

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caspase-1 (Homo sapiens (Human)) | BDBM50071542 ((S)-3-(2-Acetylamino-3-methyl-butyrylamino)-5-[2-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against N-His (D381E) Interleukin -1 beta converting enzyme (resynthesized compound) | Bioorg Med Chem Lett 8: 2309-14 (1999) BindingDB Entry DOI: 10.7270/Q2F47N9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50071543 ((S)-3-(2-Acetylamino-3-methyl-butyrylamino)-4-oxo-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against N-His (D381E) Interleukin -1 beta converting enzyme (resynthesized compound) | Bioorg Med Chem Lett 8: 2309-14 (1999) BindingDB Entry DOI: 10.7270/Q2F47N9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50071541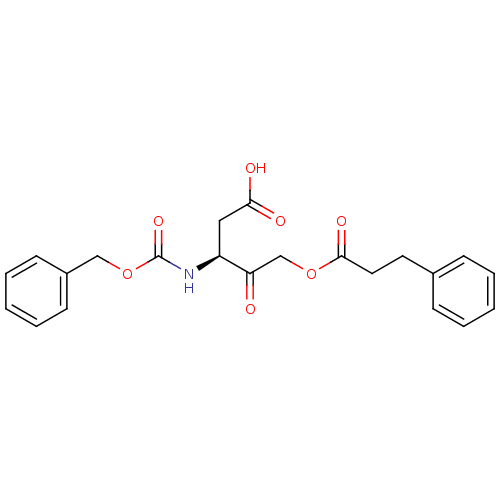 ((S)-3-(benzyloxycarbonylamino)-4-oxo-5-(3-phenylpr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Compound was tested for binding affinity against N-His (D381E) Interleukin -1 beta converting enzyme | Bioorg Med Chem Lett 8: 2309-14 (1999) BindingDB Entry DOI: 10.7270/Q2F47N9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50071543 ((S)-3-(2-Acetylamino-3-methyl-butyrylamino)-4-oxo-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against N-His (D381E) Interleukin -1 beta converting enzyme (combinatorially prepared compound) | Bioorg Med Chem Lett 8: 2309-14 (1999) BindingDB Entry DOI: 10.7270/Q2F47N9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50071542 ((S)-3-(2-Acetylamino-3-methyl-butyrylamino)-5-[2-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against N-His (D381E) Interleukin -1 beta converting enzyme (combinatorially prepared compound) | Bioorg Med Chem Lett 8: 2309-14 (1999) BindingDB Entry DOI: 10.7270/Q2F47N9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50071543 ((S)-3-(2-Acetylamino-3-methyl-butyrylamino)-4-oxo-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against N-His (D381E) Interleukin -1 beta converting enzyme (resynthesized compound) | Bioorg Med Chem Lett 8: 2309-14 (1999) BindingDB Entry DOI: 10.7270/Q2F47N9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50071542 ((S)-3-(2-Acetylamino-3-methyl-butyrylamino)-5-[2-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Compound was tested for binding affinity against N-His (D381E) Interleukin -1 beta converting enzyme | Bioorg Med Chem Lett 8: 2309-14 (1999) BindingDB Entry DOI: 10.7270/Q2F47N9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439246 (CHEMBL2418830) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439247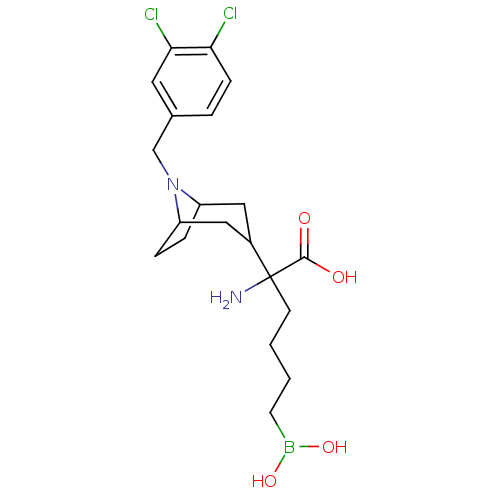 (CHEMBL2418831) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439245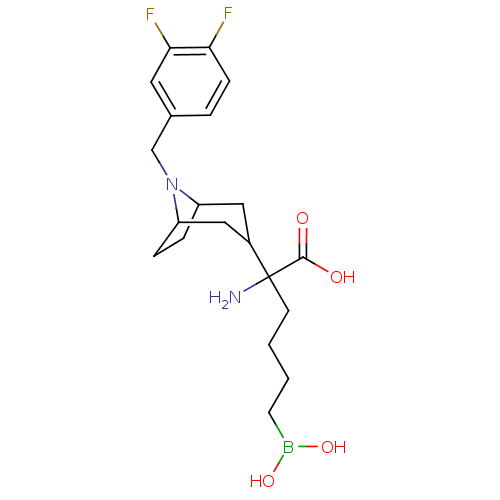 (CHEMBL2418991) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50439247 (CHEMBL2418831) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439244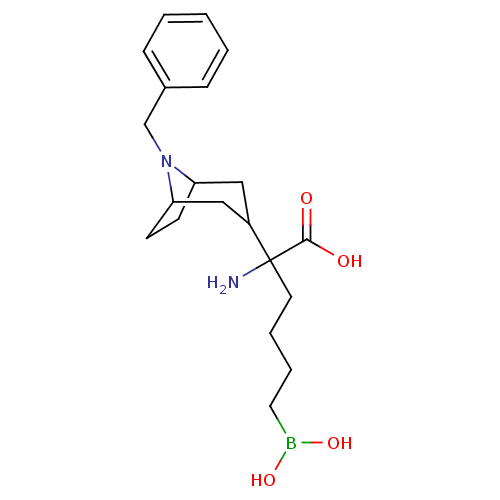 (CHEMBL2418829) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50439246 (CHEMBL2418830) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50439245 (CHEMBL2418991) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent N-type calcium channel subunit alpha-1B (Homo sapiens (Human)) | BDBM50080037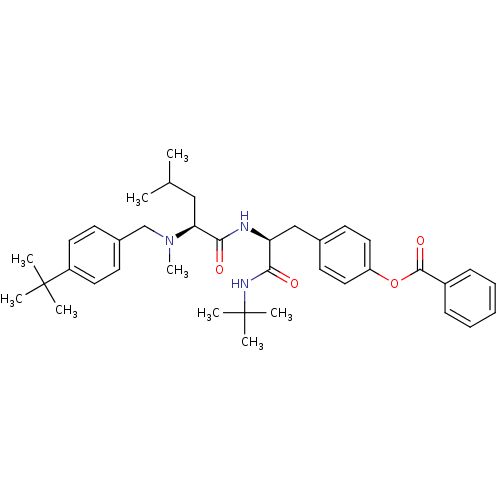 (Benzoic acid 4-((S)-2-{(S)-2-[(4-tert-butyl-benzyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of N-type calcium channels in IMR-32 human neuroblastoma cells | Bioorg Med Chem Lett 9: 2151-6 (1999) BindingDB Entry DOI: 10.7270/Q2H70F02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent N-type calcium channel subunit alpha-1B (Homo