 Found 433 hits with Last Name = 'rydzewski' and Initial = 'rm'
Found 433 hits with Last Name = 'rydzewski' and Initial = 'rm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19854

(CHEMBL426819 | CRA-013783/L-006235 | N-{1-[(cyanom...)Show SMILES CN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C24H30N6O2S/c1-29-13-15-30(16-14-29)23-27-20(17-33-23)18-5-7-19(8-6-18)21(31)28-24(9-3-2-4-10-24)22(32)26-12-11-25/h5-8,17H,2-4,9-10,12-16H2,1H3,(H,26,32)(H,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410611
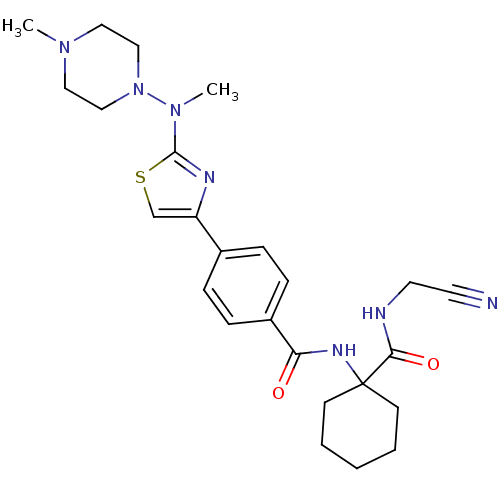
(CHEMBL414669)Show SMILES CN(N1CCN(C)CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C25H33N7O2S/c1-30-14-16-32(17-15-30)31(2)24-28-21(18-35-24)19-6-8-20(9-7-19)22(33)29-25(10-4-3-5-11-25)23(34)27-13-12-26/h6-9,18H,3-5,10-11,13-17H2,1-2H3,(H,27,34)(H,29,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410588
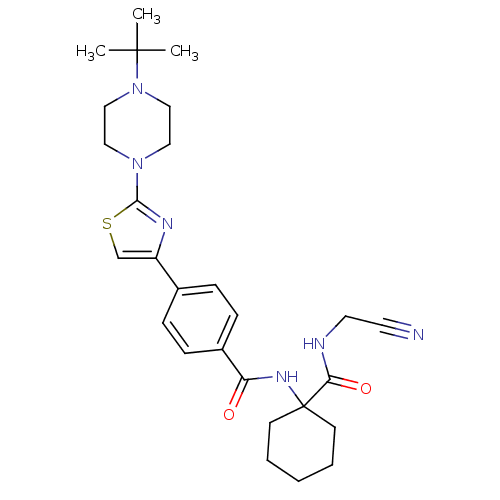
(CHEMBL200708)Show SMILES CC(C)(C)N1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C27H36N6O2S/c1-26(2,3)33-17-15-32(16-18-33)25-30-22(19-36-25)20-7-9-21(10-8-20)23(34)31-27(11-5-4-6-12-27)24(35)29-14-13-28/h7-10,19H,4-6,11-12,14-18H2,1-3H3,(H,29,35)(H,31,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410609
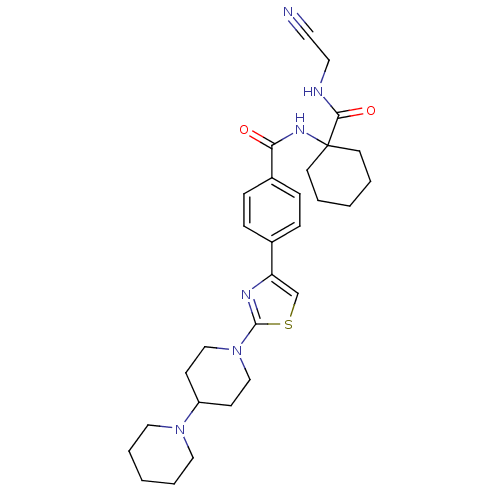
(CHEMBL198798)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1csc(n1)N1CCC(CC1)N1CCCCC1 Show InChI InChI=1S/C29H38N6O2S/c30-15-16-31-27(37)29(13-3-1-4-14-29)33-26(36)23-9-7-22(8-10-23)25-21-38-28(32-25)35-19-11-24(12-20-35)34-17-5-2-6-18-34/h7-10,21,24H,1-6,11-14,16-20H2,(H,31,37)(H,33,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410590
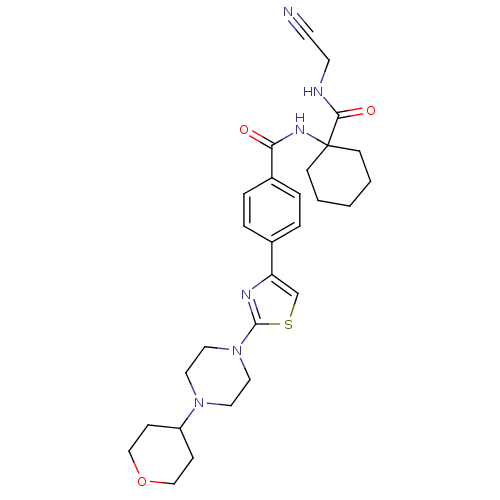
(CHEMBL200543)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1csc(n1)N1CCN(CC1)C1CCOCC1 Show InChI InChI=1S/C28H36N6O3S/c29-12-13-30-26(36)28(10-2-1-3-11-28)32-25(35)22-6-4-21(5-7-22)24-20-38-27(31-24)34-16-14-33(15-17-34)23-8-18-37-19-9-23/h4-7,20,23H,1-3,8-11,13-19H2,(H,30,36)(H,32,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410587
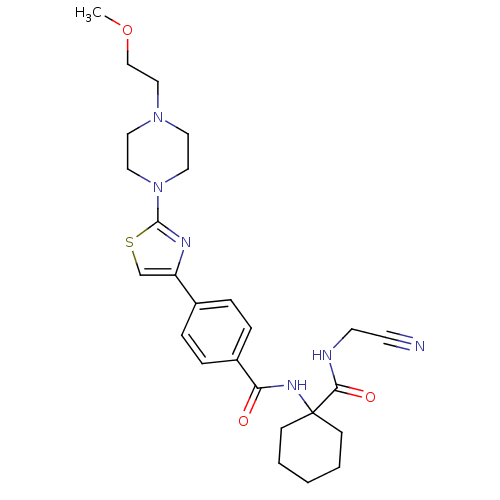
(CHEMBL200602)Show SMILES COCCN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C26H34N6O3S/c1-35-18-17-31-13-15-32(16-14-31)25-29-22(19-36-25)20-5-7-21(8-6-20)23(33)30-26(9-3-2-4-10-26)24(34)28-12-11-27/h5-8,19H,2-4,9-10,12-18H2,1H3,(H,28,34)(H,30,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19854

(CHEMBL426819 | CRA-013783/L-006235 | N-{1-[(cyanom...)Show SMILES CN1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C24H30N6O2S/c1-29-13-15-30(16-14-29)23-27-20(17-33-23)18-5-7-19(8-6-18)21(31)28-24(9-3-2-4-10-24)22(32)26-12-11-25/h5-8,17H,2-4,9-10,12-16H2,1H3,(H,26,32)(H,28,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50410903
