 Found 2122 hits with Last Name = 'sabat' and Initial = 'm'
Found 2122 hits with Last Name = 'sabat' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
DNA polymerase beta
(Rattus norvegicus) | BDBM50241570
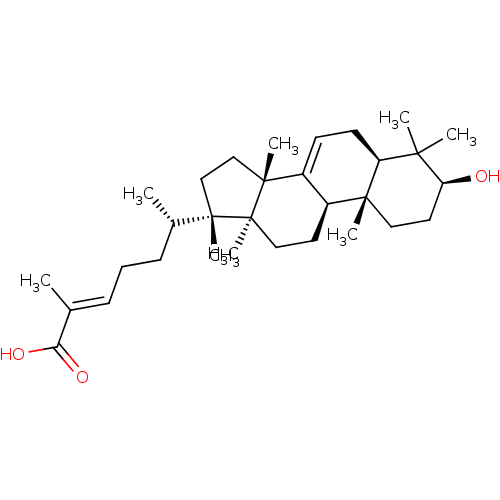
((24E)-3beta-hydroxy-7,24-euphadien-26-oic acid | C...)Show SMILES C[C@@H](CC\C=C(/C)C(O)=O)[C@]1(C)CC[C@]2(C)C3=CC[C@H]4C(C)(C)[C@@H](O)CC[C@]4(C)[C@H]3CC[C@@]12C |r,t:16| Show InChI InChI=1S/C31H50O3/c1-20(26(33)34)10-9-11-21(2)29(6)18-19-30(7)23-12-13-24-27(3,4)25(32)15-16-28(24,5)22(23)14-17-31(29,30)8/h10,12,21-22,24-25,32H,9,11,13-19H2,1-8H3,(H,33,34)/b20-10+/t21-,22-,24-,25-,28+,29-,30+,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant DNA polymerase beta after 20 mins by uncompetitive inhibition assay in presence of activated calf thymus DNA and 0.1 mg... |
J Nat Prod 63: 1356-60 (2000)
BindingDB Entry DOI: 10.7270/Q2X066S2 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM30344
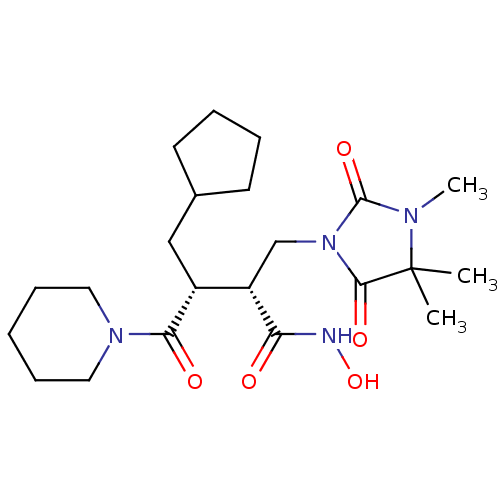
(Cipemastat | Trocade)Show SMILES CN1C(=O)N(C[C@@H]([C@@H](CC2CCCC2)C(=O)N2CCCCC2)C(=O)NO)C(=O)C1(C)C |r| Show InChI InChI=1S/C22H36N4O5/c1-22(2)20(29)26(21(30)24(22)3)14-17(18(27)23-31)16(13-15-9-5-6-10-15)19(28)25-11-7-4-8-12-25/h15-17,31H,4-14H2,1-3H3,(H,23,27)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-1 (MMP1) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Rattus norvegicus) | BDBM50241570

((24E)-3beta-hydroxy-7,24-euphadien-26-oic acid | C...)Show SMILES C[C@@H](CC\C=C(/C)C(O)=O)[C@]1(C)CC[C@]2(C)C3=CC[C@H]4C(C)(C)[C@@H](O)CC[C@]4(C)[C@H]3CC[C@@]12C |r,t:16| Show InChI InChI=1S/C31H50O3/c1-20(26(33)34)10-9-11-21(2)29(6)18-19-30(7)23-12-13-24-27(3,4)25(32)15-16-28(24,5)22(23)14-17-31(29,30)8/h10,12,21-22,24-25,32H,9,11,13-19H2,1-8H3,(H,33,34)/b20-10+/t21-,22-,24-,25-,28+,29-,30+,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant DNA polymerase beta after 20 mins by noncompetitive inhibition assay in presence of activated calf thymus DNA and 0.1 m... |
J Nat Prod 63: 1356-60 (2000)
BindingDB Entry DOI: 10.7270/Q2X066S2 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Rattus norvegicus) | BDBM50241570

((24E)-3beta-hydroxy-7,24-euphadien-26-oic acid | C...)Show SMILES C[C@@H](CC\C=C(/C)C(O)=O)[C@]1(C)CC[C@]2(C)C3=CC[C@H]4C(C)(C)[C@@H](O)CC[C@]4(C)[C@H]3CC[C@@]12C |r,t:16| Show InChI InChI=1S/C31H50O3/c1-20(26(33)34)10-9-11-21(2)29(6)18-19-30(7)23-12-13-24-27(3,4)25(32)15-16-28(24,5)22(23)14-17-31(29,30)8/h10,12,21-22,24-25,32H,9,11,13-19H2,1-8H3,(H,33,34)/b20-10+/t21-,22-,24-,25-,28+,29-,30+,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant DNA polymerase beta after 20 mins by uncompetitive inhibition assay in presence of [3H]dTTP and 0.1 mg/mL BSA |
J Nat Prod 63: 1356-60 (2000)
BindingDB Entry DOI: 10.7270/Q2X066S2 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Rattus norvegicus) | BDBM50241570

((24E)-3beta-hydroxy-7,24-euphadien-26-oic acid | C...)Show SMILES C[C@@H](CC\C=C(/C)C(O)=O)[C@]1(C)CC[C@]2(C)C3=CC[C@H]4C(C)(C)[C@@H](O)CC[C@]4(C)[C@H]3CC[C@@]12C |r,t:16| Show InChI InChI=1S/C31H50O3/c1-20(26(33)34)10-9-11-21(2)29(6)18-19-30(7)23-12-13-24-27(3,4)25(32)15-16-28(24,5)22(23)14-17-31(29,30)8/h10,12,21-22,24-25,32H,9,11,13-19H2,1-8H3,(H,33,34)/b20-10+/t21-,22-,24-,25-,28+,29-,30+,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
Inhibition of rat recombinant DNA polymerase beta after 20 mins by noncompetitive inhibition assay in presence of [3H]dTTP and 0.1 mg/mL BSA |
J Nat Prod 63: 1356-60 (2000)
BindingDB Entry DOI: 10.7270/Q2X066S2 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM350321
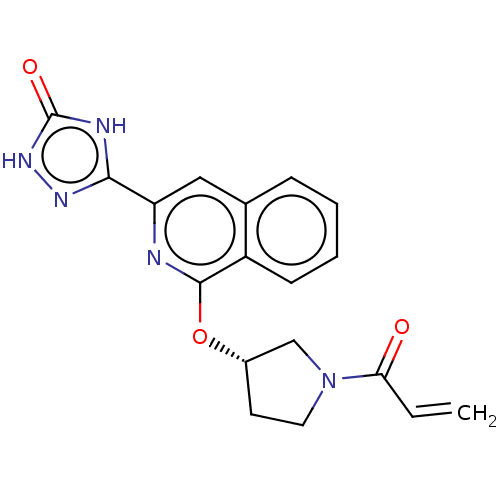
((S)-3-(1-((1-acryloylpyrrolidin-3-yl)oxy)isoquinol...)Show SMILES C=CC(=O)N1CC[C@@H](C1)Oc1nc(cc2ccccc12)-c1n[nH]c(=O)[nH]1 |r| Show InChI InChI=1S/C18H15N5O3/c1-2-15(24)23-8-7-12(10-23)26-17-13-6-4-3-5-11(13)9-14(19-17)16-20-18(25)22-21-16/h2-6,9,12H,1,7-8,10H2/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human Jak3 |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM350321