sapiens (Human)) | BDBM50078723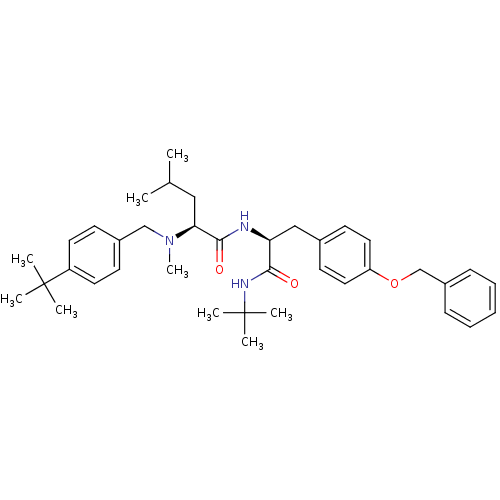 ((S)-2-[(4-tert-Butyl-benzyl)-methyl-amino]-4-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro antagonism of N-type voltage sensitive calcium channel in IMR-32 assay using fluorescent [Ca2+] indicator Indo-11 in the presence of 5 micro... | Bioorg Med Chem Lett 9: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2KD1X3X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50439244 (CHEMBL2418829) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50439243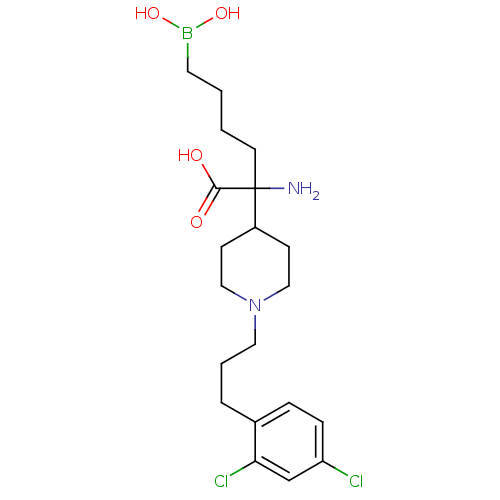 (CHEMBL2418998) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50427899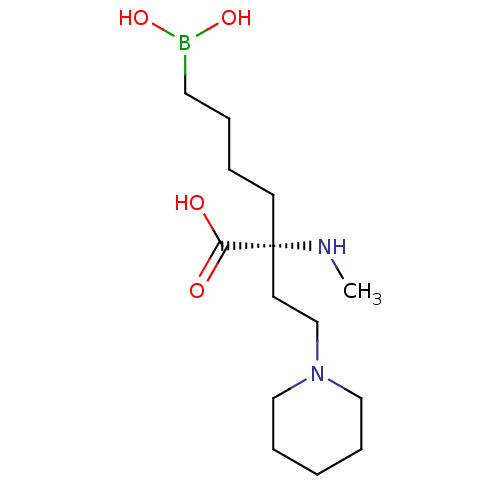 (CHEMBL2326090) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery , LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant full length arginase I overexpressed in Escherichia coli BL21(DE3) assessed as inhibition of urea formation after 60 ... | J Med Chem 56: 2568-80 (2013) Article DOI: 10.1021/jm400014c BindingDB Entry DOI: 10.7270/Q2BC40WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50427899 (CHEMBL2326090) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery , LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant fully active truncated form of arginase 2 overexpressed in Escherichia coli BL21(DE3) assessed as inhibition of urea ... | J Med Chem 56: 2568-80 (2013) Article DOI: 10.1021/jm400014c BindingDB Entry DOI: 10.7270/Q2BC40WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50439241 (CHEMBL2418999) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439242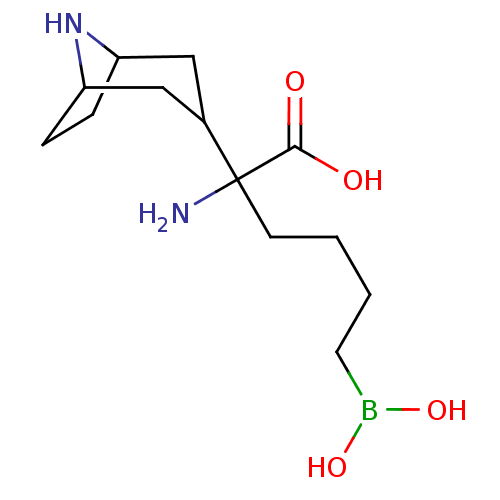 (CHEMBL2418828) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50439242 (CHEMBL2418828) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent N-type