

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Mycobacterium tuberculosis) | BDBM50336507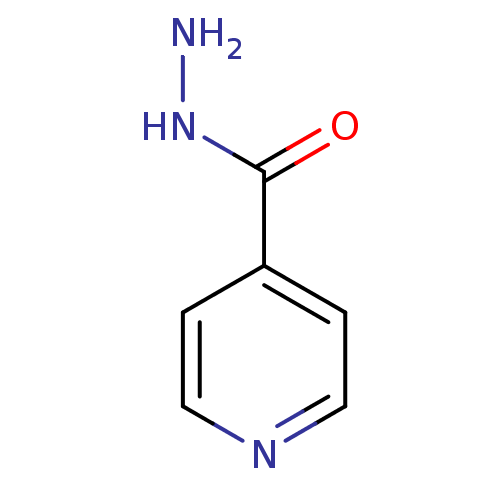 (4-pyridinecarbohydrazide(Isoniazid) | CHEMBL64 | D...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis dihydrofolate reductase | Antimicrob Agents Chemother 54: 3776-82 (2010) Article DOI: 10.1128/AAC.00453-10 BindingDB Entry DOI: 10.7270/Q2F47PFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524390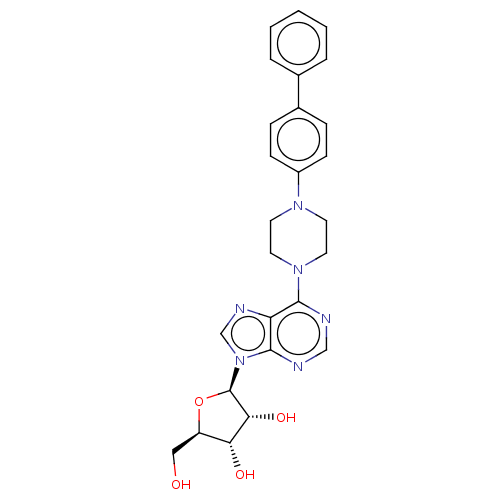 (CHEMBL4475619) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524393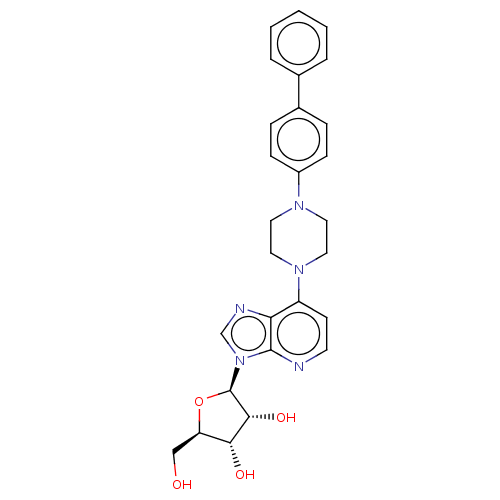 (CHEMBL4448092) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524392 (CHEMBL4475059) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524387 (CHEMBL4566815) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524388 (CHEMBL4449169) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524389 (CHEMBL4525944) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524394 (CHEMBL4539148) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524383 (CHEMBL4442160) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524384 (CHEMBL4458246) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50375654 (CHEMBL99203 | US11633415, Compound 5-iodotubercidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of purified human adenosine kinase using varying levels of [3H]Ado as substrate in presence of adenosine deaminase inhibitor deoxycoformyc... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524385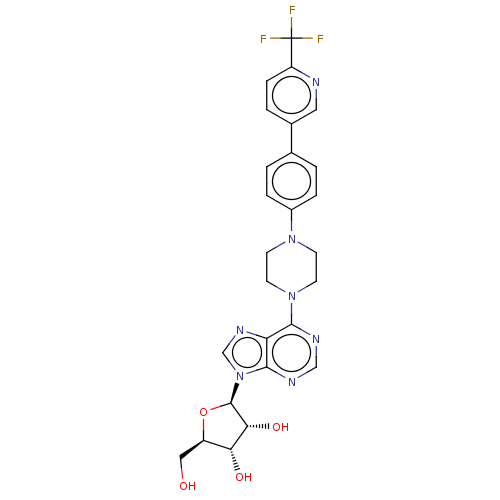 (CHEMBL4463459) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524386 (CHEMBL4474951) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50524391 (CHEMBL2042164) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of wild-type N-terminal TEV cleavage site-fused/His-tagged Mycobacterium tuberculosis H37Rv adenosine kinase expressed in Escherichia coli... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine kinase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50375654 (CHEMBL99203 | US11633415, Compound 5-iodotubercidi...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis H37Ra ATCC 25177 adenosine kinase using varying levels of [3H]Ado as substrate in presence of adenosine deam... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50524383 (CHEMBL4442160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of recombinant human adenosine kinase expressed in Escherichia coli BL21 (DE3) using adenosine as substrate in presence of ATP and PEP by ... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50524385 (CHEMBL4463459) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of recombinant human adenosine kinase expressed in Escherichia coli BL21 (DE3) using adenosine as substrate in presence of ATP and PEP by ... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50524391 (CHEMBL2042164) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of recombinant human adenosine kinase expressed in Escherichia coli BL21 (DE3) using adenosine as substrate in presence of ATP and PEP by ... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50524392 (CHEMBL4475059) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of recombinant human adenosine kinase expressed in Escherichia coli BL21 (DE3) using adenosine as substrate in presence of ATP and PEP by ... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50524393 (CHEMBL4448092) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of recombinant human adenosine kinase expressed in Escherichia coli BL21 (DE3) using adenosine as substrate in presence of ATP and PEP by ... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50524388 (CHEMBL4449169) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of recombinant human adenosine kinase expressed in Escherichia coli BL21 (DE3) using adenosine as substrate in presence of ATP and PEP by ... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50524386 (CHEMBL4474951) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of recombinant human adenosine kinase expressed in Escherichia coli BL21 (DE3) using adenosine as substrate in presence of ATP and PEP by ... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50524387 (CHEMBL4566815) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of recombinant human adenosine kinase expressed in Escherichia coli BL21 (DE3) using adenosine as substrate in presence of ATP and PEP by ... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50524390 (CHEMBL4475619) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of recombinant human adenosine kinase expressed in Escherichia coli BL21 (DE3) using adenosine as substrate in presence of ATP and PEP by ... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50524389 (CHEMBL4525944) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of recombinant human adenosine kinase expressed in Escherichia coli BL21 (DE3) using adenosine as substrate in presence of ATP and PEP by ... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine kinase (Homo sapiens (Human)) | BDBM50524384 (CHEMBL4458246) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Texas A&M University Curated by ChEMBL | Assay Description Inhibition of recombinant human adenosine kinase expressed in Escherichia coli BL21 (DE3) using adenosine as substrate in presence of ATP and PEP by ... | J Med Chem 62: 4483-4499 (2019) Article DOI: 10.1021/acs.jmedchem.9b00020 BindingDB Entry DOI: 10.7270/Q2028VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM25418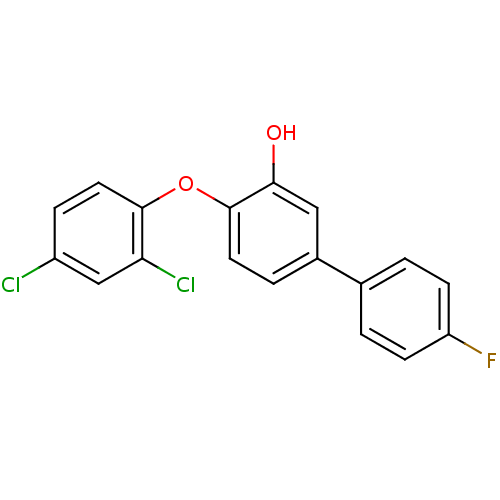 (2-(2,4-dichlorophenoxy)-5-(4-fluorophenyl)phenol |...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Jacobus Pharmaceutical Company | Assay Description PfENR inhibition assays were carried out on a Cary 100 Bio Spectrophotometer or POLARstar Optima by monitoring oxidation of NADH at 340 nm. The IC50 ... | J Biol Chem 282: 25436-44 (2007) Article DOI: 10.1074/jbc.M701813200 BindingDB Entry DOI: 10.7270/Q22V2DFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM25403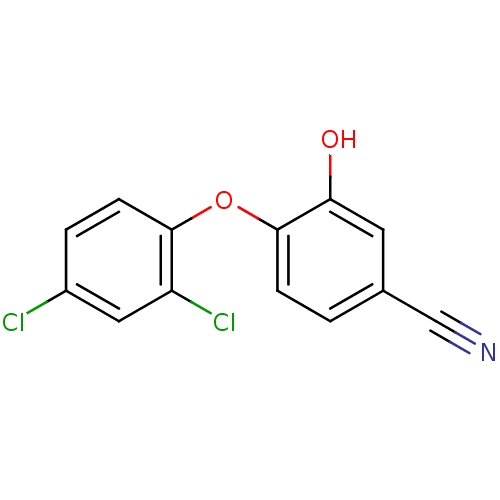 (4‐(2,4‐dichlorophenoxy)‐3‐...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Jacobus Pharmaceutical Company | Assay Description PfENR inhibition assays were carried out on a Cary 100 Bio Spectrophotometer or POLARstar Optima by monitoring oxidation of NADH at 340 nm. The IC50 ... | J Biol Chem 282: 25436-44 (2007) Article DOI: 10.1074/jbc.M701813200 BindingDB Entry DOI: 10.7270/Q22V2DFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM50174772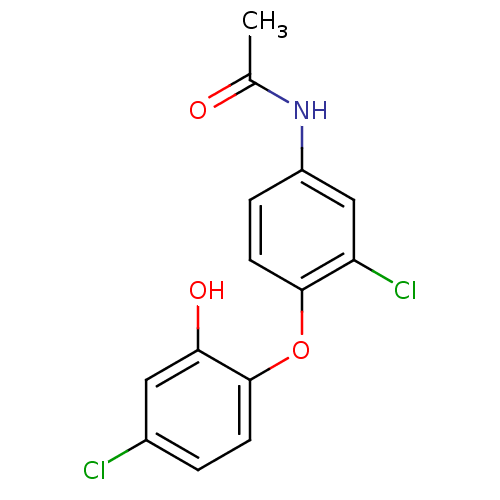 (CHEMBL200658 | N-(3-chloro-4-(4-chloro-2-hydroxyph...