

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (Homo sapiens (Human)) | BDBM50279775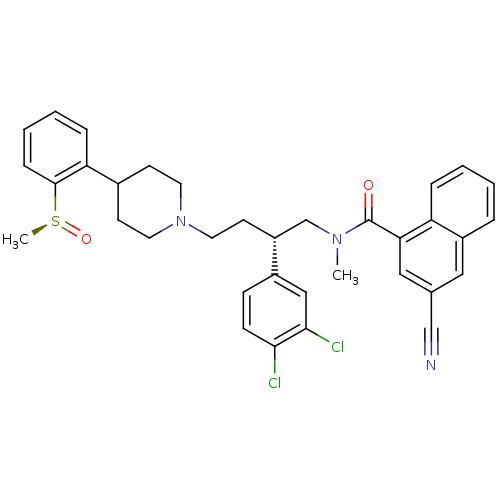 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Binding affinity against human cloned Tachykinin receptor 1 expressed in MEL cells | Bioorg Med Chem Lett 11: 2769-73 (2001) BindingDB Entry DOI: 10.7270/Q2SJ1M4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50105595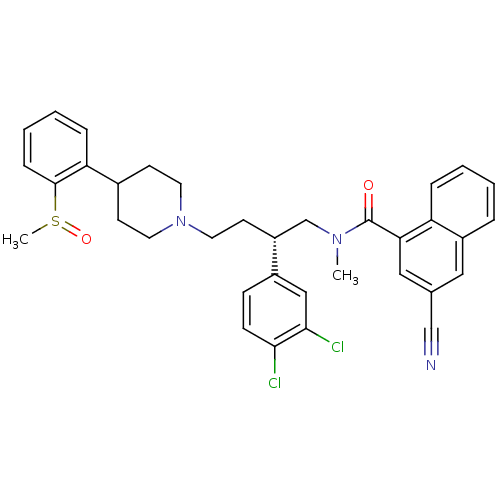 ((R,S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 307-15 (2001) BindingDB Entry DOI: 10.7270/Q2H130JX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50279775 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]SP to Tachykinin receptor 1 of human tachykinin receptor expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50118098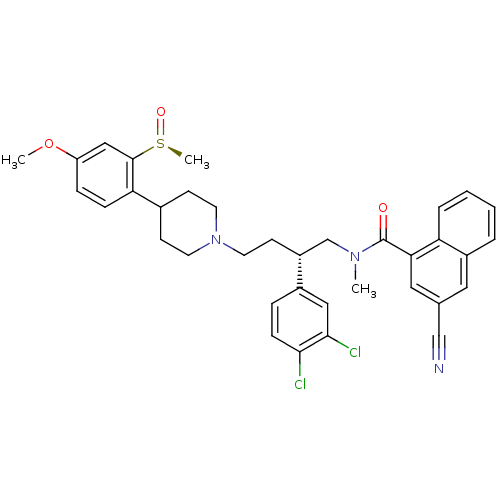 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]SP to Tachykinin receptor 1 of human tachykinin receptor expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50118100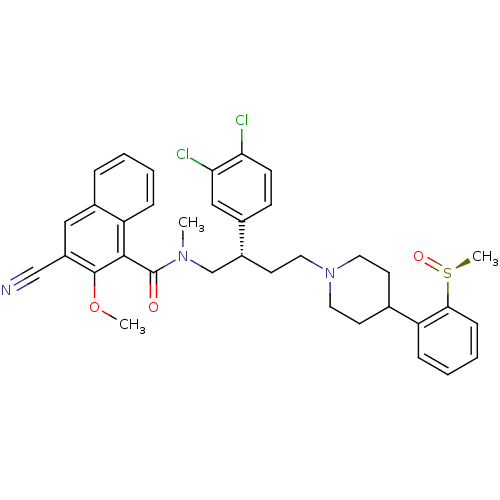 (3-Cyano-2-methoxy-naphthalene-1-carboxylic acid ((...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]SP to Tachykinin receptor 1 of human tachykinin receptor expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50118099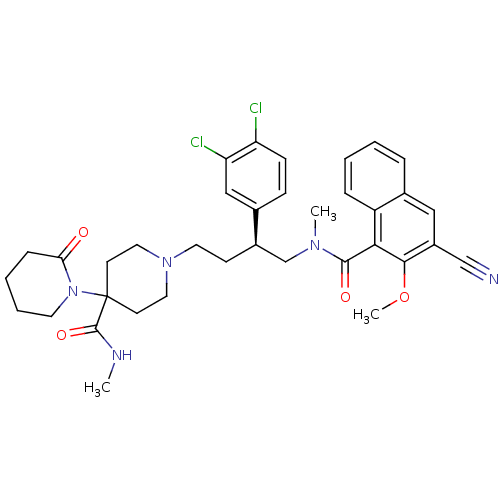 (1'-[4-[(3-Cyano-2-methoxy-naphthalene-1-carbonyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]SP to Tachykinin receptor 1 of human tachykinin receptor expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50175494 (1-{2-[(R)-3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Binding affinity against human cloned Tachykinin receptor 1 expressed in MEL cells | Bioorg Med Chem Lett 11: 2769-73 (2001) BindingDB Entry DOI: 10.7270/Q2SJ1M4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50118098 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]NKA to human Tachykinin receptor 2 (NK2) in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50138823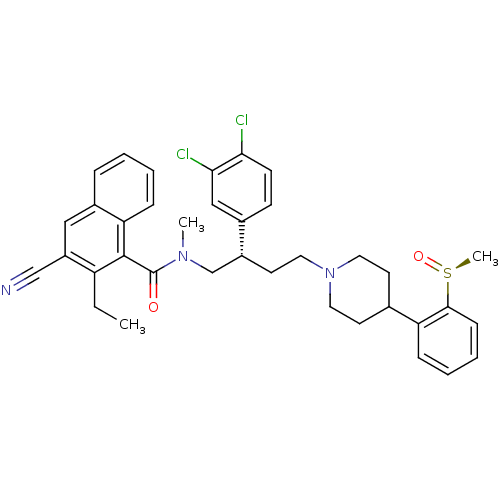 (3-Cyano-2-ethyl-naphthalene-1-carboxylic acid ((S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description In vitro