calcium channel subunit alpha-1B (Homo sapiens (Human)) | BDBM50078730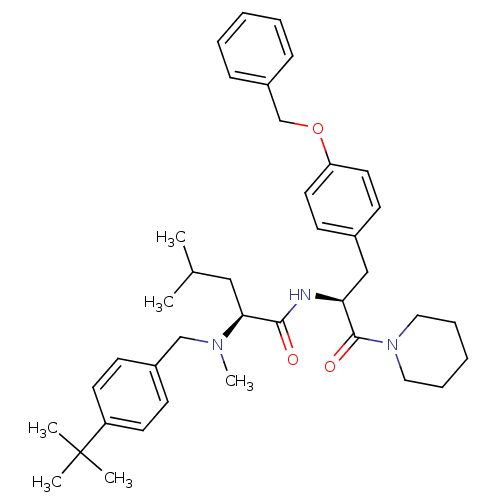 ((S)-2-[(4-tert-Butyl-benzyl)-methyl-amino]-4-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro antagonism of N-type voltage sensitive calcium channel in IMR-32 assay using fluorescent [Ca2+] indicator Indo-11 in the presence of 5 micro... | Bioorg Med Chem Lett 9: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2KD1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439243 (CHEMBL2418998) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50427903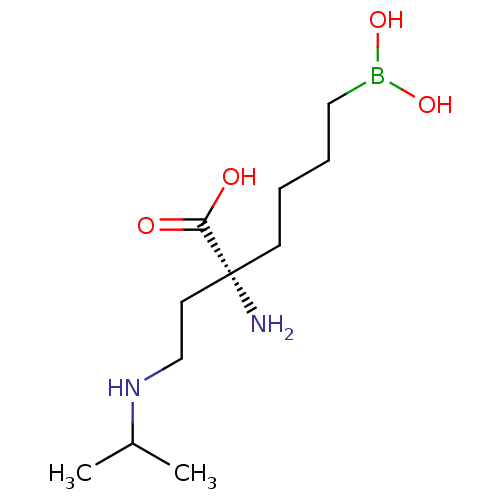 (CHEMBL2326087) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery , LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant full length arginase I overexpressed in Escherichia coli BL21(DE3) assessed as inhibition of urea formation after 60 ... | J Med Chem 56: 2568-80 (2013) Article DOI: 10.1021/jm400014c BindingDB Entry DOI: 10.7270/Q2BC40WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439241 (CHEMBL2418999) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent N-type calcium channel subunit alpha-1B (Homo sapiens (Human)) | BDBM50080046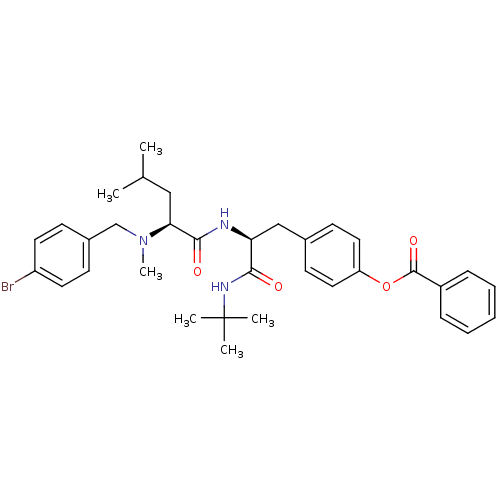 (Benzoic acid 4-((S)-2-{(S)-2-[(4-bromo-benzyl)-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of N-type calcium channels in IMR-32 human neuroblastoma cells | Bioorg Med Chem Lett 9: 2151-6 (1999) BindingDB Entry DOI: 10.7270/Q2H70F02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50427904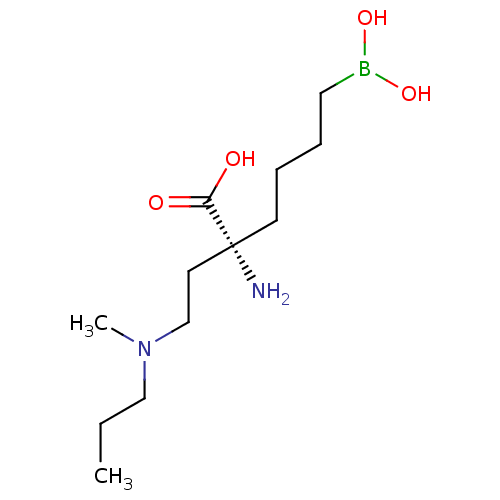 (CHEMBL2326086) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery , LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant full length arginase I overexpressed in Escherichia coli BL21(DE3) assessed as inhibition of urea formation