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of PfENR enzymatic activity | Bioorg Med Chem Lett 15: 5247-52 (2005) Article DOI: 10.1016/j.bmcl.2005.08.044 BindingDB Entry DOI: 10.7270/Q2NC60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM25419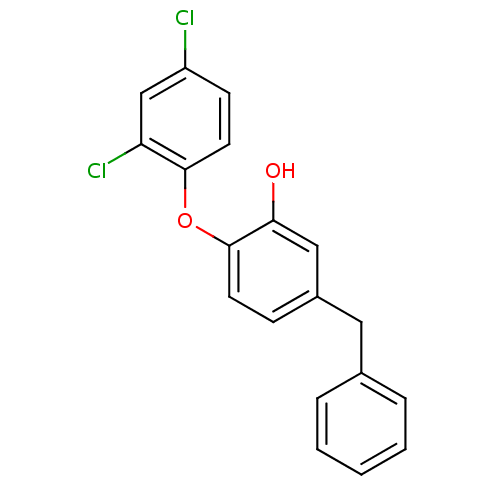 (5-benzyl-2-(2,4-dichlorophenoxy)phenol | Triclosan...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Jacobus Pharmaceutical Company | Assay Description PfENR inhibition assays were carried out on a Cary 100 Bio Spectrophotometer or POLARstar Optima by monitoring oxidation of NADH at 340 nm. The IC50 ... | J Biol Chem 282: 25436-44 (2007) Article DOI: 10.1074/jbc.M701813200 BindingDB Entry DOI: 10.7270/Q22V2DFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM8726 (5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of PfENR enzymatic activity | Bioorg Med Chem Lett 15: 5247-52 (2005) Article DOI: 10.1016/j.bmcl.2005.08.044 BindingDB Entry DOI: 10.7270/Q2NC60RF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM8726 (5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Jacobus Pharmaceutical Company | Assay Description PfENR inhibition assays were carried out on a Cary 100 Bio Spectrophotometer or POLARstar Optima by monitoring oxidation of NADH at 340 nm. The IC50 ... | J Biol Chem 282: 25436-44 (2007) Article DOI: 10.1074/jbc.M701813200 BindingDB Entry DOI: 10.7270/Q22V2DFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM8726 (5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum ENR enzymatic activity | Bioorg Med Chem Lett 16: 2163-9 (2006) Article DOI: 10.1016/j.bmcl.2006.01.051 BindingDB Entry DOI: 10.7270/Q2154GNG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM25420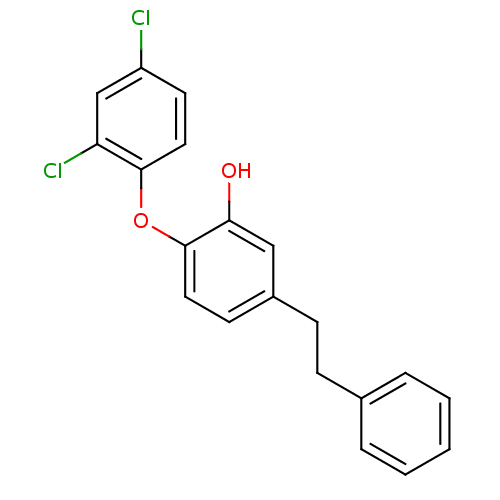 (2-(2,4-dichlorophenoxy)-5-(2-phenylethyl)phenol | ...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Jacobus Pharmaceutical Company | Assay Description PfENR inhibition assays were carried out on a Cary 100 Bio Spectrophotometer or POLARstar Optima by monitoring oxidation of NADH at 340 nm. The IC50 ... | J Biol Chem 282: 25436-44 (2007) Article DOI: 10.1074/jbc.M701813200 BindingDB Entry DOI: 10.7270/Q22V2DFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM25407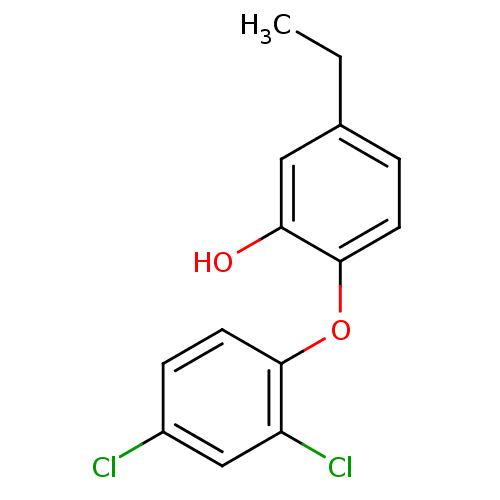 (2‐(2,4‐dichlorophenoxy)‐5‐...