

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110121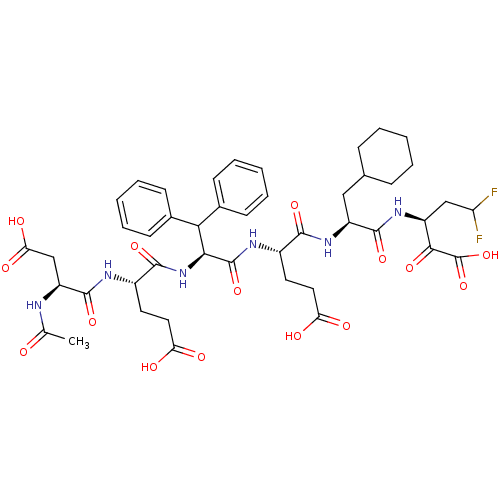 (3-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110121 (3-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110117 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110117 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110122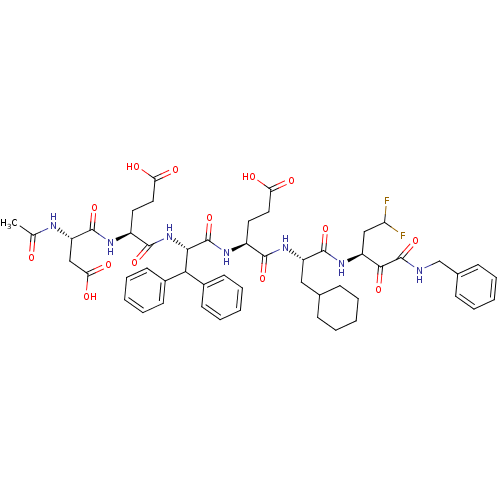 ((S)-4-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110120 (3-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110126 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150997 (4-{2-[(S)-4,4-Difluoro-2-({(2S,4R)-1-((S)-2-isobut...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110125 (2-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150993 (4-[2-(4,4-Difluoro-2-{[(S)-(R)-1-(2-isobutoxycarbo...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50084685 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110125 (2-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 705-8 (2002) BindingDB Entry DOI: 10.7270/Q24X572V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110123 (2-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150992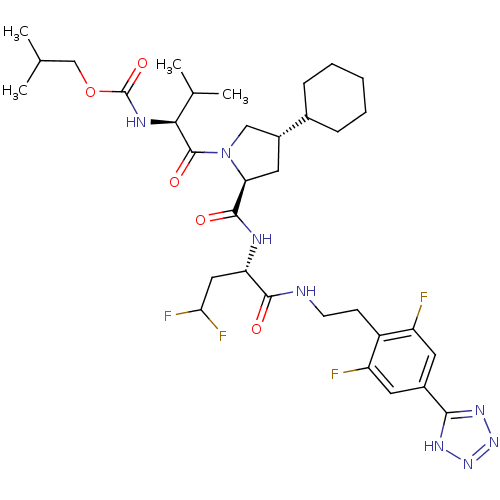 (CHEMBL366279 | {(S)-1-[(2S,4S)-4-Cyclohexyl-2-((S)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150991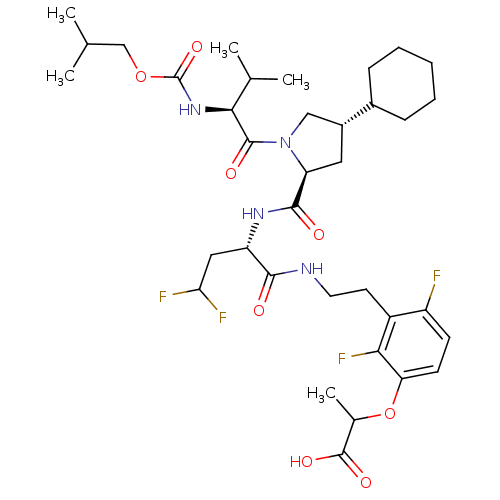 (2-{3-[2-((S)-2-{[(2S,4S)-4-Cyclohexyl-1-((S)-2-iso...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110124 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-[1-(1...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150989 (CHEMBL362406 | {3-[2-((S)-2-{[(2S,4S)-4-Cyclohexyl...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 (Homo sapiens (Human)) | BDBM50551201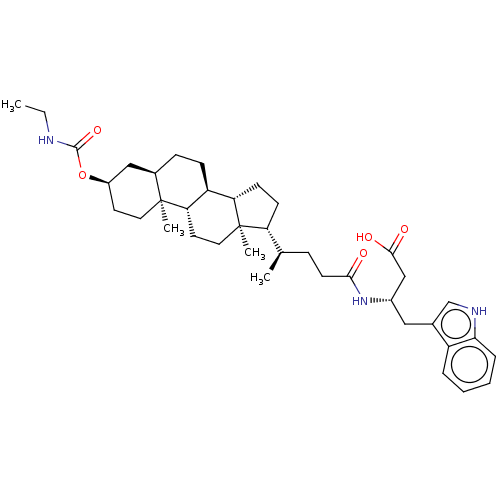 (CHEMBL4761556) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of biotinylated ephrin-A1-Fc binding to EphA2 (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112083 BindingDB Entry DOI: 10.7270/Q2X35233 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150990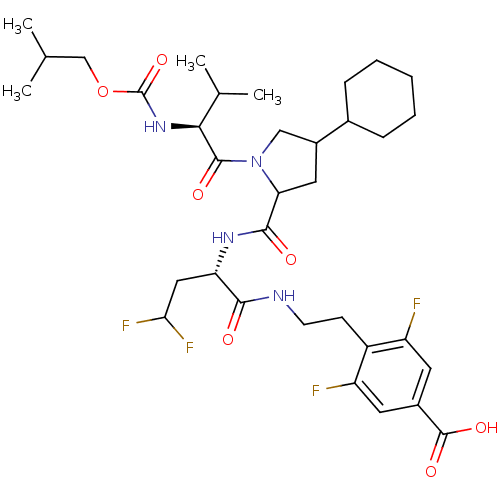 (4-[2-((S)-2-{[(R)-4-Cyclohexyl-1-((S)-2-isobutoxyc...