after 60 ... | J Med Chem 56: 2568-80 (2013) Article DOI: 10.1021/jm400014c BindingDB Entry DOI: 10.7270/Q2BC40WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50427911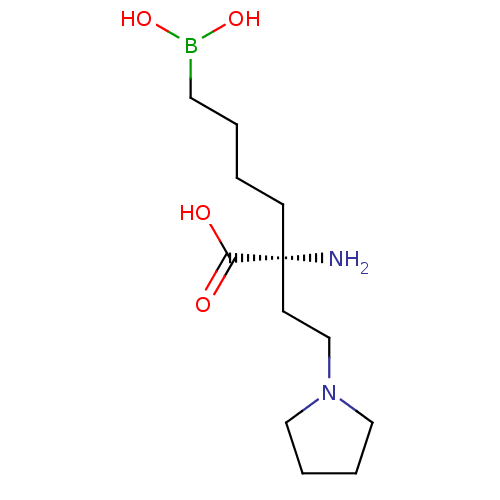 (CHEMBL2326095) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery , LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant full length arginase I overexpressed in Escherichia coli BL21(DE3) assessed as inhibition of urea formation after 60 ... | J Med Chem 56: 2568-80 (2013) Article DOI: 10.1021/jm400014c BindingDB Entry DOI: 10.7270/Q2BC40WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent N-type calcium channel subunit alpha-1B (Homo sapiens (Human)) | BDBM50080305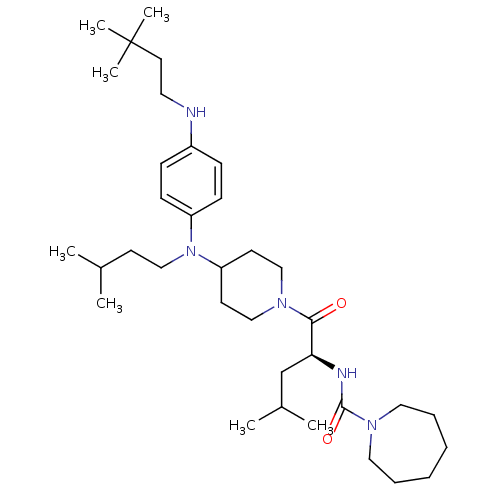 (Azepane-1-carboxylic acid (1-{4-[[4-(3,3-dimethyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description Inhibition of N-type Voltage Sensitive Calcium Channel (VSCC) using IMR-32 assay | Bioorg Med Chem Lett 9: 2453-8 (1999) BindingDB Entry DOI: 10.7270/Q29K49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439240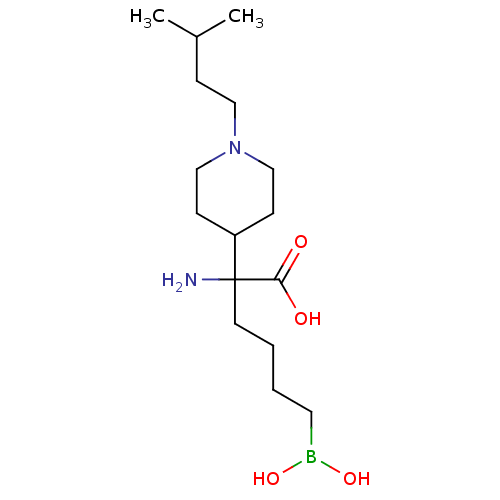 (CHEMBL2418993) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent N-type calcium channel subunit alpha-1B (Homo sapiens (Human)) | BDBM50080309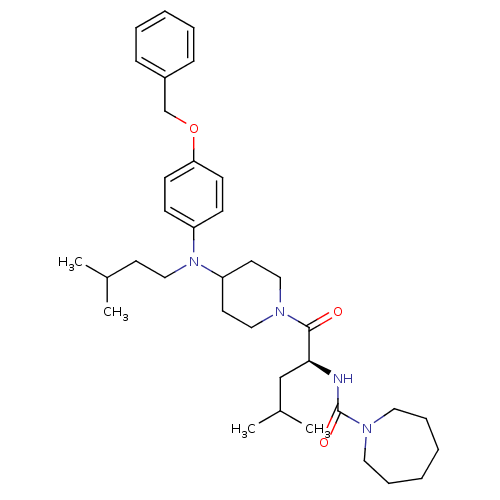 (Azepane-1-carboxylic acid (1-{4-[(4-benzyloxy-phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description Inhibition of N-type Voltage Sensitive Calcium Channel (VSCC) using IMR-32 assay | Bioorg Med Chem Lett 9: 2453-8 (1999) BindingDB Entry DOI: 10.7270/Q29K49FB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439238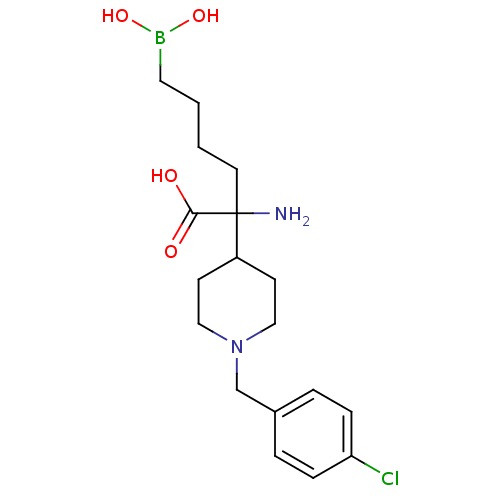 (CHEMBL2418995) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50427903 (CHEMBL2326087) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery , LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant fully active truncated form of arginase 2 overexpressed in Escherichia coli BL21(DE3) assessed as inhibition of urea ... | J Med Chem 56: 2568-80 (2013) Article DOI: 10.1021/jm400014c BindingDB Entry DOI: 10.7270/Q2BC40WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent N-type calcium channel subunit alpha-1B (Homo sapiens (Human)) | BDBM50076117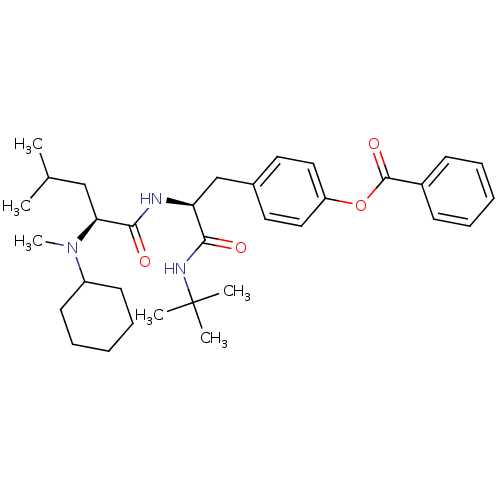 (Benzoic acid 4-{(S)-2-tert-butylcarbamoyl-2-[(S)-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro inhibitory activity against N-type calcium channel in the IMR-32 human neuroblastoma cells | Bioorg Med Chem Lett 9: 907-12 (1999) BindingDB Entry DOI: 10.7270/Q2D799MZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439239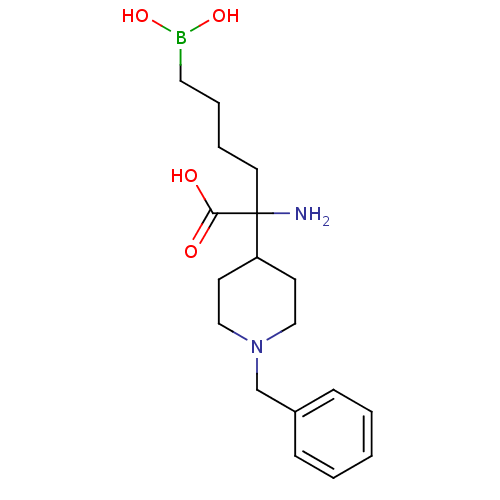 (CHEMBL2418994) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439237 (CHEMBL2418996) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Branched-chain-amino-acid aminotransferase, cytosolic (Rattus norvegicus) | BDBM50172933 (5-chloro-N'-(2-(trifluoromethyl)phenylsulfonyl)-1H...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of rat BCATc | Bioorg Med Chem Lett 16: 2337-40 (2006) Article DOI: 10.1016/j.bmcl.2005.07.058 BindingDB Entry DOI: 10.7270/Q2F47NQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent N-type calcium channel subunit alpha-1B (Homo sapiens (Human)) | BDBM50078727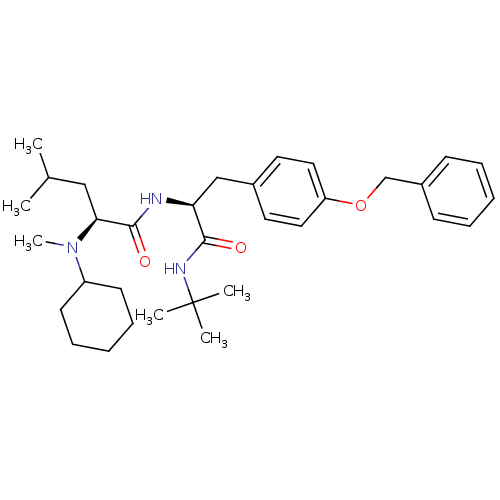 ((S)-2-(Cyclohexyl-methyl-amino)-4-methyl-pentanoic...