((S)-3-(1-((1-acryloylpyrrolidin-3-yl)oxy)isoquinol...)Show SMILES C=CC(=O)N1CC[C@@H](C1)Oc1nc(cc2ccccc12)-c1n[nH]c(=O)[nH]1 |r| Show InChI InChI=1S/C18H15N5O3/c1-2-15(24)23-8-7-12(10-23)26-17-13-6-4-3-5-11(13)9-14(19-17)16-20-18(25)22-21-16/h2-6,9,12H,1,7-8,10H2/t12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human EGFR |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM350321

((S)-3-(1-((1-acryloylpyrrolidin-3-yl)oxy)isoquinol...)Show SMILES C=CC(=O)N1CC[C@@H](C1)Oc1nc(cc2ccccc12)-c1n[nH]c(=O)[nH]1 |r| Show InChI InChI=1S/C18H15N5O3/c1-2-15(24)23-8-7-12(10-23)26-17-13-6-4-3-5-11(13)9-14(19-17)16-20-18(25)22-21-16/h2-6,9,12H,1,7-8,10H2/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human ITK |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01026
BindingDB Entry DOI: 10.7270/Q2JM2FHN |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50096646
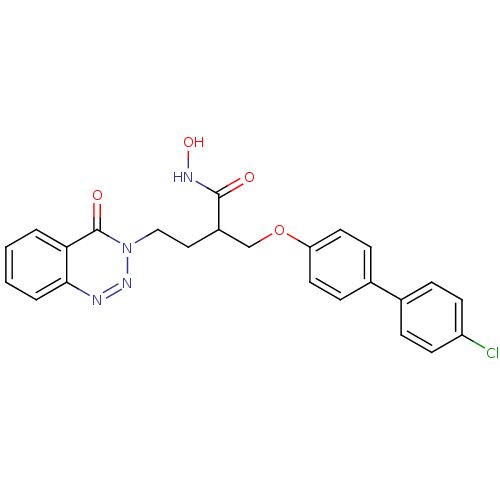
(2-(4'-Chloro-biphenyl-4-yloxymethyl)-N-hydroxy-4-(...)Show SMILES ONC(=O)C(CCn1nnc2ccccc2c1=O)COc1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H21ClN4O4/c25-19-9-5-16(6-10-19)17-7-11-20(12-8-17)33-15-18(23(30)27-32)13-14-29-24(31)21-3-1-2-4-22(21)26-28-29/h1-12,18,32H,13-15H2,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-13 |
Bioorg Med Chem Lett 11: 295-9 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KD9 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50131941
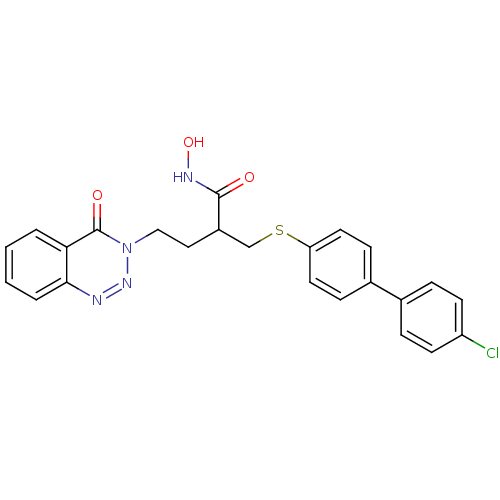
(2-(4'-Chloro-biphenyl-4-ylsulfanylmethyl)-N-hydrox...)Show SMILES ONC(=O)C(CCn1nnc2ccccc2c1=O)CSc1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H21ClN4O3S/c25-19-9-5-16(6-10-19)17-7-11-20(12-8-17)33-15-18(23(30)27-32)13-14-29-24(31)21-3-1-2-4-22(21)26-28-29/h1-12,18,32H,13-15H2,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-2 (MMP2) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50096646