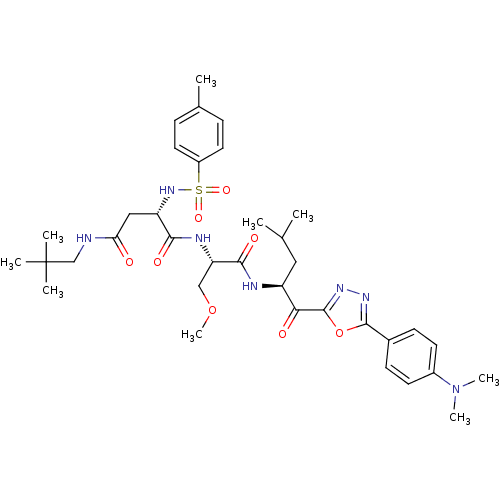
(CHEMBL207598)Show SMILES COC[C@H](NC(=O)[C@H](CC(=O)NCC(C)(C)C)NS(=O)(=O)c1ccc(C)cc1)C(=O)N[C@@H](CC(C)C)C(=O)c1nnc(o1)-c1ccc(cc1)N(C)C Show InChI InChI=1S/C36H51N7O8S/c1-22(2)18-27(31(45)35-41-40-34(51-35)24-12-14-25(15-13-24)43(7)8)38-33(47)29(20-50-9)39-32(46)28(19-30(44)37-21-36(4,5)6)42-52(48,49)26-16-10-23(3)11-17-26/h10-17,22,27-29,42H,18-21H2,1-9H3,(H,37,44)(H,38,47)(H,39,46)/t27-,28-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like proteasome activity of human 20S proteasome |
J Med Chem 49: 2953-68 (2006)
Article DOI: 10.1021/jm058289o
BindingDB Entry DOI: 10.7270/Q2FF3S0S |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50410898
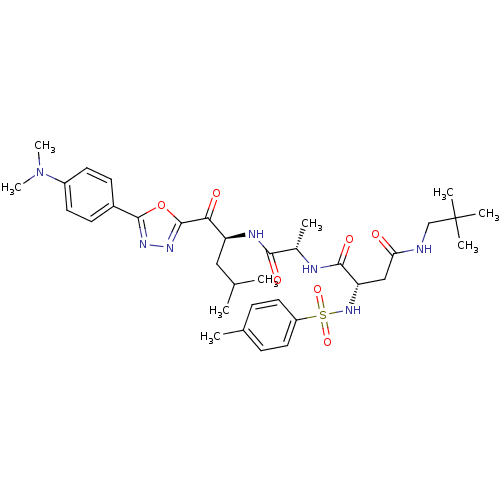
(CHEMBL205757)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(=O)NCC(C)(C)C)NS(=O)(=O)c1ccc(C)cc1)C(=O)c1nnc(o1)-c1ccc(cc1)N(C)C Show InChI InChI=1S/C35H49N7O7S/c1-21(2)18-27(30(44)34-40-39-33(49-34)24-12-14-25(15-13-24)42(8)9)38-31(45)23(4)37-32(46)28(19-29(43)36-20-35(5,6)7)41-50(47,48)26-16-10-22(3)11-17-26/h10-17,21,23,27-28,41H,18-20H2,1-9H3,(H,36,43)(H,37,46)(H,38,45)/t23-,27-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like proteasome activity of human 20S proteasome |
J Med Chem 49: 2953-68 (2006)
Article DOI: 10.1021/jm058289o
BindingDB Entry DOI: 10.7270/Q2FF3S0S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410607
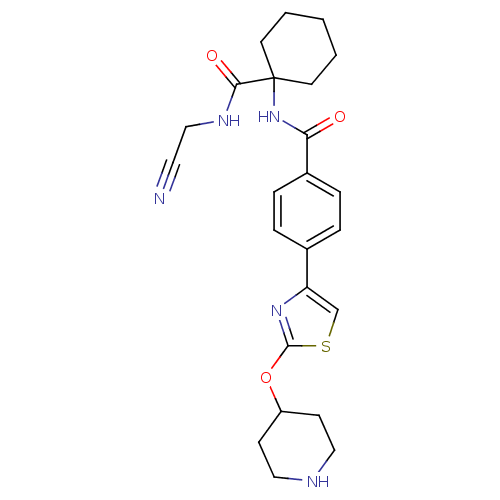
(CHEMBL200744)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1csc(OC2CCNCC2)n1 Show InChI InChI=1S/C24H29N5O3S/c25-12-15-27-22(31)24(10-2-1-3-11-24)29-21(30)18-6-4-17(5-7-18)20-16-33-23(28-20)32-19-8-13-26-14-9-19/h4-7,16,19,26H,1-3,8-11,13-15H2,(H,27,31)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410571
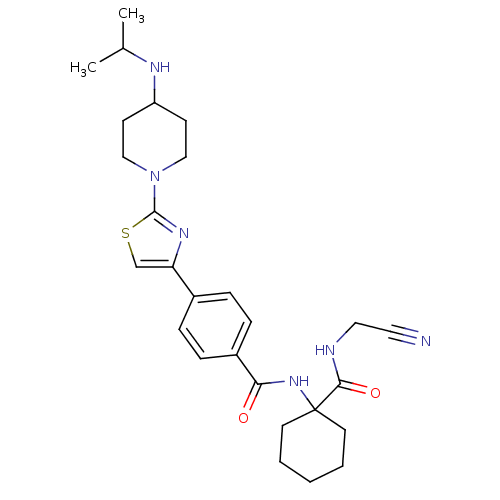
(CHEMBL200287)Show SMILES CC(C)NC1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C27H36N6O2S/c1-19(2)30-22-10-16-33(17-11-22)26-31-23(18-36-26)20-6-8-21(9-7-20)24(34)32-27(12-4-3-5-13-27)25(35)29-15-14-28/h6-9,18-19,22,30H,3-5,10-13,15-17H2,1-2H3,(H,29,35)(H,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50410901
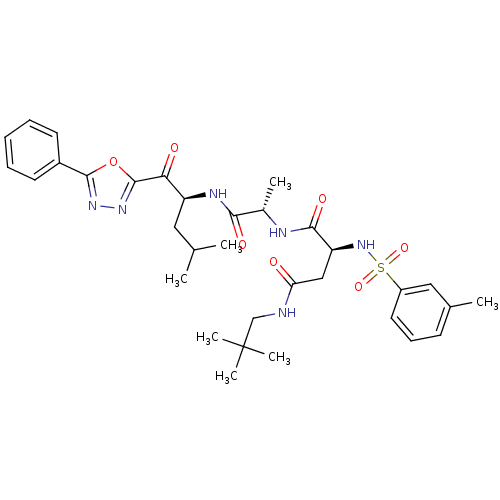
(CHEMBL206413)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(=O)NCC(C)(C)C)NS(=O)(=O)c1cccc(C)c1)C(=O)c1nnc(o1)-c1ccccc1 Show InChI InChI=1S/C33H44N6O7S/c1-20(2)16-25(28(41)32-38-37-31(46-32)23-13-9-8-10-14-23)36-29(42)22(4)35-30(43)26(18-27(40)34-19-33(5,6)7)39-47(44,45)24-15-11-12-21(3)17-24/h8-15,17,20,22,25-26,39H,16,18-19H2,1-7H3,(H,34,40)(H,35,43)(H,36,42)/t22-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like proteasome activity of human 20S proteasome |