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Jacobus Pharmaceutical Company | Assay Description PfENR inhibition assays were carried out on a Cary 100 Bio Spectrophotometer or POLARstar Optima by monitoring oxidation of NADH at 340 nm. The IC50 ... | J Biol Chem 282: 25436-44 (2007) Article DOI: 10.1074/jbc.M701813200 BindingDB Entry DOI: 10.7270/Q22V2DFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM50174766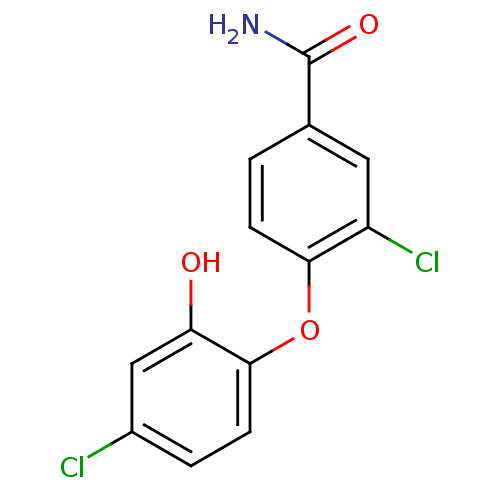 (3-chloro-4-(4-chloro-2-hydroxyphenoxy)benzamide | ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of PfENR enzymatic activity | Bioorg Med Chem Lett 15: 5247-52 (2005) Article DOI: 10.1016/j.bmcl.2005.08.044 BindingDB Entry DOI: 10.7270/Q2NC60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM25412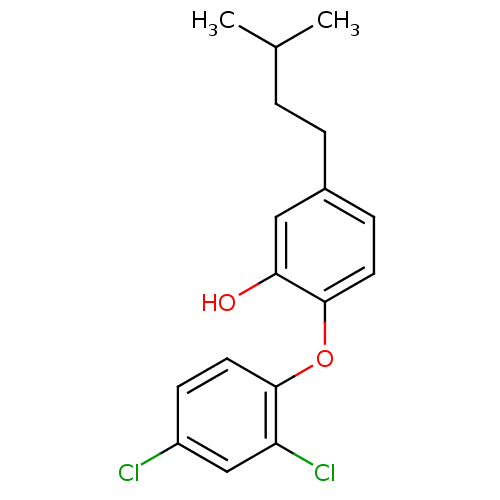 (2-(2,4-dichlorophenoxy)-5-(3-methylbutyl)phenol | ...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Jacobus Pharmaceutical Company | Assay Description PfENR inhibition assays were carried out on a Cary 100 Bio Spectrophotometer or POLARstar Optima by monitoring oxidation of NADH at 340 nm. The IC50 ... | J Biol Chem 282: 25436-44 (2007) Article DOI: 10.1074/jbc.M701813200 BindingDB Entry DOI: 10.7270/Q22V2DFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM50174775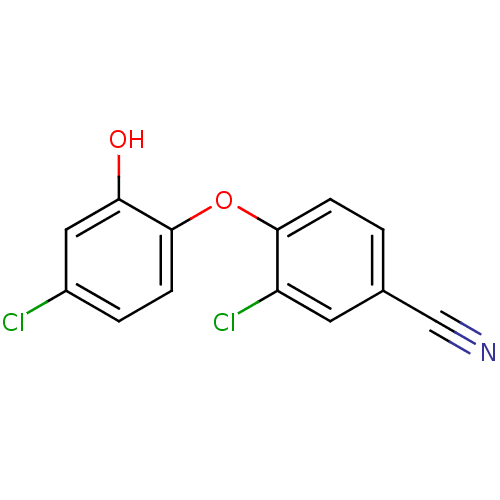 (3-chloro-4-(4-chloro-2-hydroxyphenoxy)benzonitrile...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of PfENR enzymatic activity | Bioorg Med Chem Lett 15: 5247-52 (2005) Article DOI: 10.1016/j.bmcl.2005.08.044 BindingDB Entry DOI: 10.7270/Q2NC60RF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM50174760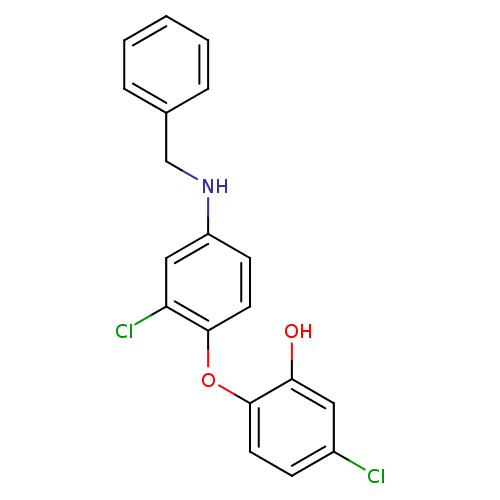 (2‐[4‐(benzylamino)‐2‐chlor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of PfENR enzymatic activity | Bioorg Med Chem Lett 15: 5247-52 (2005) Article DOI: 10.1016/j.bmcl.2005.08.044 BindingDB Entry DOI: 10.7270/Q2NC60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM25402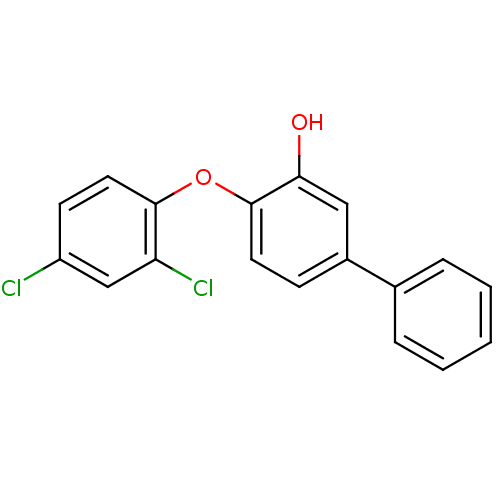 (2-(2,4-dichlorophenoxy)-5-phenylphenol | Triclosan...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Jacobus Pharmaceutical Company | Assay Description PfENR inhibition assays were carried out on a Cary 100 Bio Spectrophotometer or POLARstar Optima by monitoring oxidation of NADH at 340 nm. The IC50 ... | J Biol Chem 282: 25436-44 (2007) Article DOI: 10.1074/jbc.M701813200 BindingDB Entry DOI: 10.7270/Q22V2DFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM50174769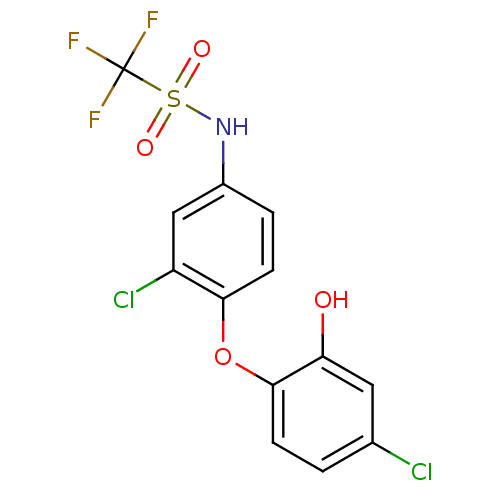 (CHEMBL198781 | N-(3-chloro-4-(4-chloro-2-hydroxyph...