binding affinity towards human Tachykinin receptor 1 was determined by using [3H]-SP as a radioligand | J Med Chem 47: 519-29 (2004) Article DOI: 10.1021/jm030197g BindingDB Entry DOI: 10.7270/Q2FJ2G6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50279775 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Binding affinity against human cloned Tachykinin receptor 2 expressed in MEL cells | Bioorg Med Chem Lett 11: 2769-73 (2001) BindingDB Entry DOI: 10.7270/Q2SJ1M4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50105595 ((R,S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by PDSP Ki Database | J Pharmacol Exp Ther 298: 307-15 (2001) BindingDB Entry DOI: 10.7270/Q2H130JX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50279775 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]NKA to human Tachykinin receptor 2 (NK2) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50409575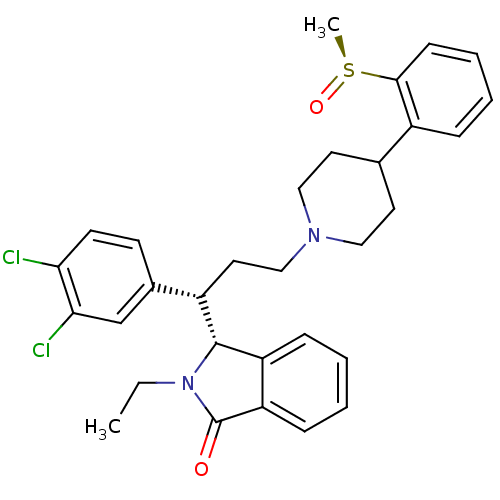 (CHEMBL339767 | ZD-7944) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]NKA to human Tachykinin receptor 2 (NK2) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50175494 (1-{2-[(R)-3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Binding affinity against human cloned Tachykinin receptor 2 expressed in MEL cells | Bioorg Med Chem Lett 11: 2769-73 (2001) BindingDB Entry DOI: 10.7270/Q2SJ1M4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50138823 (3-Cyano-2-ethyl-naphthalene-1-carboxylic acid ((S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description In vitro binding affinity towards human Tachykinin receptor 1 was determined by using [3H]-SP as a radioligand | J Med Chem 47: 519-29 (2004) Article DOI: 10.1021/jm030197g BindingDB Entry DOI: 10.7270/Q2FJ2G6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50138823 (3-Cyano-2-ethyl-naphthalene-1-carboxylic acid ((S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description In vitro binding affinity towards human Tachykinin receptor 1 was determined by using [3H]-SP as a radioligand | J Med Chem 47: 519-29 (2004) Article DOI: 10.1021/jm030197g BindingDB Entry DOI: 10.7270/Q2FJ2G6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50138823 (3-Cyano-2-ethyl-naphthalene-1-carboxylic acid ((S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description In vitro binding affinity towards human Tachykinin receptor 1 was determined by using [3H]-SP as a radioligand | J Med Chem 47: 519-29 (2004) Article DOI: 10.1021/jm030197g BindingDB Entry DOI: 10.7270/Q2FJ2G6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50138823 (3-Cyano-2-ethyl-naphthalene-1-carboxylic acid ((S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description In vitro binding affinity towards human Tachykinin receptor 1 was determined by using [3H]-SP as a radioligand | J Med Chem 47: 519-29 (2004) Article DOI: 10.1021/jm030197g BindingDB Entry DOI: 10.7270/Q2FJ2G6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50118099 (1'-[4-[(3-Cyano-2-methoxy-naphthalene-1-carbonyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [125I]MPNI to human Tachykinin receptor 3 (NK3) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50138823 (3-Cyano-2-ethyl-naphthalene-1-carboxylic acid ((S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description In vitro binding affinity towards human Tachykinin receptor 2 was determined by using [125I]-NKA as a radioligand | J Med Chem 47: 519-29 (2004) Article DOI: 10.1021/jm030197g BindingDB Entry DOI: 10.7270/Q2FJ2G6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50138823 (3-Cyano-2-ethyl-naphthalene-1-carboxylic acid ((S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description In vitro binding affinity towards human Tachykinin receptor 2 was determined by using [125I]-NKA as a radioligand | J Med Chem 47: 519-29 (2004) Article DOI: 10.1021/jm030197g BindingDB Entry DOI: 10.7270/Q2FJ2G6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50138823 (3-Cyano-2-ethyl-naphthalene-1-carboxylic acid ((S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description In vitro binding affinity towards human Tachykinin receptor 2 was determined by using [125I]-NKA as a radioligand | J Med Chem 47: 519-29 (2004) Article DOI: 10.1021/jm030197g BindingDB Entry DOI: 10.7270/Q2FJ2G6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50138823 (3-Cyano-2-ethyl-naphthalene-1-carboxylic acid ((S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description In vitro binding affinity towards human Tachykinin receptor 2 was determined by using [125I]-NKA as a radioligand | J Med Chem 47: 519-29 (2004) Article DOI: 10.1021/jm030197g BindingDB Entry DOI: 10.7270/Q2FJ2G6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50138823 (3-Cyano-2-ethyl-naphthalene-1-carboxylic acid ((S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description In vitro binding affinity towards human Tachykinin receptor 2 was determined by using [125I]-NKA as a radioligand | J Med Chem 47: 519-29 (2004) Article DOI: 10.1021/jm030197g BindingDB Entry DOI: 10.7270/Q2FJ2G6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50118099 (1'-[4-[(3-Cyano-2-methoxy-naphthalene-1-carbonyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]NKA to human Tachykinin receptor 2 (NK2) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50118098 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [125I]MPNI to human Tachykinin receptor 3 (NK3) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50118100 (3-Cyano-2-methoxy-naphthalene-1-carboxylic acid ((...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]NKA to human Tachykinin receptor 2 (NK2) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50279775 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [125I]MPNI to human Tachykinin receptor 3 (NK3) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50279775 ((S)-3-Cyano-naphthalene-1-carboxylic acid {2-(3,4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Binding affinity against human cloned Tachykinin receptor 3 expressed in MEL cells | Bioorg Med Chem Lett 11: 2769-73 (2001) BindingDB Entry DOI: 10.7270/Q2SJ1M4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50138823 (3-Cyano-2-ethyl-naphthalene-1-carboxylic acid ((S)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description In vitro binding affinity towards human Tachykinin receptor 3 was determined by using [125I]-MePhe-NKB as a radioligand | J Med Chem 47: 519-29 (2004) Article DOI: 10.1021/jm030197g