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150986 ((E)-3-{3-[2-((S)-4,4-Difluoro-2-{[(2S,4S)-1-((S)-2...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 (Homo sapiens (Human)) | BDBM50234957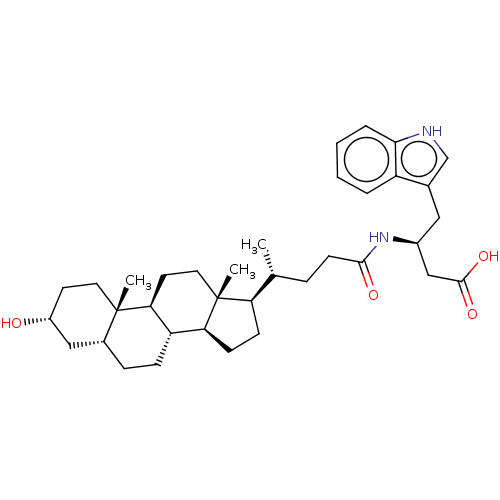 (CHEMBL3735125) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of biotinylated ephrin-A1-Fc binding to EphA2 (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112083 BindingDB Entry DOI: 10.7270/Q2X35233 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150984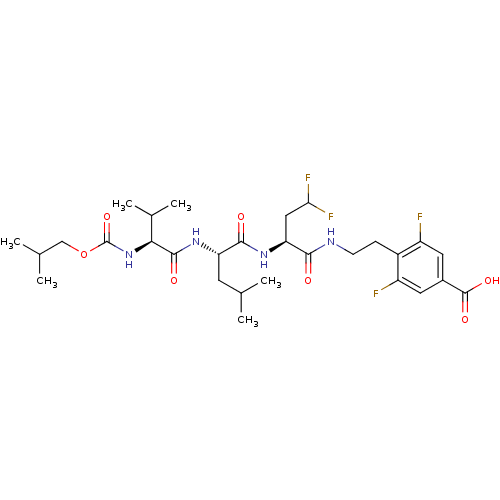 (4-(2-{(S)-4,4-Difluoro-2-[(S)-2-((S)-2-isobutoxyca...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150994 ((E)-3-{3-[2-((S)-4,4-Difluoro-2-{[(2S,4S)-1-((S)-2...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150995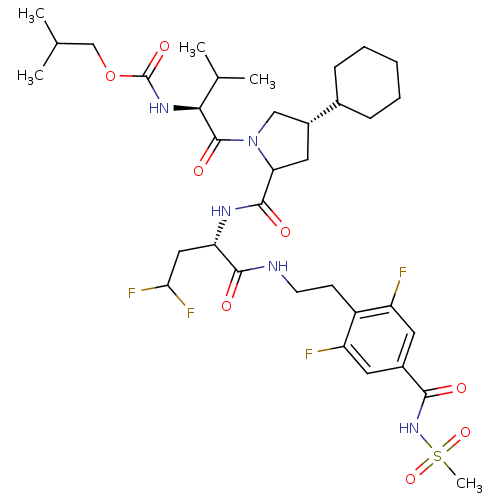 (CHEMBL263183 | [(S)-1-((2S,4S)-4-Cyclohexyl-2-{(S)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50144349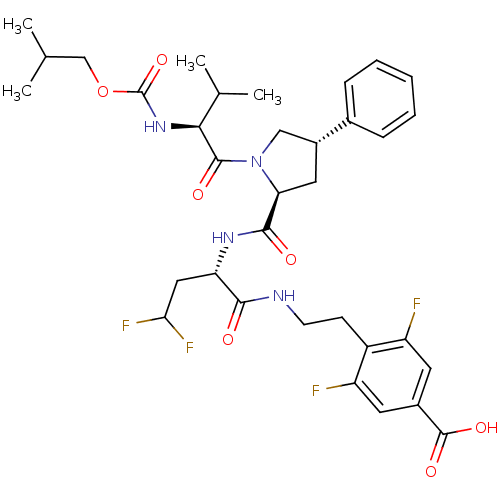 (4-[2-((S)-4,4-Difluoro-2-{[(2S,4S)-1-(2-isobutoxyc...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150983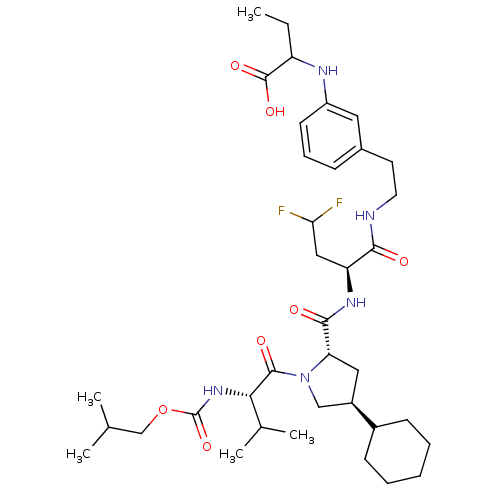 (2-{3-[2-((S)-2-{[(2S,4S)-4-Cyclohexyl-1-((S)-2-iso...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50122891 (4-(2-{2-[2-(4-Carboxy-2-isobutoxycarbonylamino-but...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome Curated by ChEMBL | Assay Description Biinding affinity of the compound for enzyme NS3 protease wild-type | J Med Chem 46: 345-8 (2003) Article DOI: 10.1021/jm025594q BindingDB Entry DOI: 10.7270/Q2CF9PF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110128 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-[1-(1...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110127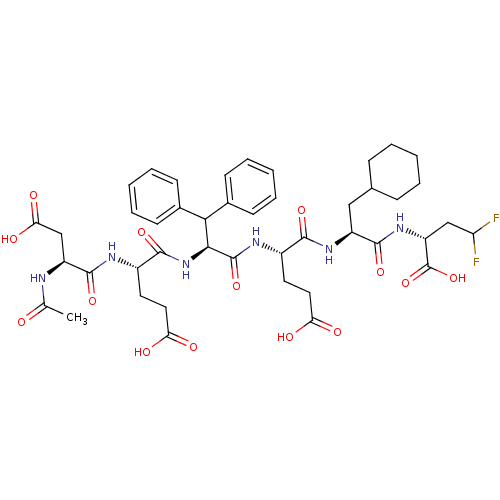 (2-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150996 (CHEMBL181465 | {3-[2-((S)-2-{[(2S,4S)-4-Cyclohexyl...