(2-(4'-Chloro-biphenyl-4-yloxymethyl)-N-hydroxy-4-(...)Show SMILES ONC(=O)C(CCn1nnc2ccccc2c1=O)COc1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H21ClN4O4/c25-19-9-5-16(6-10-19)17-7-11-20(12-8-17)33-15-18(23(30)27-32)13-14-29-24(31)21-3-1-2-4-22(21)26-28-29/h1-12,18,32H,13-15H2,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-2 |
Bioorg Med Chem Lett 11: 295-9 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KD9 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50593468
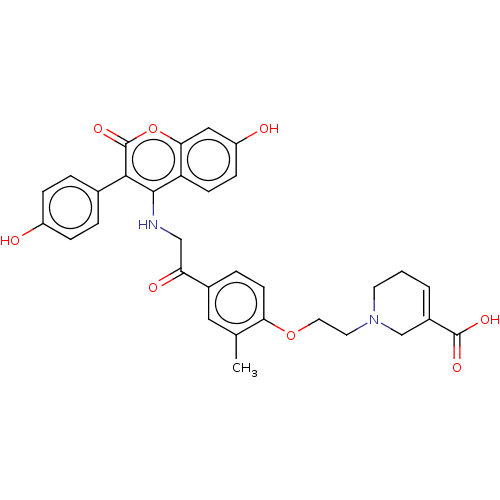
(CHEMBL5199803)Show SMILES Cc1cc(ccc1OCCN1CCC=C(C1)C(O)=O)C(=O)CNc1c(-c2ccc(O)cc2)c(=O)oc2cc(O)ccc12 |c:14| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113869
BindingDB Entry DOI: 10.7270/Q2K0788F |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50450688
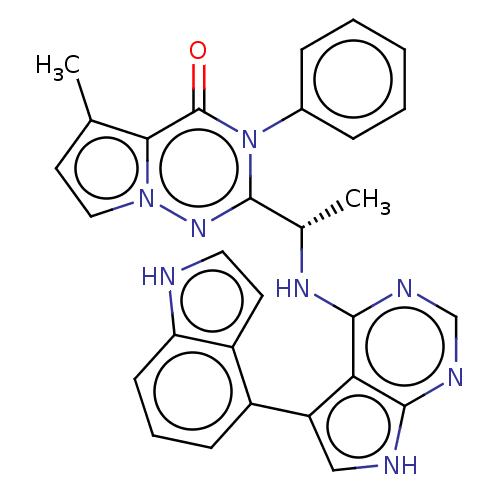
(CHEMBL4168086)Show SMILES C[C@H](Nc1ncnc2[nH]cc(-c3cccc4[nH]ccc34)c12)c1nn2ccc(C)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C29H24N8O/c1-17-12-14-36-25(17)29(38)37(19-7-4-3-5-8-19)28(35-36)18(2)34-27-24-22(15-31-26(24)32-16-33-27)20-9-6-10-23-21(20)11-13-30-23/h3-16,18,30H,1-2H3,(H2,31,32,33,34)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged full length p110delta/recombinant human full length p85alpha using PIP2 as substrate preincubat... |
J Med Chem 61: 9551-9567 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00873
BindingDB Entry DOI: 10.7270/Q2GB26MQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50450687
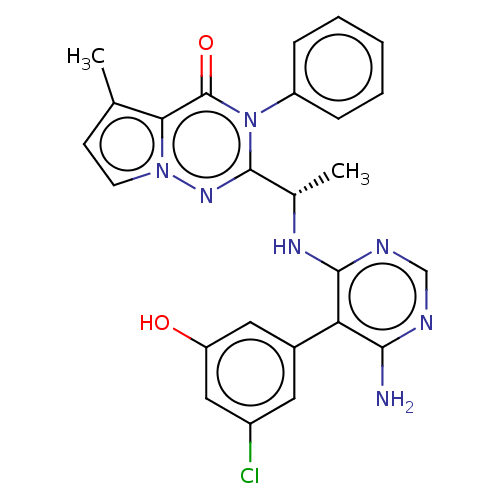
(CHEMBL4164818)Show SMILES C[C@H](Nc1ncnc(N)c1-c1cc(O)cc(Cl)c1)c1nn2ccc(C)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C25H22ClN7O2/c1-14-8-9-32-21(14)25(35)33(18-6-4-3-5-7-18)24(31-32)15(2)30-23-20(22(27)28-13-29-23)16-10-17(26)12-19(34)11-16/h3-13,15,34H,1-2H3,(H3,27,28,29,30)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged full length p110delta/recombinant human full length p85alpha using PIP2 as substrate preincubat... |
J Med Chem 61: 9551-9567 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00873
BindingDB Entry DOI: 10.7270/Q2GB26MQ |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 295-9 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KD9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM236935
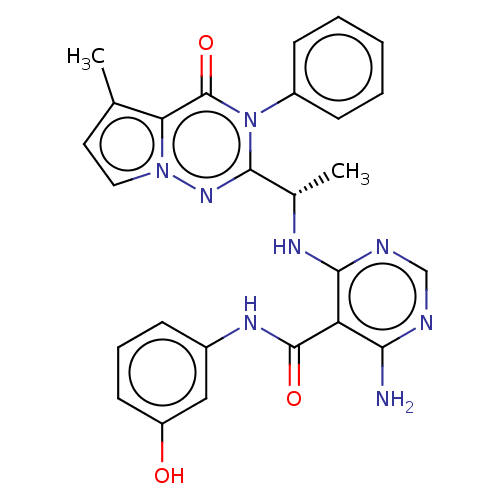
(US9388189, 36)Show SMILES C[C@H](Nc1ncnc(N)c1C(=O)Nc1cccc(O)c1)c1nn2ccc(C)c2c(=O)n1-c1ccccc1 Show InChI InChI=1S/C26H24N8O3/c1-15-11-12-33-21(15)26(37)34(18-8-4-3-5-9-18)24(32-33)16(2)30-23-20(22(27)28-14-29-23)25(36)31-17-7-6-10-19(35)13-17/h3-14,16,35H,1-2H3,(H,31,36)(H3,27,28,29,30)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged full length p110delta/recombinant human full length p85alpha using PIP2 as substrate preincubat... |
J Med Chem 61: 9551-9567 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00873
BindingDB Entry DOI: 10.7270/Q2GB26MQ |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-9 (MMP9) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM236931
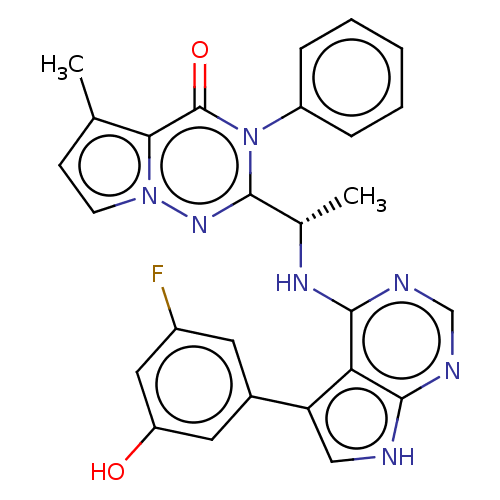
(US9388189, 27)Show SMILES C[C@H](Nc1ncnc2[nH]cc(-c3cc(O)cc(F)c3)c12)c1nn2ccc(C)c2c(=O)n1-c1ccccc1 Show InChI InChI=1S/C27H22FN7O2/c1-15-8-9-34-23(15)27(37)35(19-6-4-3-5-7-19)26(33-34)16(2)32-25-22-21(13-29-24(22)30-14-31-25)17-10-18(28)12-20(36)11-17/h3-14,16,36H,1-2H3,(H2,29,30,31,32)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged full length p110delta/recombinant human full length p85alpha using PIP2 as substrate preincubat... |
J Med Chem 61: 9551-9567 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00873
BindingDB Entry DOI: 10.7270/Q2GB26MQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50450679
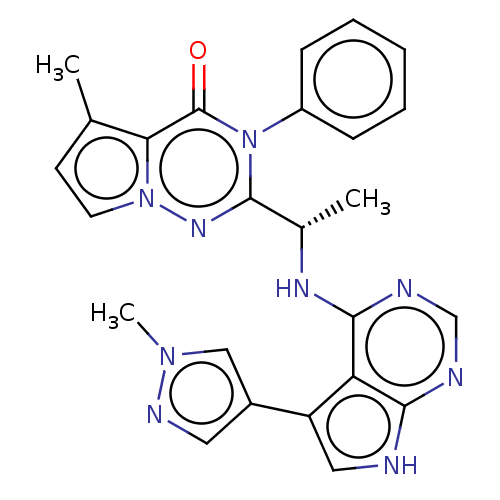
(CHEMBL4164652)Show SMILES C[C@H](Nc1ncnc2[nH]cc(-c3cnn(C)c3)c12)c1nn2ccc(C)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C25H23N9O/c1-15-9-10-33-21(15)25(35)34(18-7-5-4-6-8-18)24(31-33)16(2)30-23-20-19(17-11-29-32(3)13-17)12-26-22(20)27-14-28-23/h4-14,16H,1-3H3,(H2,26,27,28,30)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged full length p110delta/recombinant human full length p85alpha using PIP2 as substrate preincubat... |
J Med Chem 61: 9551-9567 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00873
BindingDB Entry DOI: 10.7270/Q2GB26MQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50450680
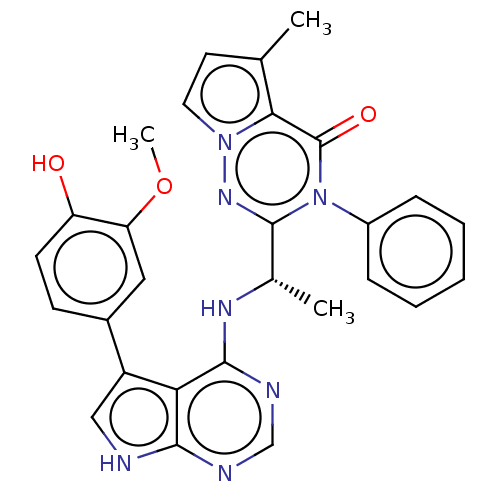
(CHEMBL4168702)Show SMILES COc1cc(ccc1O)-c1c[nH]c2ncnc(N[C@@H](C)c3nn4ccc(C)c4c(=O)n3-c3ccccc3)c12 |r| Show InChI InChI=1S/C28H25N7O3/c1-16-11-12-34-24(16)28(37)35(19-7-5-4-6-8-19)27(33-34)17(2)32-26-23-20(14-29-25(23)30-15-31-26)18-9-10-21(36)22(13-18)38-3/h4-15,17,36H,1-3H3,(H2,29,30,31,32)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged full length p110delta/recombinant human full length p85alpha using PIP2 as substrate preincubat... |
J Med Chem 61: 9551-9567 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00873
BindingDB Entry DOI: 10.7270/Q2GB26MQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50450675