J Med Chem 49: 2953-68 (2006)
Article DOI: 10.1021/jm058289o
BindingDB Entry DOI: 10.7270/Q2FF3S0S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410580
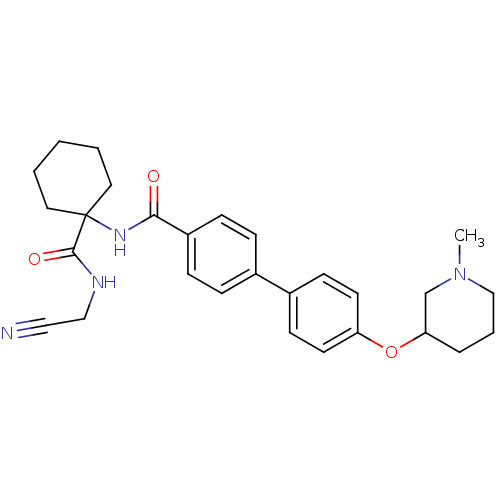
(CHEMBL435913)Show SMILES CN1CCCC(C1)Oc1ccc(cc1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C28H34N4O3/c1-32-19-5-6-25(20-32)35-24-13-11-22(12-14-24)21-7-9-23(10-8-21)26(33)31-28(15-3-2-4-16-28)27(34)30-18-17-29/h7-14,25H,2-6,15-16,18-20H2,1H3,(H,30,34)(H,31,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50410904
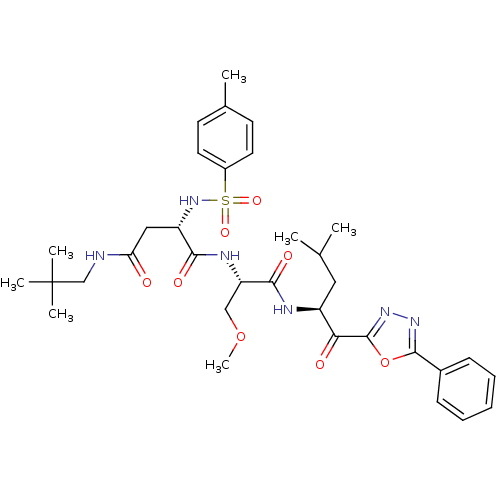
(CHEMBL377532)Show SMILES COC[C@H](NC(=O)[C@H](CC(=O)NCC(C)(C)C)NS(=O)(=O)c1ccc(C)cc1)C(=O)N[C@@H](CC(C)C)C(=O)c1nnc(o1)-c1ccccc1 Show InChI InChI=1S/C34H46N6O8S/c1-21(2)17-25(29(42)33-39-38-32(48-33)23-11-9-8-10-12-23)36-31(44)27(19-47-7)37-30(43)26(18-28(41)35-20-34(4,5)6)40-49(45,46)24-15-13-22(3)14-16-24/h8-16,21,25-27,40H,17-20H2,1-7H3,(H,35,41)(H,36,44)(H,37,43)/t25-,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like proteasome activity of human 20S proteasome |
J Med Chem 49: 2953-68 (2006)
Article DOI: 10.1021/jm058289o
BindingDB Entry DOI: 10.7270/Q2FF3S0S |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50410899

(CHEMBL383674)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(=O)NCC(C)(C)C)NS(=O)(=O)c1ccc(C)cc1)C(=O)c1nnc(o1)-c1ccccc1 Show InChI InChI=1S/C33H44N6O7S/c1-20(2)17-25(28(41)32-38-37-31(46-32)23-11-9-8-10-12-23)36-29(42)22(4)35-30(43)26(18-27(40)34-19-33(5,6)7)39-47(44,45)24-15-13-21(3)14-16-24/h8-16,20,22,25-26,39H,17-19H2,1-7H3,(H,34,40)(H,35,43)(H,36,42)/t22-,25-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like proteasome activity of human 20S proteasome |
J Med Chem 49: 2953-68 (2006)
Article DOI: 10.1021/jm058289o
BindingDB Entry DOI: 10.7270/Q2FF3S0S |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410575
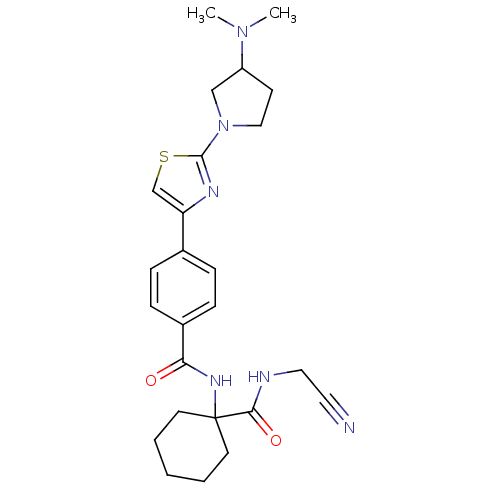
(CHEMBL199470)Show SMILES CN(C)C1CCN(C1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C25H32N6O2S/c1-30(2)20-10-15-31(16-20)24-28-21(17-34-24)18-6-8-19(9-7-18)22(32)29-25(11-4-3-5-12-25)23(33)27-14-13-26/h6-9,17,20H,3-5,10-12,14-16H2,1-2H3,(H,27,33)(H,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410591

(CHEMBL200506)Show SMILES CN1CCN(Cc2nc(cs2)-c2ccc(cc2)C(=O)NC2(CCCCC2)C(=O)NCC#N)CC1 Show InChI InChI=1S/C25H32N6O2S/c1-30-13-15-31(16-14-30)17-22-28-21(18-34-22)19-5-7-20(8-6-19)23(32)29-25(9-3-2-4-10-25)24(33)27-12-11-26/h5-8,18H,2-4,9-10,12-17H2,1H3,(H,27,33)(H,29,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410612
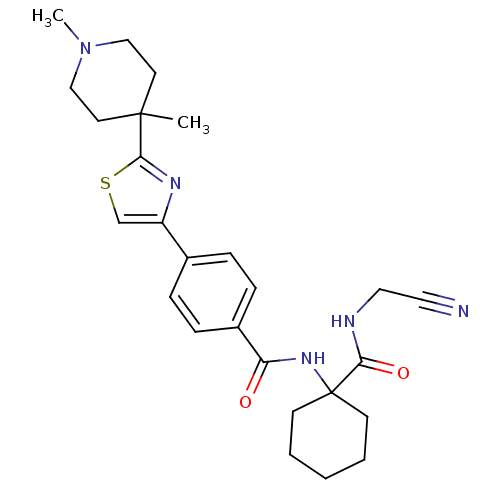