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110118 (2-[2-(2-{2-[2-(2-Acetylamino-3-carboxy-propionylam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ephrin type-A receptor 2 (Homo sapiens (Human)) | BDBM50234948 (CHEMBL4078429) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of biotinylated ephrin-A1-Fc binding to EphA2 (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112083 BindingDB Entry DOI: 10.7270/Q2X35233 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50122891 (4-(2-{2-[2-(4-Carboxy-2-isobutoxycarbonylamino-but...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome Curated by ChEMBL | Assay Description Binding affinity for enzyme K136R | J Med Chem 46: 345-8 (2003) Article DOI: 10.1021/jm025594q BindingDB Entry DOI: 10.7270/Q2CF9PF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150985 (CHEMBL362404 | {3-[2-((S)-4,4-Difluoro-2-{[(2S,4S)...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50144357 (3-Chloro-4-(2-{(S)-4,4-difluoro-2-[(S)-2-((S)-2-is...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150988 ((E)-3-{3-[2-((S)-4,4-Difluoro-2-{[(2S,4S)-1-((S)-2...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50122891 (4-(2-{2-[2-(4-Carboxy-2-isobutoxycarbonylamino-but...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome Curated by ChEMBL | Assay Description Biinding affinity of the compound for enzyme K136M | J Med Chem 46: 345-8 (2003) Article DOI: 10.1021/jm025594q BindingDB Entry DOI: 10.7270/Q2CF9PF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50110119 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-[1-(3...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM, MRL Rome Curated by ChEMBL | Assay Description Inhibition of hepatitis C virus (HCV) NS3/NS4A serine protease | Bioorg Med Chem Lett 12: 701-4 (2002) BindingDB Entry DOI: 10.7270/Q28P5ZSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50122883 (3-Chloro-4-(2-{4,4-difluoro-2-[2-(2-isobutoxycarbo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome Curated by ChEMBL | Assay Description Biinding affinity of the compound for wild type NS3 protease | J Med Chem 46: 345-8 (2003) Article DOI: 