(CHEMBL4166977)Show SMILES C[C@H](Nc1ncnc2[nH]cc(-c3cc(O)cc(NS(C)(=O)=O)c3)c12)c1nn2ccc(C)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C28H26N8O4S/c1-16-9-10-35-24(16)28(38)36(20-7-5-4-6-8-20)27(33-35)17(2)32-26-23-22(14-29-25(23)30-15-31-26)18-11-19(13-21(37)12-18)34-41(3,39)40/h4-15,17,34,37H,1-3H3,(H2,29,30,31,32)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged full length p110delta/recombinant human full length p85alpha using PIP2 as substrate preincubat... |
J Med Chem 61: 9551-9567 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00873
BindingDB Entry DOI: 10.7270/Q2GB26MQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50450693
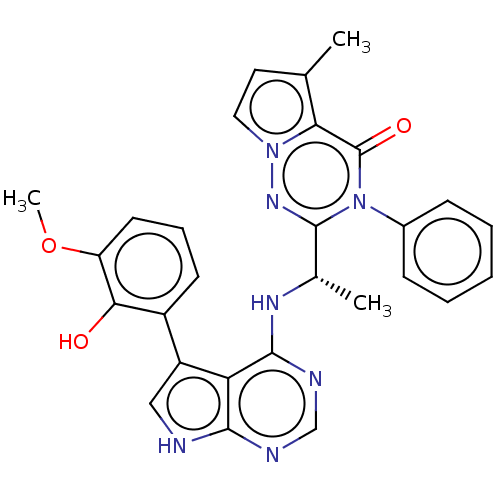
(CHEMBL4164842)Show SMILES COc1cccc(-c2c[nH]c3ncnc(N[C@@H](C)c4nn5ccc(C)c5c(=O)n4-c4ccccc4)c23)c1O |r| Show InChI InChI=1S/C28H25N7O3/c1-16-12-13-34-23(16)28(37)35(18-8-5-4-6-9-18)27(33-34)17(2)32-26-22-20(14-29-25(22)30-15-31-26)19-10-7-11-21(38-3)24(19)36/h4-15,17,36H,1-3H3,(H2,29,30,31,32)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged full length p110delta/recombinant human full length p85alpha using PIP2 as substrate preincubat... |
J Med Chem 61: 9551-9567 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00873
BindingDB Entry DOI: 10.7270/Q2GB26MQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM236932
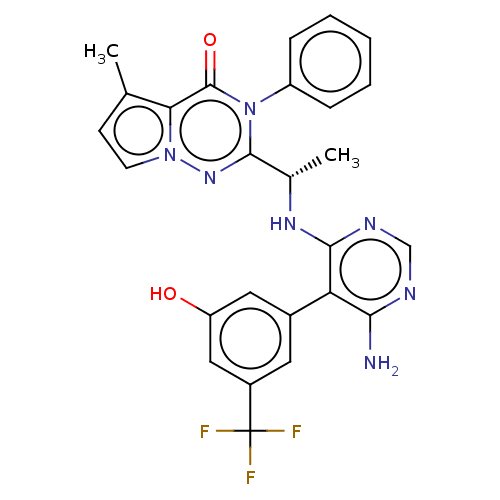
(US9388189, 28)Show SMILES C[C@H](Nc1ncnc(N)c1-c1cc(O)cc(c1)C(F)(F)F)c1nn2ccc(C)c2c(=O)n1-c1ccccc1 Show InChI InChI=1S/C26H22F3N7O2/c1-14-8-9-35-21(14)25(38)36(18-6-4-3-5-7-18)24(34-35)15(2)33-23-20(22(30)31-13-32-23)16-10-17(26(27,28)29)12-19(37)11-16/h3-13,15,37H,1-2H3,(H3,30,31,32,33)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged full length p110delta/recombinant human full length p85alpha using PIP2 as substrate preincubat... |
J Med Chem 61: 9551-9567 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00873
BindingDB Entry DOI: 10.7270/Q2GB26MQ |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50593464
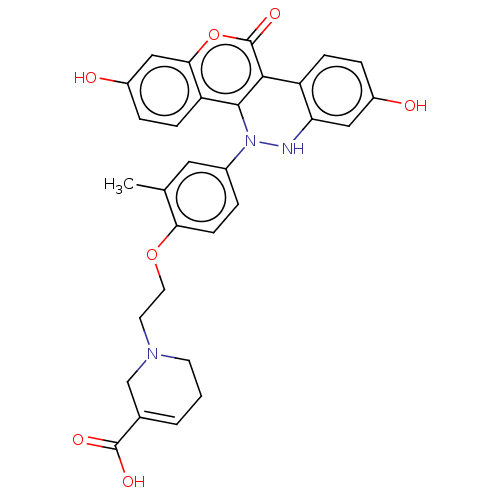
(CHEMBL5184399)Show SMILES Cc1cc(ccc1OCCN1CCC=C(C1)C(O)=O)N1Nc2cc(O)ccc2-c2c1c1ccc(O)cc1oc2=O |c:14| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113869
BindingDB Entry DOI: 10.7270/Q2K0788F |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50328584
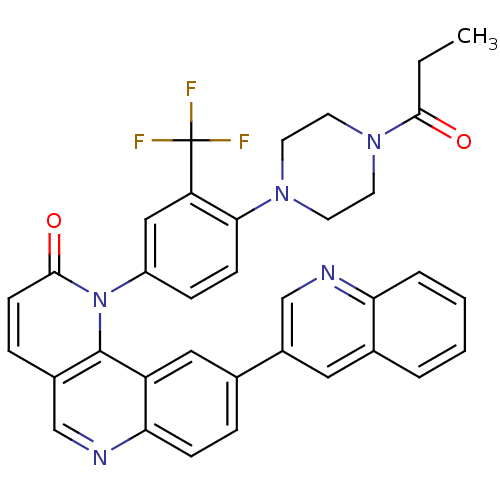
(1-(4-(4-Propionylpiperazin-1-yl)-3-(trifluoromethy...)Show SMILES CCC(=O)N1CCN(CC1)c1ccc(cc1C(F)(F)F)-n1c2c(ccc1=O)cnc1ccc(cc21)-c1cnc2ccccc2c1 Show InChI InChI=1S/C35H28F3N5O2/c1-2-32(44)42-15-13-41(14-16-42)31-11-9-26(19-28(31)35(36,37)38)43-33(45)12-8-24-20-40-30-10-7-22(18-27(30)34(24)43)25-17-23-5-3-4-6-29(23)39-21-25/h3-12,17-21H,2,13-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of human mTOR complex 1 after 30 mins by FRET assay |
J Med Chem 53: 7146-55 (2010)
Article DOI: 10.1021/jm101144f
BindingDB Entry DOI: 10.7270/Q2M32W0R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50450681
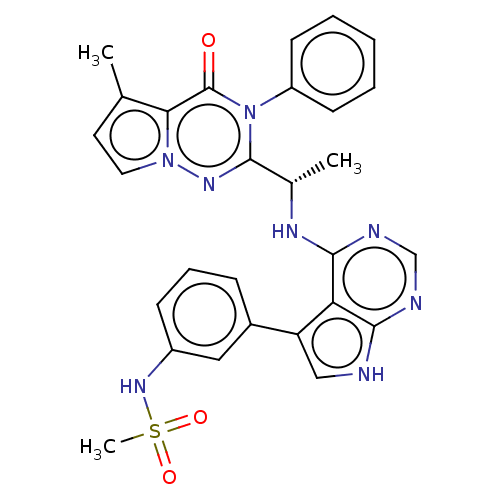
(CHEMBL4171428)Show SMILES C[C@H](Nc1ncnc2[nH]cc(-c3cccc(NS(C)(=O)=O)c3)c12)c1nn2ccc(C)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C28H26N8O3S/c1-17-12-13-35-24(17)28(37)36(21-10-5-4-6-11-21)27(33-35)18(2)32-26-23-22(15-29-25(23)30-16-31-26)19-8-7-9-20(14-19)34-40(3,38)39/h4-16,18,34H,1-3H3,(H2,29,30,31,32)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged full length p110delta/recombinant human full length p85alpha using PIP2 as substrate preincubat... |
J Med Chem 61: 9551-9567 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00873
BindingDB Entry DOI: 10.7270/Q2GB26MQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50450680