(CHEMBL200166)Show SMILES CN1CCC(C)(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C26H33N5O2S/c1-25(12-16-31(2)17-13-25)24-29-21(18-34-24)19-6-8-20(9-7-19)22(32)30-26(10-4-3-5-11-26)23(33)28-15-14-27/h6-9,18H,3-5,10-13,15-17H2,1-2H3,(H,28,33)(H,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19855
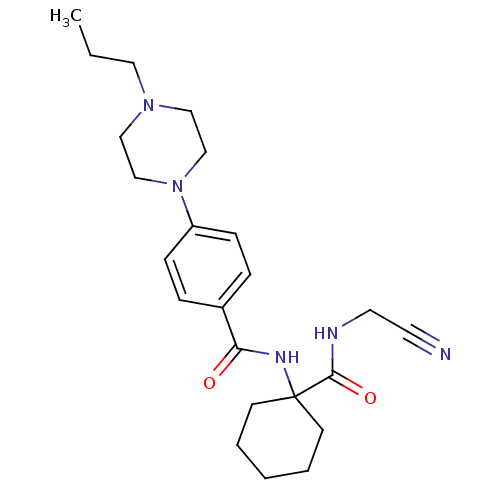
(Balicatib | CHEMBL371064 | N-[1-(cyanomethylcarbam...)Show SMILES CCCN1CCN(CC1)c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C23H33N5O2/c1-2-14-27-15-17-28(18-16-27)20-8-6-19(7-9-20)21(29)26-23(10-4-3-5-11-23)22(30)25-13-12-24/h6-9H,2-5,10-11,13-18H2,1H3,(H,25,30)(H,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410595
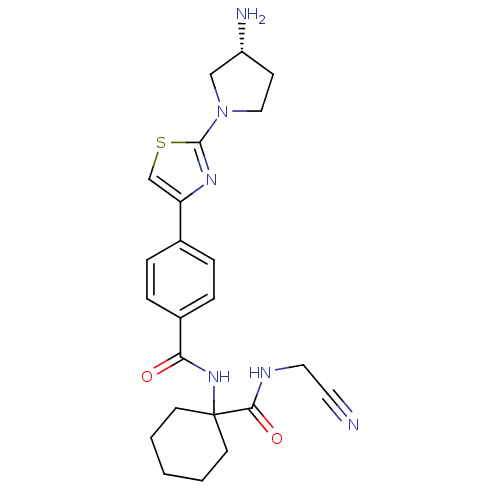
(CHEMBL200507)Show SMILES N[C@@H]1CCN(C1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C23H28N6O2S/c24-11-12-26-21(31)23(9-2-1-3-10-23)28-20(30)17-6-4-16(5-7-17)19-15-32-22(27-19)29-13-8-18(25)14-29/h4-7,15,18H,1-3,8-10,12-14,25H2,(H,26,31)(H,28,30)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410572
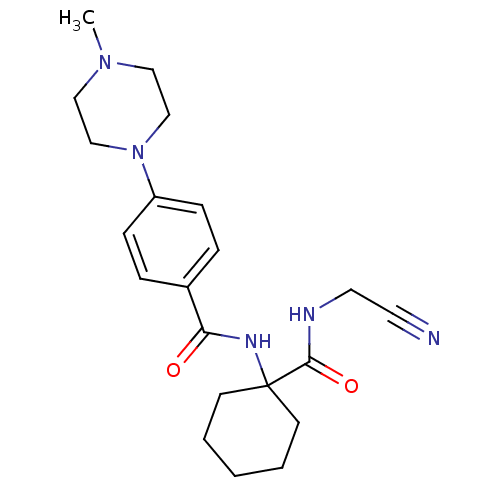
(CHEMBL440035)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C21H29N5O2/c1-25-13-15-26(16-14-25)18-7-5-17(6-8-18)19(27)24-21(9-3-2-4-10-21)20(28)23-12-11-22/h5-8H,2-4,9-10,12-16H2,1H3,(H,23,28)(H,24,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410592
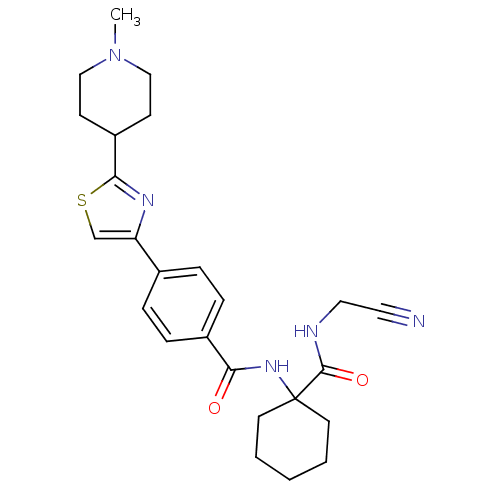
(CHEMBL200455)Show SMILES CN1CCC(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C25H31N5O2S/c1-30-15-9-20(10-16-30)23-28-21(17-33-23)18-5-7-19(8-6-18)22(31)29-25(11-3-2-4-12-25)24(32)27-14-13-26/h5-8,17,20H,2-4,9-12,14-16H2,1H3,(H,27,32)(H,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410594
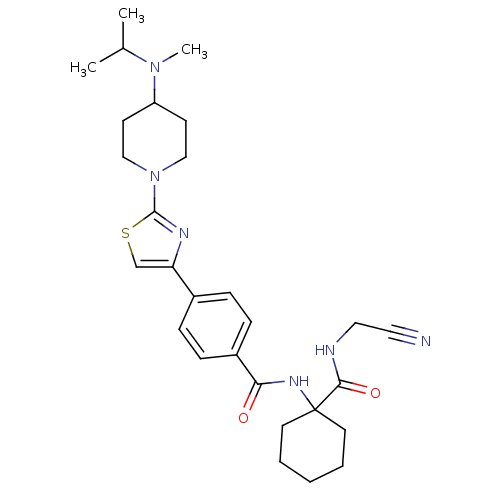
(CHEMBL200596)Show SMILES CC(C)N(C)C1CCN(CC1)c1nc(cs1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C28H38N6O2S/c1-20(2)33(3)23-11-17-34(18-12-23)27-31-24(19-37-27)21-7-9-22(10-8-21)25(35)32-28(13-5-4-6-14-28)26(36)30-16-15-29/h7-10,19-20,23H,4-6,11-14,16-18H2,1-3H3,(H,30,36)(H,32,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410606
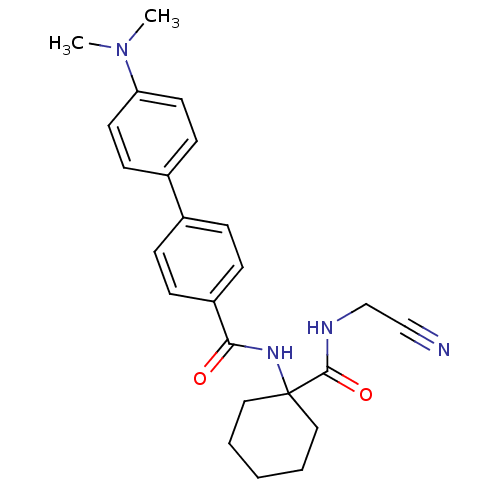
(CHEMBL383186)Show SMILES CN(C)c1ccc(cc1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C24H28N4O2/c1-28(2)21-12-10-19(11-13-21)18-6-8-20(9-7-18)22(29)27-24(14-4-3-5-15-24)23(30)26-17-16-25/h6-13H,3-5,14-15,17H2,1-2H3,(H,26,30)(H,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM19857
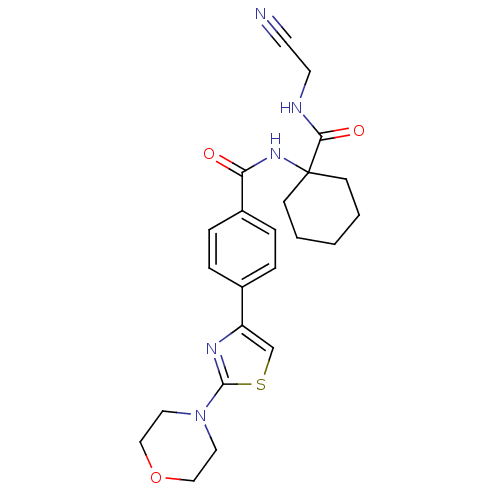
(N-{1-[(cyanomethyl)carbamoyl]cyclohexyl}-4-[2-(mor...)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1csc(n1)N1CCOCC1 Show InChI InChI=1S/C23H27N5O3S/c24-10-11-25-21(30)23(8-2-1-3-9-23)27-20(29)18-6-4-17(5-7-18)19-16-32-22(26-19)28-12-14-31-15-13-28/h4-7,16H,1-3,8-9,11-15H2,(H,25,30)(H,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50069984