10.1021/jm025594q BindingDB Entry DOI: 10.7270/Q2CF9PF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50122883 (3-Chloro-4-(2-{4,4-difluoro-2-[2-(2-isobutoxycarbo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome Curated by ChEMBL | Assay Description Biinding affinity of the compound for NS3 protease K136R | J Med Chem 46: 345-8 (2003) Article DOI: 10.1021/jm025594q BindingDB Entry DOI: 10.7270/Q2CF9PF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50122883 (3-Chloro-4-(2-{4,4-difluoro-2-[2-(2-isobutoxycarbo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome Curated by ChEMBL | Assay Description Biinding affinity of the compound NS3 protease K136M | J Med Chem 46: 345-8 (2003) Article DOI: 10.1021/jm025594q BindingDB Entry DOI: 10.7270/Q2CF9PF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150987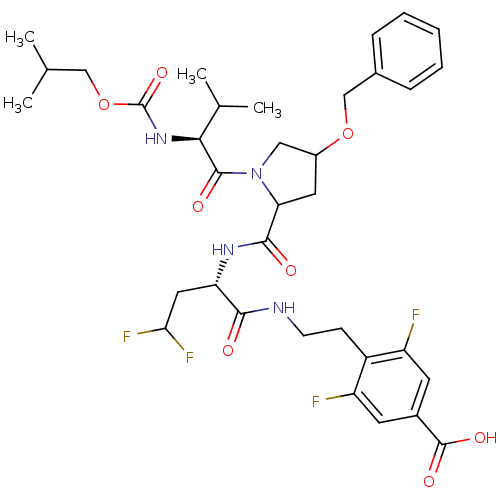 (4-[2-((S)-2-{[(R)-4-Benzyloxy-1-((S)-2-isobutoxyca...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein/Non-structural protein 4A (Hepatitis C virus) | BDBM50150987 (4-[2-((S)-2-{[(R)-4-Benzyloxy-1-((S)-2-isobutoxyca...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Hepatitis C virus Non structural protein 3 serine protease/Non structural protein 4A serine protease | Bioorg Med Chem Lett 14: 4575-9 (2004) Article DOI: 10.1016/j.bmcl.2004.05.093 BindingDB Entry DOI: 10.7270/Q27S7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50122882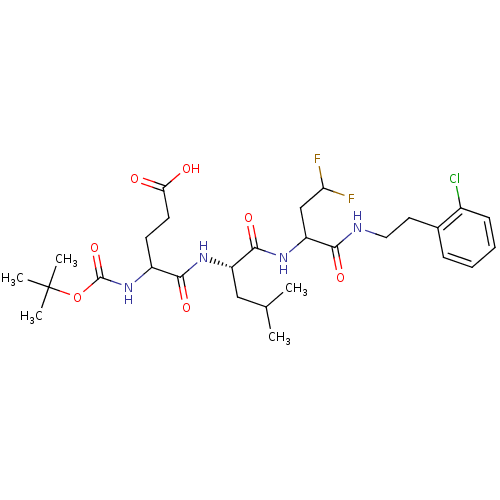 (4-(1-{1-[2-(2-Chloro-phenyl)-ethylcarbamoyl]-2-mer...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome Curated by ChEMBL | Assay Description Biinding affinity of the compound for enzyme NS3 protease wild-type | J Med Chem 46: 345-8 (2003) Article DOI: 10.1021/jm025594q BindingDB Entry DOI: 10.7270/Q2CF9PF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50122882 (4-(1-{1-[2-(2-Chloro-phenyl)-ethylcarbamoyl]-2-mer...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome Curated by ChEMBL | Assay Description Binding affinity for enzyme K136R | J Med Chem 46: 345-8 (2003) Article DOI: 10.1021/jm025594q BindingDB Entry DOI: 10.7270/Q2CF9PF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50122882 (4-(1-{1-[2-(2-Chloro-phenyl)-ethylcarbamoyl]-2-mer...