(CHEMBL4168702)Show SMILES COc1cc(ccc1O)-c1c[nH]c2ncnc(N[C@@H](C)c3nn4ccc(C)c4c(=O)n3-c3ccccc3)c12 |r| Show InChI InChI=1S/C28H25N7O3/c1-16-11-12-34-24(16)28(37)35(19-7-5-4-6-8-19)27(33-34)17(2)32-26-23-20(14-29-25(23)30-15-31-26)18-9-10-21(36)22(13-18)38-3/h4-15,17,36H,1-3H3,(H2,29,30,31,32)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length p110gamma using PIP2 as substrate preincubated for 30 mins followed by substrate a... |
J Med Chem 61: 9551-9567 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00873
BindingDB Entry DOI: 10.7270/Q2GB26MQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50450674
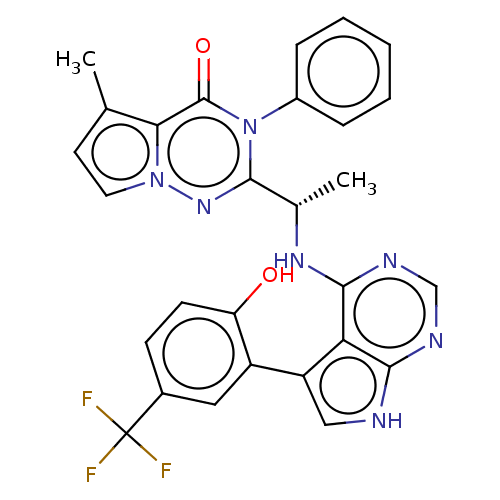
(CHEMBL4163332)Show SMILES C[C@H](Nc1ncnc2[nH]cc(-c3cc(ccc3O)C(F)(F)F)c12)c1nn2ccc(C)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C28H22F3N7O2/c1-15-10-11-37-23(15)27(40)38(18-6-4-3-5-7-18)26(36-37)16(2)35-25-22-20(13-32-24(22)33-14-34-25)19-12-17(28(29,30)31)8-9-21(19)39/h3-14,16,39H,1-2H3,(H2,32,33,34,35)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged full length p110delta/recombinant human full length p85alpha using PIP2 as substrate preincubat... |
J Med Chem 61: 9551-9567 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00873
BindingDB Entry DOI: 10.7270/Q2GB26MQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50450691
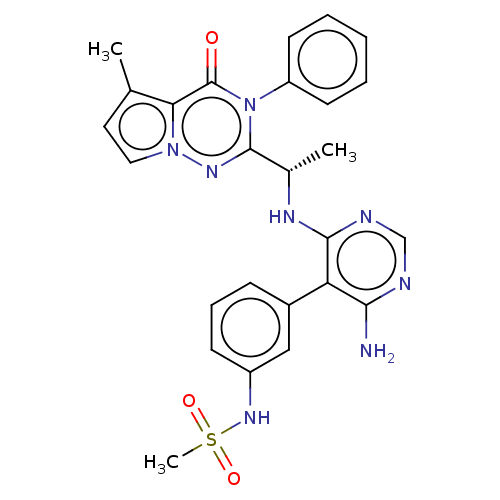
(CHEMBL4168514)Show SMILES C[C@H](Nc1ncnc(N)c1-c1cccc(NS(C)(=O)=O)c1)c1nn2ccc(C)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C26H26N8O3S/c1-16-12-13-33-22(16)26(35)34(20-10-5-4-6-11-20)25(31-33)17(2)30-24-21(23(27)28-15-29-24)18-8-7-9-19(14-18)32-38(3,36)37/h4-15,17,32H,1-3H3,(H3,27,28,29,30)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged full length p110delta/recombinant human full length p85alpha using PIP2 as substrate preincubat... |
J Med Chem 61: 9551-9567 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00873
BindingDB Entry DOI: 10.7270/Q2GB26MQ |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50593462