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description In vitro antagonism of N-type voltage sensitive calcium channel in IMR-32 assay using fluorescent [Ca2+] indicator Indo-11 in the presence of 5 micro... | Bioorg Med Chem Lett 9: 1813-8 (1999) BindingDB Entry DOI: 10.7270/Q2KD1X3X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50427909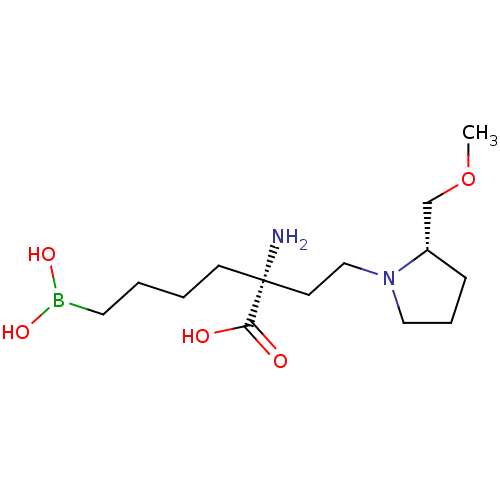 (CHEMBL2326097) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery , LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant full length arginase I overexpressed in Escherichia coli BL21(DE3) assessed as inhibition of urea formation after 60 ... | J Med Chem 56: 2568-80 (2013) Article DOI: 10.1021/jm400014c BindingDB Entry DOI: 10.7270/Q2BC40WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50439236 (CHEMBL2418997) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-1 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50427900 ((R)-2-amino-6-borono-2-[2-(piperidin-1-yl)ethyl]he...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 223 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery , LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant full length arginase I overexpressed in Escherichia coli BL21(DE3) assessed as inhibition of urea formation after 60 ... | J Med Chem 56: 2568-80 (2013) Article DOI: 10.1021/jm400014c BindingDB Entry DOI: 10.7270/Q2BC40WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50439239 (CHEMBL2418994) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50439240 (CHEMBL2418993) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent N-type calcium channel subunit alpha-1B (Homo sapiens (Human)) | BDBM50080035 (Benzoic acid 4-((S)-2-tert-butylcarbamoyl-2-{(S)-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of N-type calcium channels in IMR-32 human neuroblastoma cells | Bioorg Med Chem Lett 9: 2151-6 (1999) BindingDB Entry DOI: 10.7270/Q2H70F02 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50427913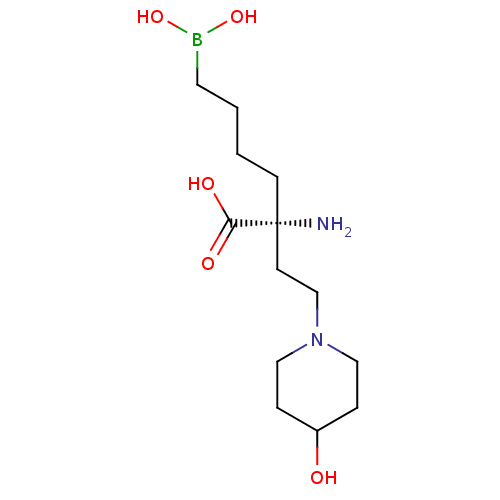 (CHEMBL2326093) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery , LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant full length arginase I overexpressed in Escherichia coli BL21(DE3) assessed as inhibition of urea formation after 60 ... | J Med Chem 56: 2568-80 (2013) Article DOI: 10.1021/jm400014c BindingDB Entry DOI: 10.7270/Q2BC40WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-2, mitochondrial (Homo sapiens (Human)) | BDBM50439238 (CHEMBL2418995) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery Curated by ChEMBL | Assay Description Inhibition of human arginase-2 assessed as L-arginine conversion to L-ornithine measured as urea level after 1 hr by colorimetric assay | Bioorg Med Chem Lett 23: 4837-41 (2013) Article DOI: 10.1016/j.bmcl.2013.06.092 BindingDB Entry DOI: 10.7270/Q2Z89DT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arginase-1 (Homo sapiens (Human)) | BDBM50427910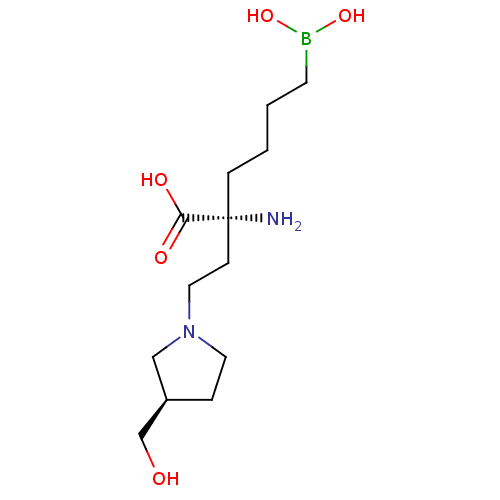 (CHEMBL2326096) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Institutes for Pharmaceutical Discovery , LLC Curated by ChEMBL | Assay Description Inhibition of human recombinant full length arginase I overexpressed in Escherichia coli BL21(DE3) assessed as inhibition of urea formation after 60 ... | J Med Chem 56: 2568-80 (2013) Article DOI: 10.1021/jm400014c BindingDB Entry DOI: 10.7270/Q2BC40WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent N-type calcium channel subunit alpha-1B (Homo sapiens (Human)) | BDBM50081687 (Azepane-1-carboxylic acid ((S)-1-{2-[(4-benzyloxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of N-type calcium channels in IMR32 human neuroblastoma cells | J Med Chem 42: 4239-49 (1999) BindingDB Entry DOI: 10.7270/Q2QF8S22 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 240 total ) | Next | Last >> |