((R)-1-((S)-2-((S)-2-(benzyloxycarbonyl)-4-methylpe...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)B(O)O Show InChI InChI=1S/C25H42BN3O6/c1-16(2)12-20(24(31)29-22(26(33)34)14-18(5)6)27-23(30)21(13-17(3)4)28-25(32)35-15-19-10-8-7-9-11-19/h7-11,16-18,20-22,33-34H,12-15H2,1-6H3,(H,27,30)(H,28,32)(H,29,31)/t20-,21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like proteasome activity of human 20S proteasome |
J Med Chem 49: 2953-68 (2006)
Article DOI: 10.1021/jm058289o
BindingDB Entry DOI: 10.7270/Q2FF3S0S |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411015
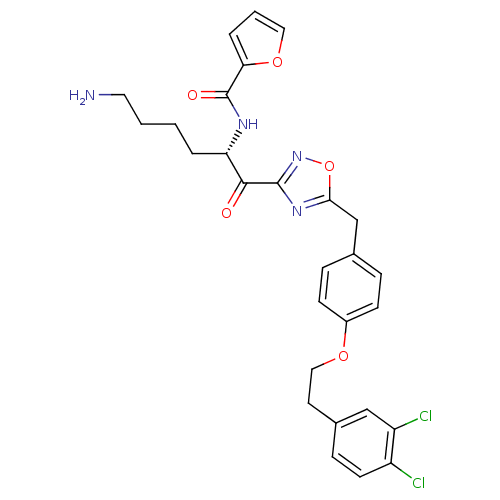
(CHEMBL539086)Show SMILES NCCCC[C@H](NC(=O)c1ccco1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C28H28Cl2N4O5/c29-21-11-8-19(16-22(21)30)12-15-37-20-9-6-18(7-10-20)17-25-33-27(34-39-25)26(35)23(4-1-2-13-31)32-28(36)24-5-3-14-38-24/h3,5-11,14,16,23H,1-2,4,12-13,15,17,31H2,(H,32,36)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411006
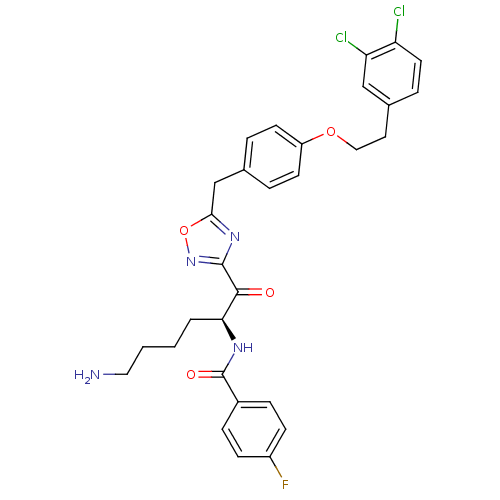
(CHEMBL539088)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)cc1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H29Cl2FN4O4/c31-24-13-6-20(17-25(24)32)14-16-40-23-11-4-19(5-12-23)18-27-36-29(37-41-27)28(38)26(3-1-2-15-34)35-30(39)21-7-9-22(33)10-8-21/h4-13,17,26H,1-3,14-16,18,34H2,(H,35,39)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411011
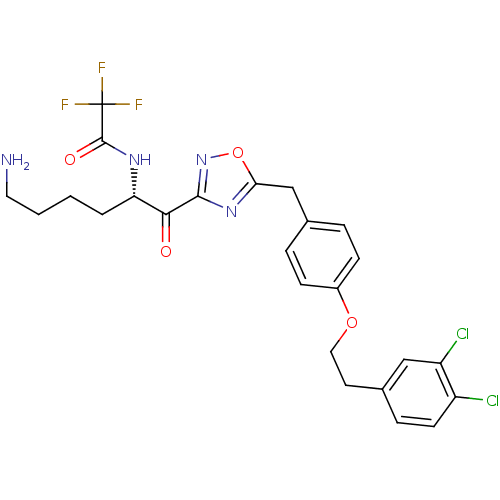
(CHEMBL537868)Show SMILES NCCCC[C@H](NC(=O)C(F)(F)F)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C25H25Cl2F3N4O4/c26-18-9-6-16(13-19(18)27)10-12-37-17-7-4-15(5-8-17)14-21-33-23(34-38-21)22(35)20(3-1-2-11-31)32-24(36)25(28,29)30/h4-9,13,20H,1-3,10-12,14,31H2,(H,32,36)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411017
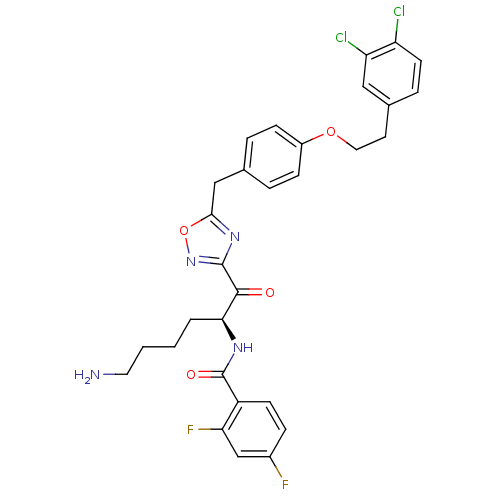
(CHEMBL539603)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)cc1F)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H28Cl2F2N4O4/c31-23-11-6-19(15-24(23)32)12-14-41-21-8-4-18(5-9-21)16-27-37-29(38-42-27)28(39)26(3-1-2-13-35)36-30(40)22-10-7-20(33)17-25(22)34/h4-11,15,17,26H,1-3,12-14,16,35H2,(H,36,40)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410603
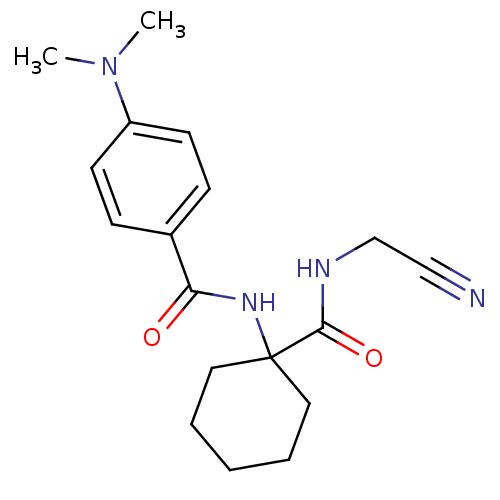
(CHEMBL198859)Show InChI InChI=1S/C18H24N4O2/c1-22(2)15-8-6-14(7-9-15)16(23)21-18(10-4-3-5-11-18)17(24)20-13-12-19/h6-9H,3-5,10-11,13H2,1-2H3,(H,20,24)(H,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50069985
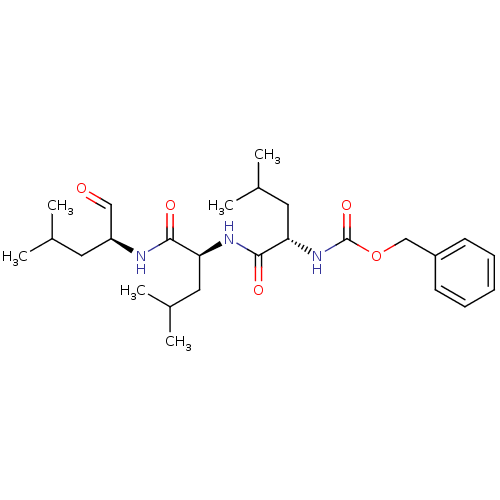