(CHEMBL5178058)Show SMILES CNCCOc1ccc(cc1)N1Nc2cc(O)ccc2-c2c1c1ccc(O)cc1oc2=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113869
BindingDB Entry DOI: 10.7270/Q2K0788F |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50593471
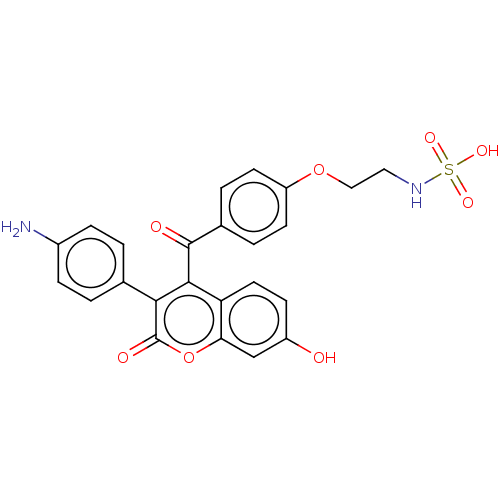
(CHEMBL5195371)Show SMILES Nc1ccc(cc1)-c1c(C(=O)c2ccc(OCCNS(O)(=O)=O)cc2)c2ccc(O)cc2oc1=O | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113869
BindingDB Entry DOI: 10.7270/Q2K0788F |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50474810
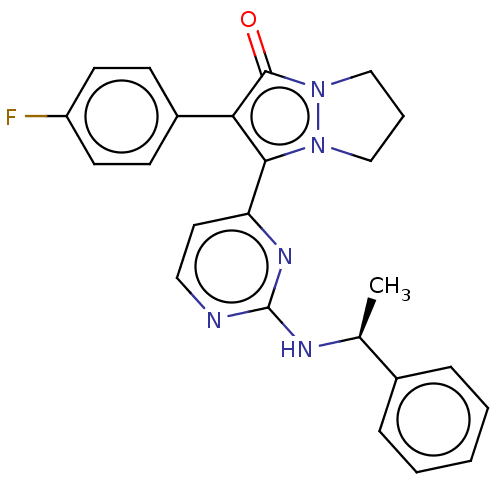
(CHEMBL92082)Show SMILES C[C@H](Nc1nccc(n1)-c1c(-c2ccc(F)cc2)c(=O)n2CCCn12)c1ccccc1 Show InChI InChI=1S/C24H22FN5O/c1-16(17-6-3-2-4-7-17)27-24-26-13-12-20(28-24)22-21(18-8-10-19(25)11-9-18)23(31)30-15-5-14-29(22)30/h2-4,6-13,16H,5,14-15H2,1H3,(H,26,27,28)/t16-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of LPS-stimulated p38-related TNF-alpha production in human peripheral blood mononuclear cells (PBMC) |
J Med Chem 47: 2724-7 (2004)
Article DOI: 10.1021/jm049968m
BindingDB Entry DOI: 10.7270/Q2ZC85NQ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50096651
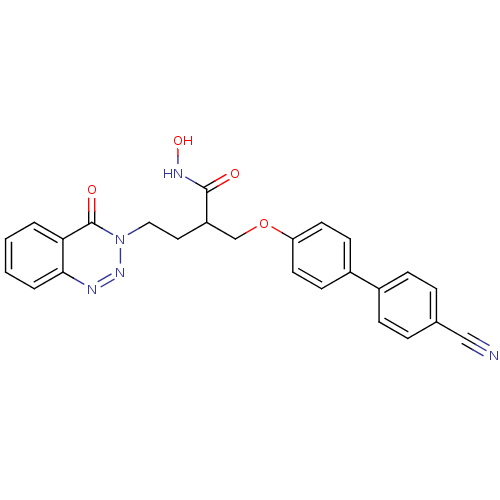
(2-(4'-Cyano-biphenyl-4-yloxymethyl)-N-hydroxy-4-(4...)Show SMILES ONC(=O)C(CCn1nnc2ccccc2c1=O)COc1ccc(cc1)-c1ccc(cc1)C#N Show InChI InChI=1S/C25H21N5O4/c26-15-17-5-7-18(8-6-17)19-9-11-21(12-10-19)34-16-20(24(31)28-33)13-14-30-25(32)22-3-1-2-4-23(22)27-29-30/h1-12,20,33H,13-14,16H2,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-2 |
Bioorg Med Chem Lett 11: 295-9 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KD9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50450695