BindingDB Entry DOI: 10.7270/Q2FJ2G6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50175494 (1-{2-[(R)-3-(3,4-Dichloro-phenyl)-1-(3,4,5-trimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Binding affinity against human cloned Tachykinin receptor 3 expressed in MEL cells | Bioorg Med Chem Lett 11: 2769-73 (2001) BindingDB Entry DOI: 10.7270/Q2SJ1M4W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50118100 (3-Cyano-2-methoxy-naphthalene-1-carboxylic acid ((...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [125I]MPNI to human Tachykinin receptor 3 (NK3) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50409575 (CHEMBL339767 | ZD-7944) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [3H]SP to human Tachykinin receptor 1 expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM50409575 (CHEMBL339767 | ZD-7944) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP Curated by ChEMBL | Assay Description Inhibition of binding of [125I]MPNI to human Tachykinin receptor 3 (NK3) expressed in mouse erythroleukemia cells | J Med Chem 45: 3972-83 (2002) BindingDB Entry DOI: 10.7270/Q2862H6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248583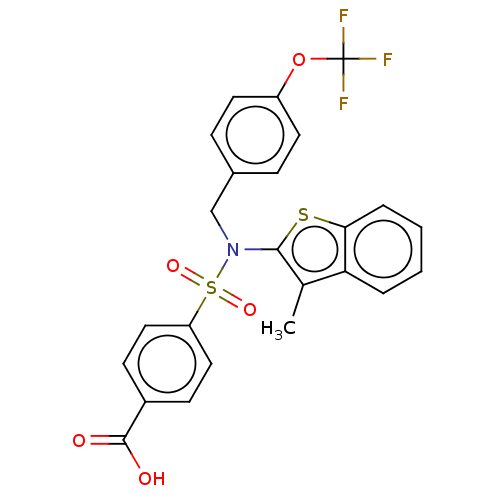 (US9434711, 496) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.183 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica, N.V. US Patent | Assay Description For patch clamp experiments, HEK293 cells are stably transfected with canine TRPM8 and cultured in DMEM supplemented with 10% fetal bovine serum, 100... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM50036555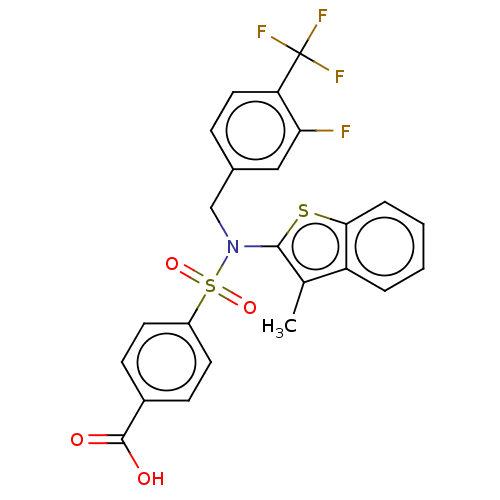 (CHEMBL3353610 | US9434711, 497) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248583 (US9434711, 496) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.554 | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Pharmaceutica, N.V. US Patent | Assay Description For patch clamp experiments, HEK293 cells are stably transfected with canine TRPM8 and cultured in DMEM supplemented with 10% fetal bovine serum, 100... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248370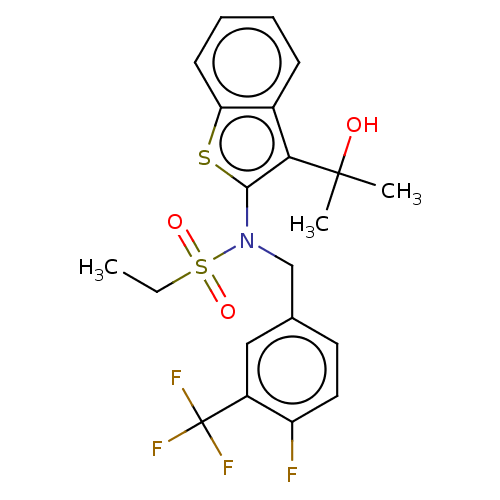 (US9434711, 227) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248576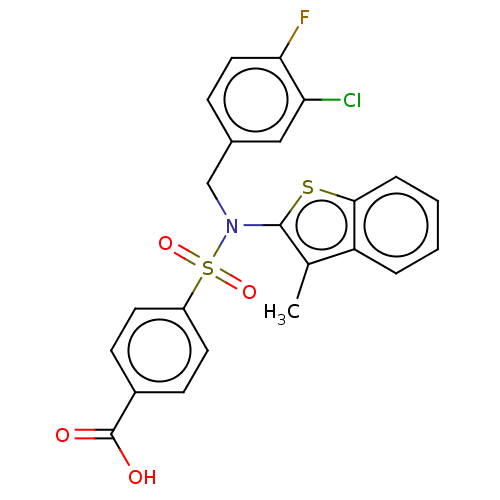 (US9434711, 489) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444549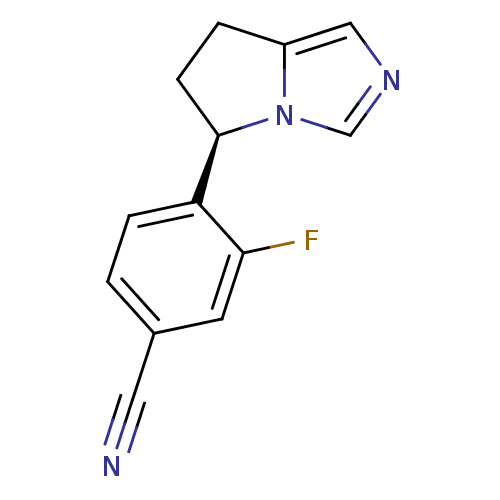 (CHEMBL3099695) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate after 2 hrs by scintillation proximity assay | J Med Chem 58: 9382-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b01545 BindingDB Entry DOI: 10.7270/Q2CC13QV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248838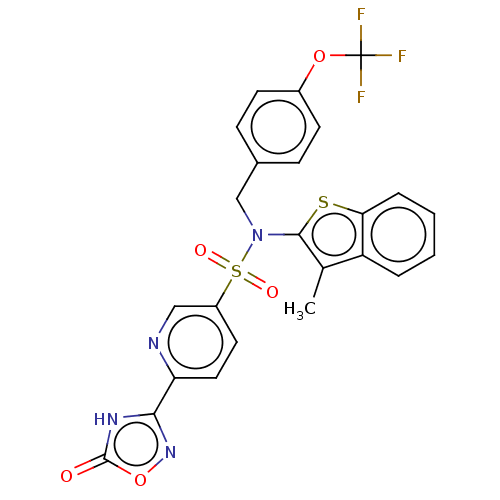 (US9434711, 808) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248599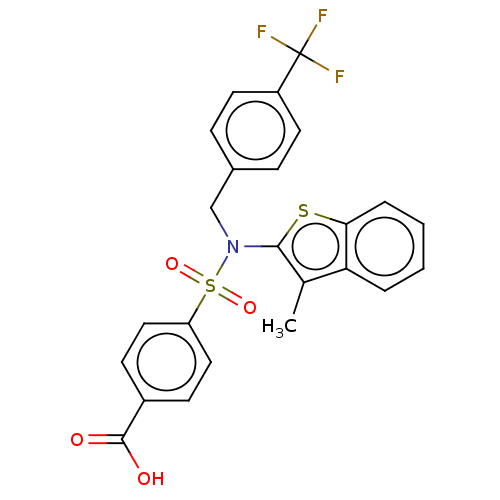 (US9434711, 518) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248580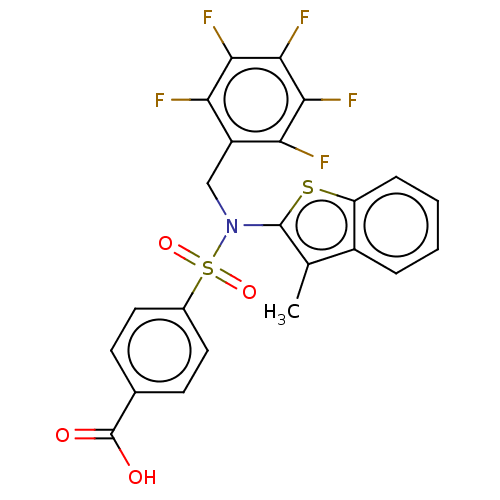 (US9434711, 493) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248480 (US9434711, 361) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248583 (US9434711, 496) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248426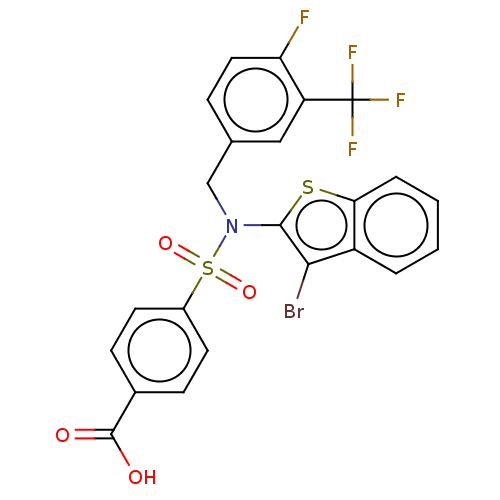 (US9434711, 292) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM50426573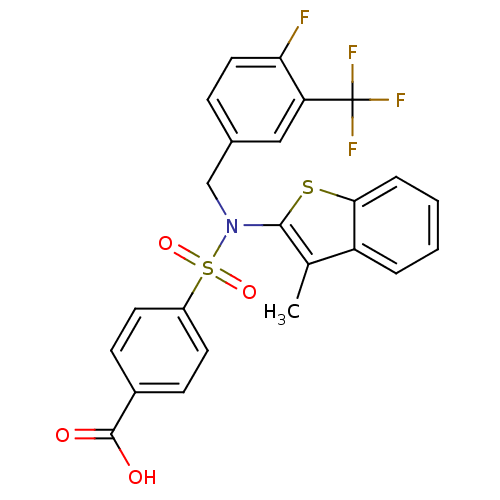 (CHEMBL2324349 | US9434711, 306) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000806 (13-[2-Amino-3-(4-hydroxy-2-methyl-phenyl)-propiony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity in comparison with [3H]- DPDPE (opioid receptor delta selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248389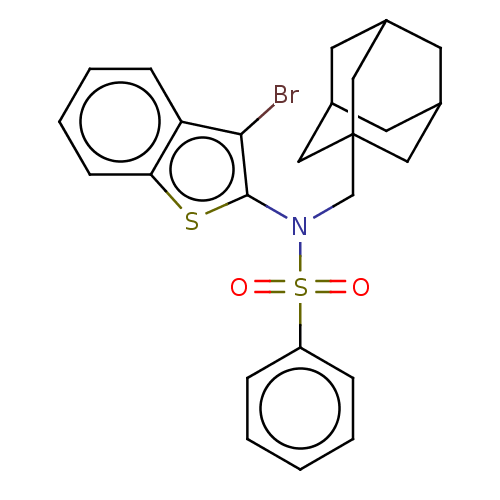 (US9434711, 249) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential M8 protein (Canis lupus familiaris (Dog)) | BDBM248581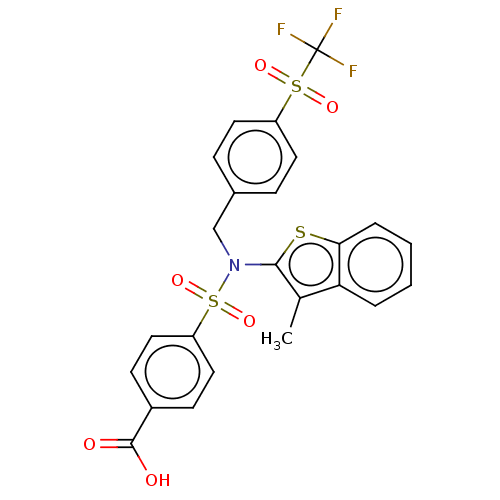 (US9434711, 494) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | 37 |
Janssen Pharmaceutica, N.V. US Patent | Assay Description The functional activity of compounds of the formula (I) was determined by measuring changes in intracellular calcium concentration using a Ca2+-sensi... | US Patent US9434711 (2016) BindingDB Entry DOI: 10.7270/Q2H41QB6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 924 total ) | Next | Last >> |