((S)-4-methyl-2-(3-phenyl-propionylamino)-pentanoic...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)OCc1ccccc1)C=O |r| Show InChI InChI=1S/C26H41N3O5/c1-17(2)12-21(15-30)27-24(31)22(13-18(3)4)28-25(32)23(14-19(5)6)29-26(33)34-16-20-10-8-7-9-11-20/h7-11,15,17-19,21-23H,12-14,16H2,1-6H3,(H,27,31)(H,28,32)(H,29,33)/t21-,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like proteasome activity of human 20S proteasome |
J Med Chem 49: 2953-68 (2006)
Article DOI: 10.1021/jm058289o
BindingDB Entry DOI: 10.7270/Q2FF3S0S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411009
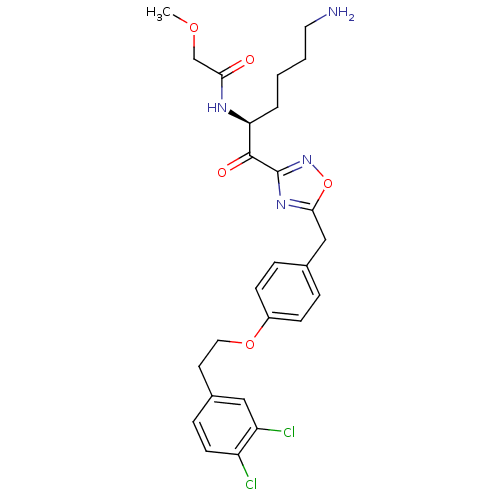
(CHEMBL538574)Show SMILES COCC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C26H30Cl2N4O5/c1-35-16-23(33)30-22(4-2-3-12-29)25(34)26-31-24(37-32-26)15-17-5-8-19(9-6-17)36-13-11-18-7-10-20(27)21(28)14-18/h5-10,14,22H,2-4,11-13,15-16,29H2,1H3,(H,30,33)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411002
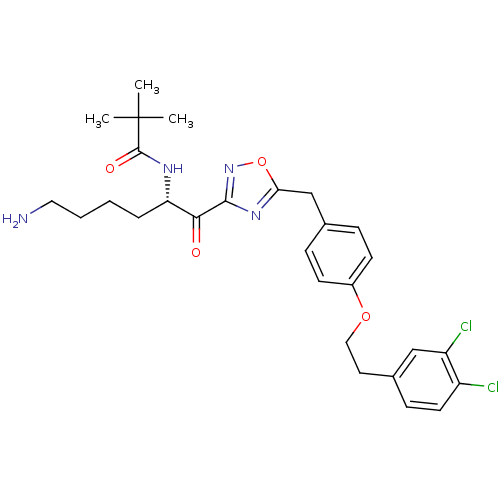
(CHEMBL556994)Show SMILES CC(C)(C)C(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C28H34Cl2N4O4/c1-28(2,3)27(36)32-23(6-4-5-14-31)25(35)26-33-24(38-34-26)17-18-7-10-20(11-8-18)37-15-13-19-9-12-21(29)22(30)16-19/h7-12,16,23H,4-6,13-15,17,31H2,1-3H3,(H,32,36)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411013
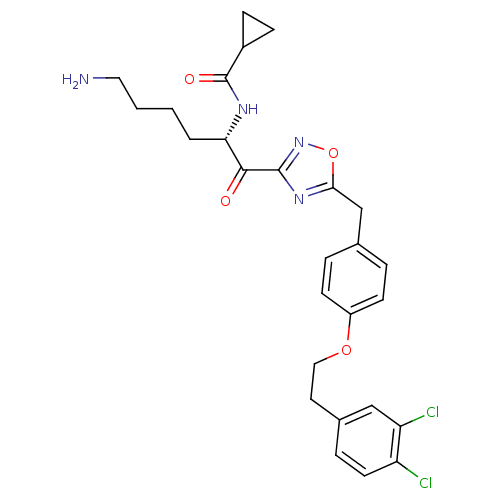
(CHEMBL534493)Show SMILES NCCCC[C@H](NC(=O)C1CC1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C27H30Cl2N4O4/c28-21-11-6-18(15-22(21)29)12-14-36-20-9-4-17(5-10-20)16-24-32-26(33-37-24)25(34)23(3-1-2-13-30)31-27(35)19-7-8-19/h4-6,9-11,15,19,23H,1-3,7-8,12-14,16,30H2,(H,31,35)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
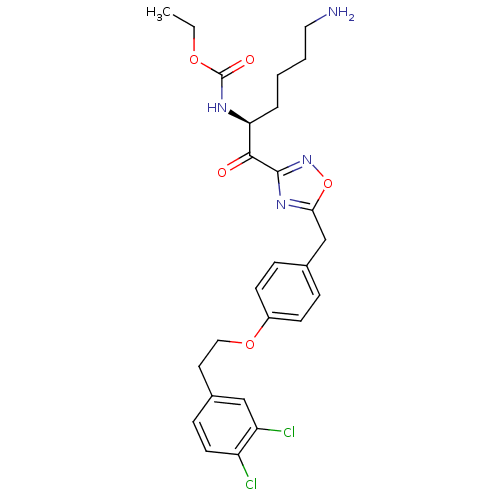
(Homo sapiens (Human)) | BDBM50410996
(CHEMBL537647)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C26H30Cl2N4O5/c1-2-35-26(34)30-22(5-3-4-13-29)24(33)25-31-23(37-32-25)16-17-6-9-19(10-7-17)36-14-12-18-8-11-20(27)21(28)15-18/h6-11,15,22H,2-5,12-14,16,29H2,1H3,(H,30,34)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411005
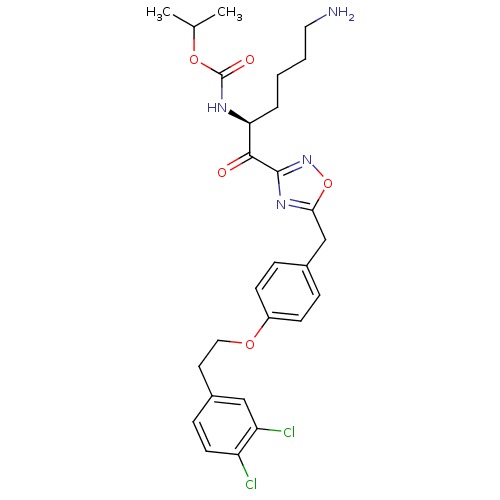
(CHEMBL537646)Show SMILES CC(C)OC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C27H32Cl2N4O5/c1-17(2)37-27(35)31-23(5-3-4-13-30)25(34)26-32-24(38-33-26)16-18-6-9-20(10-7-18)36-14-12-19-8-11-21(28)22(29)15-19/h6-11,15,17,23H,3-5,12-14,16,30H2,1-2H3,(H,31,35)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411019
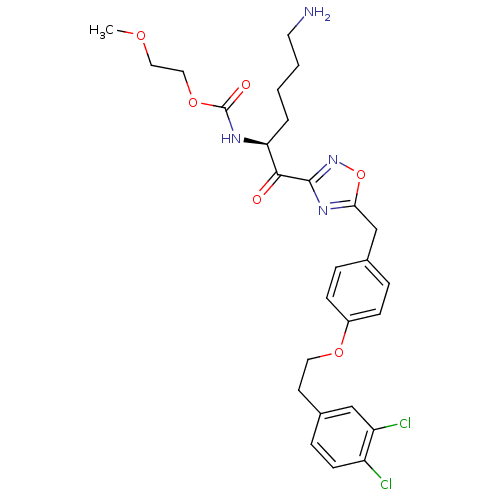
(CHEMBL558199)Show SMILES COCCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C27H32Cl2N4O6/c1-36-14-15-38-27(35)31-23(4-2-3-12-30)25(34)26-32-24(39-33-26)17-18-5-8-20(9-6-18)37-13-11-19-7-10-21(28)22(29)16-19/h5-10,16,23H,2-4,11-15,17,30H2,1H3,(H,31,35)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50187800