(CHEMBL4176898)Show SMILES C[C@H](Nc1ncnc2[nH]cc(-c3ccc(O)cc3)c12)c1nn2ccc(C)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C27H23N7O2/c1-16-12-13-33-23(16)27(36)34(19-6-4-3-5-7-19)26(32-33)17(2)31-25-22-21(14-28-24(22)29-15-30-25)18-8-10-20(35)11-9-18/h3-15,17,35H,1-2H3,(H2,28,29,30,31)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged full length p110delta/recombinant human full length p85alpha using PIP2 as substrate preincubat... |
J Med Chem 61: 9551-9567 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00873
BindingDB Entry DOI: 10.7270/Q2GB26MQ |
More data for this
Ligand-Target Pair | |
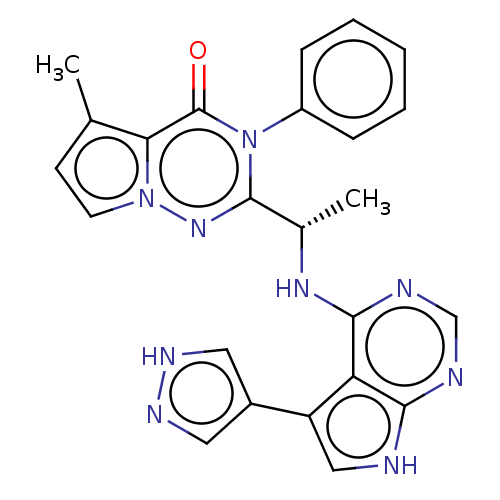
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50450685
(CHEMBL4160167)Show SMILES C[C@H](Nc1ncnc2[nH]cc(-c3cn[nH]c3)c12)c1nn2ccc(C)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C24H21N9O/c1-14-8-9-32-20(14)24(34)33(17-6-4-3-5-7-17)23(31-32)15(2)30-22-19-18(16-10-28-29-11-16)12-25-21(19)26-13-27-22/h3-13,15H,1-2H3,(H,28,29)(H2,25,26,27,30)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged full length p110delta/recombinant human full length p85alpha using PIP2 as substrate preincubat... |
J Med Chem 61: 9551-9567 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00873
BindingDB Entry DOI: 10.7270/Q2GB26MQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50450682
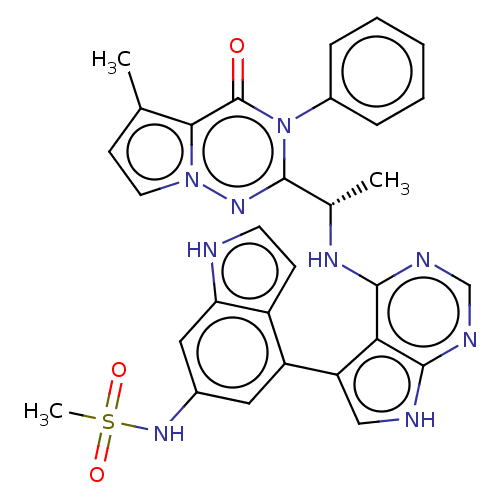
(CHEMBL4167561)Show SMILES C[C@H](Nc1ncnc2[nH]cc(-c3cc(NS(C)(=O)=O)cc4[nH]ccc34)c12)c1nn2ccc(C)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C30H27N9O3S/c1-17-10-12-38-26(17)30(40)39(20-7-5-4-6-8-20)29(36-38)18(2)35-28-25-23(15-32-27(25)33-16-34-28)22-13-19(37-43(3,41)42)14-24-21(22)9-11-31-24/h4-16,18,31,37H,1-3H3,(H2,32,33,34,35)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged full length p110delta/recombinant human full length p85alpha using PIP2 as substrate preincubat... |
J Med Chem 61: 9551-9567 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00873
BindingDB Entry DOI: 10.7270/Q2GB26MQ |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50096649
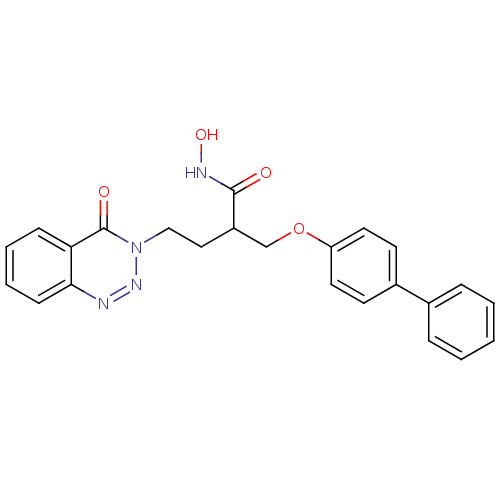
(2-(Biphenyl-4-yloxymethyl)-N-hydroxy-4-(4-oxo-4H-b...)Show SMILES ONC(=O)C(CCn1nnc2ccccc2c1=O)COc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C24H22N4O4/c29-23(26-31)19(14-15-28-24(30)21-8-4-5-9-22(21)25-27-28)16-32-20-12-10-18(11-13-20)17-6-2-1-3-7-17/h1-13,19,31H,14-16H2,(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-2 |
Bioorg Med Chem Lett 11: 295-9 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KD9 |
More data for this
Ligand-Target Pair | |
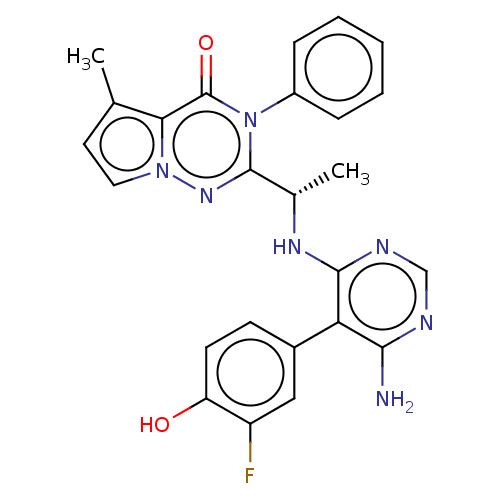
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50450697
(CHEMBL4176794)Show SMILES C[C@H](Nc1ncnc(N)c1-c1ccc(O)c(F)c1)c1nn2ccc(C)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C25H22FN7O2/c1-14-10-11-32-21(14)25(35)33(17-6-4-3-5-7-17)24(31-32)15(2)30-23-20(22(27)28-13-29-23)16-8-9-19(34)18(26)12-16/h3-13,15,34H,1-2H3,(H3,27,28,29,30)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged full length p110delta/recombinant human full length p85alpha using PIP2 as substrate preincubat... |
J Med Chem 61: 9551-9567 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00873
BindingDB Entry DOI: 10.7270/Q2GB26MQ |
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM50341209
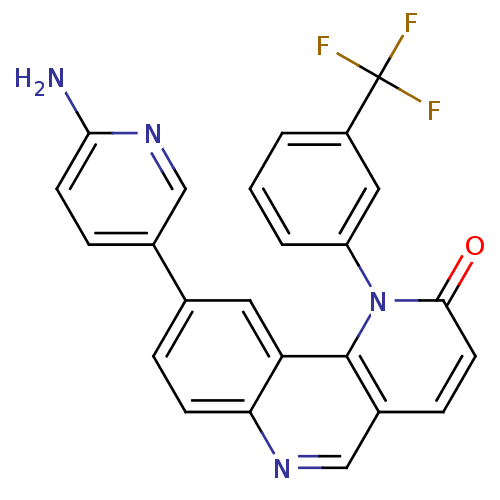
(9-(6-aminopyridin-3-yl)-1-(3-(trifluoromethyl)phen...)Show SMILES Nc1ccc(cn1)-c1ccc2ncc3ccc(=O)n(-c4cccc(c4)C(F)(F)F)c3c2c1 Show InChI InChI=1S/C24H15F3N4O/c25-24(26,27)17-2-1-3-18(11-17)31-22(32)9-6-16-13-29-20-7-4-14(10-19(20)23(16)31)15-5-8-21(28)30-12-15/h1-13H,(H2,28,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of DNA-PK |
J Med Chem 54: 1473-80 (2011)
Article DOI: 10.1021/jm101520v
BindingDB Entry DOI: 10.7270/Q2S46S8H |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-2 |
Bioorg Med Chem Lett 11: 295-9 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KD9 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-2 (MMP2) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50131941

