrs by cell proliferati... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00450 BindingDB Entry DOI: 10.7270/Q23R0XP8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM50092081 (CHEMBL3582442) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of Tel-fused TRKB (unknown origin) overexpressed in mouse BA/F3 cells assessed as inhibition of cell proliferation after 48 hrs by lucifer... | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50347126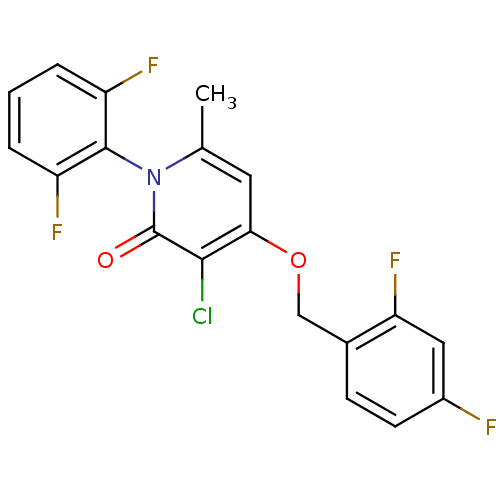 (CHEMBL1797124) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 21: 4059-65 (2011) Article DOI: 10.1016/j.bmcl.2011.04.120 BindingDB Entry DOI: 10.7270/Q25Q4WFH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50347937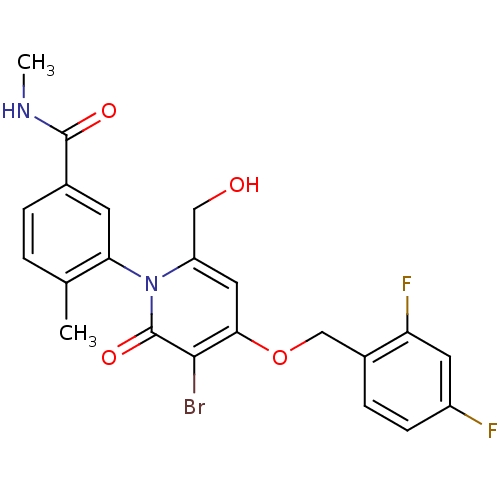 (CHEMBL1802641) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of p38alpha assessed as phosphorylation FITC-labeled Hsp27 after 60 mins by fluorescence based cascade assay | Bioorg Med Chem Lett 21: 4066-71 (2011) Article DOI: 10.1016/j.bmcl.2011.04.121 BindingDB Entry DOI: 10.7270/Q2SB46R2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-6 (Homo sapiens (Human)) | BDBM140012 (US8901310, Comparator B) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The glucocorticoid receptor fluorescence polarization ligand binding (GRFP) assay is used to evaluate direct binding of testing compounds to full-len... | US Patent US8901310 (2014) BindingDB Entry DOI: 10.7270/Q2MC8XQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM50092132 (CHEMBL3582440) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of Tel-fused TRKB (unknown origin) overexpressed in mouse BA/F3 cells assessed as inhibition of cell proliferation after 48 hrs by lucifer... | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NT-3 growth factor receptor (Homo sapiens (Human)) | BDBM50092086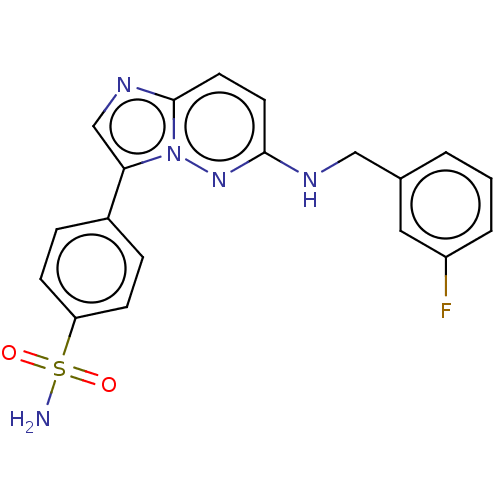 (CHEMBL3582433) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of Tel-fused TRKC (unknown origin) overexpressed in mouse BA/F3 cells assessed as inhibition of cell proliferation after 48 hrs by lucifer... | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| High affinity nerve growth factor receptor (Homo sapiens (Human)) | BDBM50092081 (CHEMBL3582442) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of Tel-fused TRKA (unknown origin) overexpressed in mouse BA/F3 cells assessed as inhibition of cell proliferation after 48 hrs by lucifer... | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-6 (Homo sapiens (Human)) | BDBM19190 ((1S,2R,10S,11S,14R,15S,17S)-14,17-dihydroxy-14-(2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The glucocorticoid receptor fluorescence polarization ligand binding (GRFP) assay is used to evaluate direct binding of testing compounds to full-len... | US Patent US8901310 (2014) BindingDB Entry DOI: 10.7270/Q2MC8XQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-6 (Homo sapiens (Human)) | BDBM140018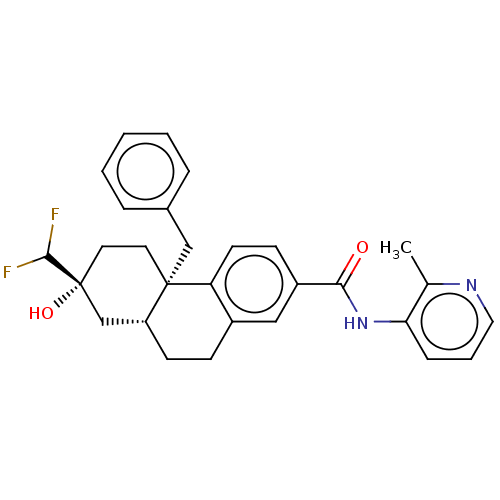 (US8901310, Comparator H) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description The glucocorticoid receptor fluorescence polarization ligand binding (GRFP) assay is used to evaluate direct binding of testing compounds to full-len... | US Patent US8901310 (2014) BindingDB Entry DOI: 10.7270/Q2MC8XQM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 731 total ) | Next | Last >> |