((S)-ethyl 1-(5-(4-phenethoxybenzyl)-1,2,4-oxadiazo...)Show SMILES CCOC(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccccc3)cc2)n1 Show InChI InChI=1S/C26H32N4O5/c1-2-33-26(32)28-22(10-6-7-16-27)24(31)25-29-23(35-30-25)18-20-11-13-21(14-12-20)34-17-15-19-8-4-3-5-9-19/h3-5,8-9,11-14,22H,2,6-7,10,15-18,27H2,1H3,(H,28,32)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410578
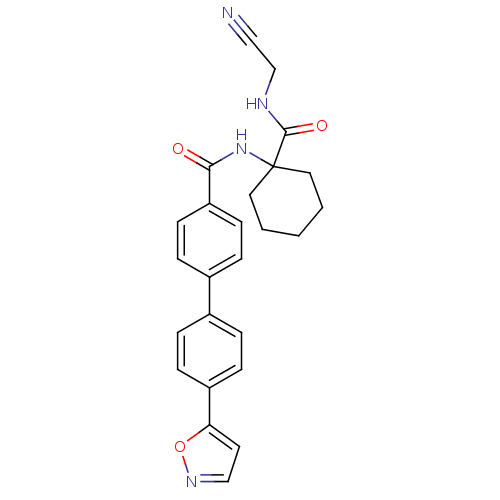
(CHEMBL199014)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1ccc(cc1)-c1ccno1 Show InChI InChI=1S/C25H24N4O3/c26-15-17-27-24(31)25(13-2-1-3-14-25)29-23(30)21-10-6-19(7-11-21)18-4-8-20(9-5-18)22-12-16-28-32-22/h4-12,16H,1-3,13-14,17H2,(H,27,31)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50410906

(CHEMBL207160)Show SMILES CC(C)C[C@H](NC(=O)[C@H](C)NC(=O)[C@H](CC(=O)NCC(C)(C)C)NS(C)(=O)=O)C(=O)c1nnc(o1)-c1ccccc1 Show InChI InChI=1S/C27H40N6O7S/c1-16(2)13-19(22(35)26-32-31-25(40-26)18-11-9-8-10-12-18)30-23(36)17(3)29-24(37)20(33-41(7,38)39)14-21(34)28-15-27(4,5)6/h8-12,16-17,19-20,33H,13-15H2,1-7H3,(H,28,34)(H,29,37)(H,30,36)/t17-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like proteasome activity of human 20S proteasome |
J Med Chem 49: 2953-68 (2006)
Article DOI: 10.1021/jm058289o
BindingDB Entry DOI: 10.7270/Q2FF3S0S |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411001
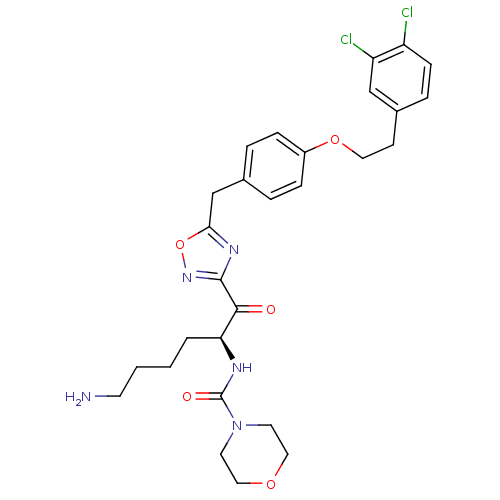
(CHEMBL558763)Show SMILES NCCCC[C@H](NC(=O)N1CCOCC1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C28H33Cl2N5O5/c29-22-9-6-20(17-23(22)30)10-14-39-21-7-4-19(5-8-21)18-25-33-27(34-40-25)26(36)24(3-1-2-11-31)32-28(37)35-12-15-38-16-13-35/h4-9,17,24H,1-3,10-16,18,31H2,(H,32,37)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411008
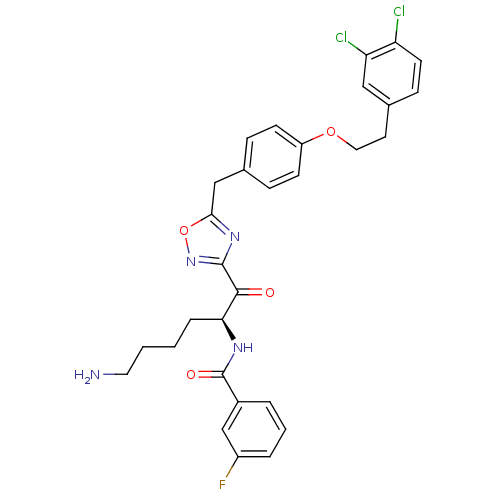
(CHEMBL535836)Show SMILES NCCCC[C@H](NC(=O)c1cccc(F)c1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H29Cl2FN4O4/c31-24-12-9-20(16-25(24)32)13-15-40-23-10-7-19(8-11-23)17-27-36-29(37-41-27)28(38)26(6-1-2-14-34)35-30(39)21-4-3-5-22(33)18-21/h3-5,7-12,16,18,26H,1-2,6,13-15,17,34H2,(H,35,39)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410593
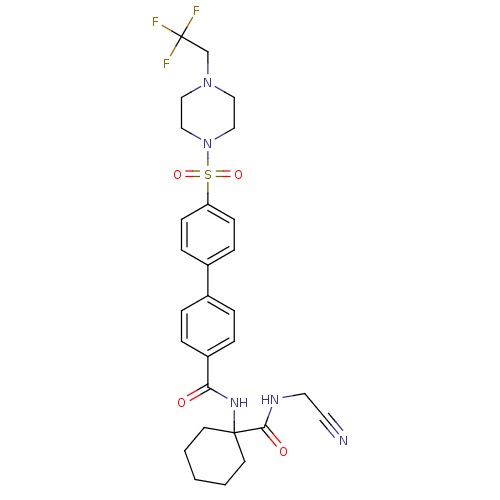
(CHEMBL197440)Show SMILES FC(F)(F)CN1CCN(CC1)S(=O)(=O)c1ccc(cc1)-c1ccc(cc1)C(=O)NC1(CCCCC1)C(=O)NCC#N Show InChI InChI=1S/C28H32F3N5O4S/c29-28(30,31)20-35-16-18-36(19-17-35)41(39,40)24-10-8-22(9-11-24)21-4-6-23(7-5-21)25(37)34-27(12-2-1-3-13-27)26(38)33-15-14-32/h4-11H,1-3,12-13,15-20H2,(H,33,38)(H,34,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Oryctolagus cuniculus (rabbit)) | BDBM50410589
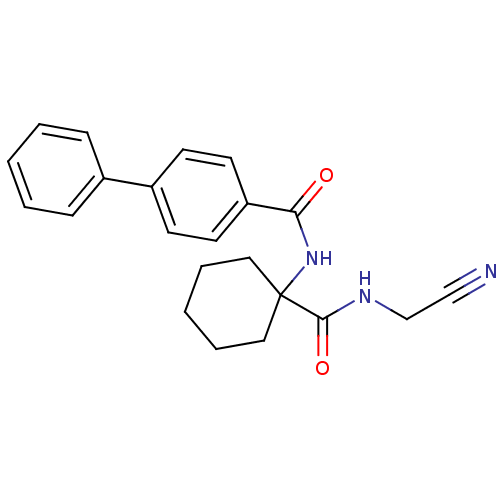