(2-(4'-Chloro-biphenyl-4-ylsulfanylmethyl)-N-hydrox...)Show SMILES ONC(=O)C(CCn1nnc2ccccc2c1=O)CSc1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H21ClN4O3S/c25-19-9-5-16(6-10-19)17-7-11-20(12-8-17)33-15-18(23(30)27-32)13-14-29-24(31)21-3-1-2-4-22(21)26-28-29/h1-12,18,32H,13-15H2,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-9 (MMP9) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50328605
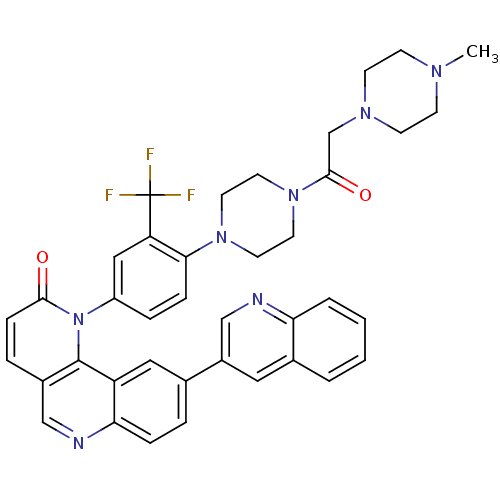
(1-(4-(4-(2-(4-methylpiperazin-1-yl)acetyl)piperazi...)Show SMILES CN1CCN(CC(=O)N2CCN(CC2)c2ccc(cc2C(F)(F)F)-n2c3c(ccc2=O)cnc2ccc(cc32)-c2cnc3ccccc3c2)CC1 Show InChI InChI=1S/C39H36F3N7O2/c1-45-12-14-46(15-13-45)25-37(51)48-18-16-47(17-19-48)35-10-8-30(22-32(35)39(40,41)42)49-36(50)11-7-28-23-44-34-9-6-26(21-31(34)38(28)49)29-20-27-4-2-3-5-33(27)43-24-29/h2-11,20-24H,12-19,25H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Dana Farber Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of human mTOR complex 1 after 30 mins by FRET assay |
J Med Chem 53: 7146-55 (2010)
Article DOI: 10.1021/jm101144f
BindingDB Entry DOI: 10.7270/Q2M32W0R |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM236931

(US9388189, 27)Show SMILES C[C@H](Nc1ncnc2[nH]cc(-c3cc(O)cc(F)c3)c12)c1nn2ccc(C)c2c(=O)n1-c1ccccc1 Show InChI InChI=1S/C27H22FN7O2/c1-15-8-9-34-23(15)27(37)35(19-6-4-3-5-7-19)26(33-34)16(2)32-25-22-21(13-29-24(22)30-14-31-25)17-10-18(28)12-20(36)11-17/h3-14,16,36H,1-2H3,(H2,29,30,31,32)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His6-tagged full length p110gamma using PIP2 as substrate preincubated for 30 mins followed by substrate a... |
J Med Chem 61: 9551-9567 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00873
BindingDB Entry DOI: 10.7270/Q2GB26MQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50450689
(CHEMBL4161674)Show SMILES C[C@H](Nc1ncnc(N)c1-c1cc(O)cc(F)c1)c1nn2ccc(C)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C25H22FN7O2/c1-14-8-9-32-21(14)25(35)33(18-6-4-3-5-7-18)24(31-32)15(2)30-23-20(22(27)28-13-29-23)16-10-17(26)12-19(34)11-16/h3-13,15,34H,1-2H3,(H3,27,28,29,30)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged full length p110delta/recombinant human full length p85alpha using PIP2 as substrate preincubat... |
J Med Chem 61: 9551-9567 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00873
BindingDB Entry DOI: 10.7270/Q2GB26MQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50450690
(CHEMBL4175709)Show SMILES C[C@H](Nc1ncnc(N)c1-c1cc(O)cc(NS(C)(=O)=O)c1)c1nn2ccc(C)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C26H26N8O4S/c1-15-9-10-33-22(15)26(36)34(19-7-5-4-6-8-19)25(31-33)16(2)30-24-21(23(27)28-14-29-24)17-11-18(13-20(35)12-17)32-39(3,37)38/h4-14,16,32,35H,1-3H3,(H3,27,28,29,30)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged full length p110delta/recombinant human full length p85alpha using PIP2 as substrate preincubat... |
J Med Chem 61: 9551-9567 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00873
BindingDB Entry DOI: 10.7270/Q2GB26MQ |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50096651

(2-(4'-Cyano-biphenyl-4-yloxymethyl)-N-hydroxy-4-(4...)Show SMILES ONC(=O)C(CCn1nnc2ccccc2c1=O)COc1ccc(cc1)-c1ccc(cc1)C#N Show InChI InChI=1S/C25H21N5O4/c26-15-17-5-7-18(8-6-17)19-9-11-21(12-10-19)34-16-20(24(31)28-33)13-14-30-25(32)22-3-1-2-4-23(22)27-29-30/h1-12,20,33H,13-14,16H2,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-13 |
Bioorg Med Chem Lett 11: 295-9 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KD9 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50096646

(2-(4'-Chloro-biphenyl-4-yloxymethyl)-N-hydroxy-4-(...)Show SMILES ONC(=O)C(CCn1nnc2ccccc2c1=O)COc1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H21ClN4O4/c25-19-9-5-16(6-10-19)17-7-11-20(12-8-17)33-15-18(23(30)27-32)13-14-29-24(31)21-3-1-2-4-22(21)26-28-29/h1-12,18,32H,13-15H2,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 295-9 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KD9 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50096651

(2-(4'-Cyano-biphenyl-4-yloxymethyl)-N-hydroxy-4-(4...)Show SMILES ONC(=O)C(CCn1nnc2ccccc2c1=O)COc1ccc(cc1)-c1ccc(cc1)C#N Show InChI InChI=1S/C25H21N5O4/c26-15-17-5-7-18(8-6-17)19-9-11-21(12-10-19)34-16-20(24(31)28-33)13-14-30-25(32)22-3-1-2-4-23(22)27-29-30/h1-12,20,33H,13-14,16H2,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 295-9 (2001)
BindingDB Entry DOI: 10.7270/Q2JH3KD9 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50593476
(CHEMBL5191405)Show SMILES CC(CN1CCCC2CCCNC12)OCC(=O)N1Nc2cc(O)ccc2C2COc3cc(O)ccc3C12 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.113869
BindingDB Entry DOI: 10.7270/Q2K0788F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data