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome Curated by ChEMBL | Assay Description Biinding affinity of the compound for enzyme K136M | J Med Chem 46: 345-8 (2003) Article DOI: 10.1021/jm025594q BindingDB Entry DOI: 10.7270/Q2CF9PF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50122882 (4-(1-{1-[2-(2-Chloro-phenyl)-ethylcarbamoyl]-2-mer...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome Curated by ChEMBL | Assay Description Binding affinity for enzyme human leukocyte HLE | J Med Chem 46: 345-8 (2003) Article DOI: 10.1021/jm025594q BindingDB Entry DOI: 10.7270/Q2CF9PF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50122891 (4-(2-{2-[2-(4-Carboxy-2-isobutoxycarbonylamino-but...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MRL Rome Curated by ChEMBL | Assay Description Binding affinity for enzyme human leukocyte HLE | J Med Chem 46: 345-8 (2003) Article DOI: 10.1021/jm025594q BindingDB Entry DOI: 10.7270/Q2CF9PF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50383051 (CHEMBL2031302) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Parma Curated by ChEMBL | Assay Description Inhibition of human recombinant EGFR using Ulight-CAGAGAIETDKEYYTVKD as substrate after 15 mins by time-resolved fluorimetric analysis | J Med Chem 55: 2251-64 (2012) Article DOI: 10.1021/jm201507x BindingDB Entry DOI: 10.7270/Q20K29M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50383049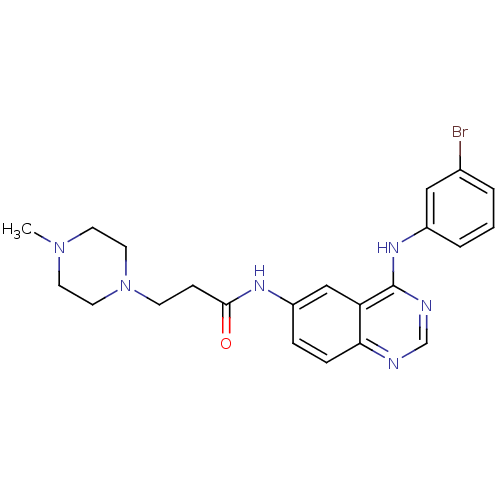 (CHEMBL2031300) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Parma Curated by ChEMBL | Assay Description Inhibition of human recombinant EGFR using Ulight-CAGAGAIETDKEYYTVKD as substrate after 15 mins by time-resolved fluorimetric analysis | J Med Chem 55: 2251-64 (2012) Article DOI: 10.1021/jm201507x BindingDB Entry DOI: 10.7270/Q20K29M1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1294 total ) | Next | Last >> |