(CHEMBL200619)Show SMILES O=C(NC1(CCCCC1)C(=O)NCC#N)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C22H23N3O2/c23-15-16-24-21(27)22(13-5-2-6-14-22)25-20(26)19-11-9-18(10-12-19)17-7-3-1-4-8-17/h1,3-4,7-12H,2,5-6,13-14,16H2,(H,24,27)(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory constant against rabbit cathepsin K using Z-Phe-Arg-AMC substrate |
J Med Chem 48: 7520-34 (2005)
Article DOI: 10.1021/jm058198r
BindingDB Entry DOI: 10.7270/Q23T9GSB |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411016
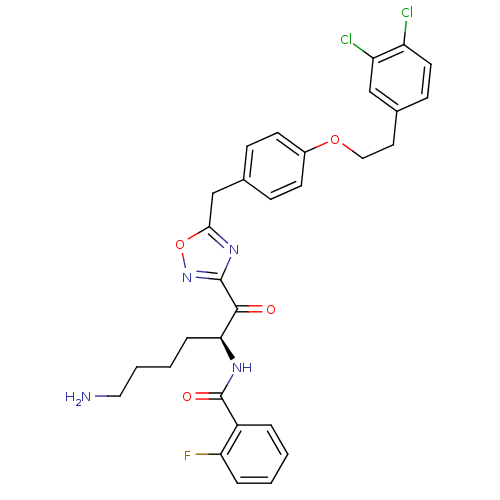
(CHEMBL559161)Show SMILES NCCCC[C@H](NC(=O)c1ccccc1F)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H29Cl2FN4O4/c31-23-13-10-20(17-24(23)32)14-16-40-21-11-8-19(9-12-21)18-27-36-29(37-41-27)28(38)26(7-3-4-15-34)35-30(39)22-5-1-2-6-25(22)33/h1-2,5-6,8-13,17,26H,3-4,7,14-16,18,34H2,(H,35,39)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411007
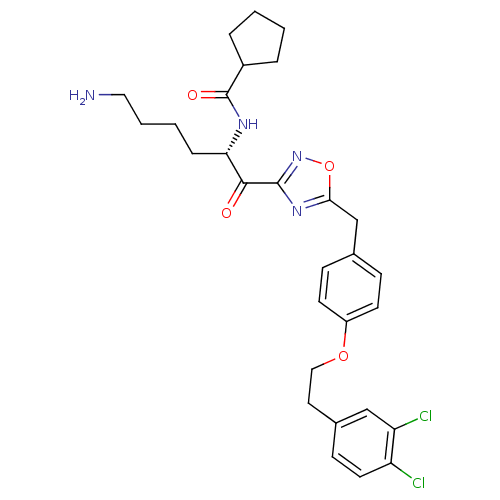
(CHEMBL534492)Show SMILES NCCCC[C@H](NC(=O)C1CCCC1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C29H34Cl2N4O4/c30-23-13-10-20(17-24(23)31)14-16-38-22-11-8-19(9-12-22)18-26-34-28(35-39-26)27(36)25(7-3-4-15-32)33-29(37)21-5-1-2-6-21/h8-13,17,21,25H,1-7,14-16,18,32H2,(H,33,37)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411018
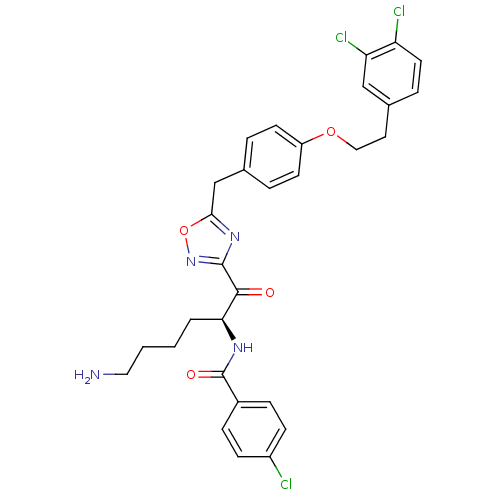
(CHEMBL559184)Show SMILES NCCCC[C@H](NC(=O)c1ccc(Cl)cc1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H29Cl3N4O4/c31-22-9-7-21(8-10-22)30(39)35-26(3-1-2-15-34)28(38)29-36-27(41-37-29)18-19-4-11-23(12-5-19)40-16-14-20-6-13-24(32)25(33)17-20/h4-13,17,26H,1-3,14-16,18,34H2,(H,35,39)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50411010

(CHEMBL535163)Show SMILES CC(C)C(=O)N[C@@H](CCCCN)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C27H32Cl2N4O4/c1-17(2)27(35)31-23(5-3-4-13-30)25(34)26-32-24(37-33-26)16-18-6-9-20(10-7-18)36-14-12-19-8-11-21(28)22(29)15-19/h6-11,15,17,23H,3-5,12-14,16,30H2,1-2H3,(H,31,35)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50410995
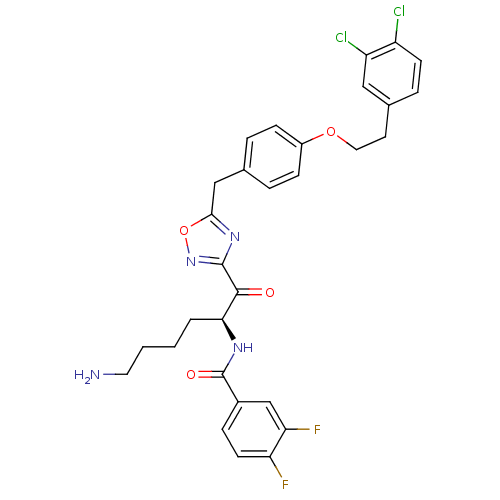
(CHEMBL536972)Show SMILES NCCCC[C@H](NC(=O)c1ccc(F)c(F)c1)C(=O)c1noc(Cc2ccc(OCCc3ccc(Cl)c(Cl)c3)cc2)n1 |r| Show InChI InChI=1S/C30H28Cl2F2N4O4/c31-22-10-6-19(15-23(22)32)12-14-41-21-8-4-18(5-9-21)16-27-37-29(38-42-27)28(39)26(3-1-2-13-35)36-30(40)20-7-11-24(33)25(34)17-20/h4-11,15,17,26H,1-3,12-14,16,35H2,(H,36,40)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics
Curated by ChEMBL
| Assay Description
Inhibition of human B tryptase |
Bioorg Med Chem Lett 16: 3434-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.013
BindingDB Entry DOI: 10.7270/Q2G44RHP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data