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of PfENR enzymatic activity | Bioorg Med Chem Lett 15: 5247-52 (2005) Article DOI: 10.1016/j.bmcl.2005.08.044 BindingDB Entry DOI: 10.7270/Q2NC60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyketide synthase Pks13 (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50582194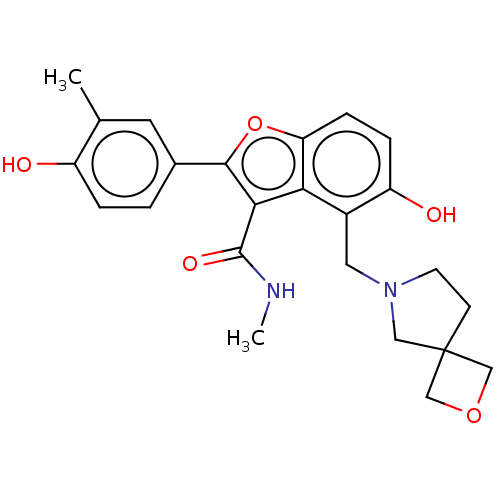 (CHEMBL5085116) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Mycobacterium tuberculosis Pks13-TE domain using 4-methylumbelliferyl heptanoate as a fluorogenic substrate measured for 110 mins at 10... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01586 BindingDB Entry DOI: 10.7270/Q24T6P88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyketide synthase Pks13 (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50582205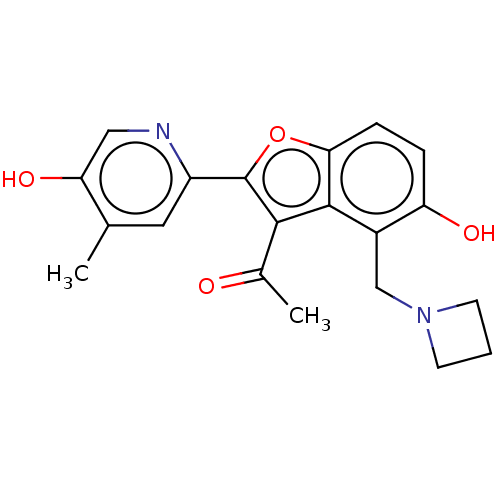 (CHEMBL5094563) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Mycobacterium tuberculosis Pks13-TE domain using 4-methylumbelliferyl heptanoate as a fluorogenic substrate measured for 110 mins at 10... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01586 BindingDB Entry DOI: 10.7270/Q24T6P88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50242245 (CHEMBL4081658) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University Curated by ChEMBL | Assay Description Inhibition of FAS thioster domain (unknown origin) | Bioorg Med Chem 25: 2901-2916 (2017) Article DOI: 10.1016/j.bmc.2017.01.020 BindingDB Entry DOI: 10.7270/Q26W9DG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50242221 (CHEMBL4084033) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University Curated by ChEMBL | Assay Description Inhibition of FAS thioster domain (unknown origin) | Bioorg Med Chem 25: 2901-2916 (2017) Article DOI: 10.1016/j.bmc.2017.01.020 BindingDB Entry DOI: 10.7270/Q26W9DG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM50174778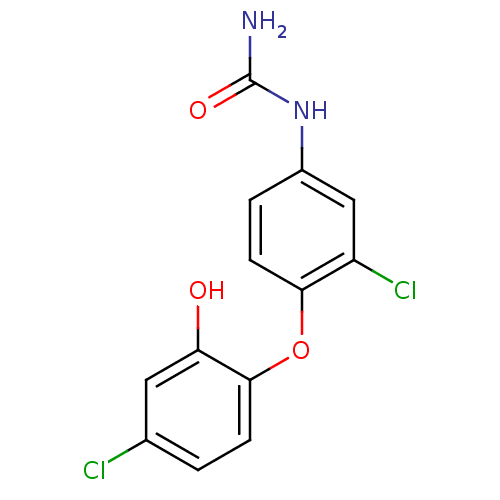 (1-(3-chloro-4-(4-chloro-2-hydroxyphenoxy)phenyl)ur...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of PfENR enzymatic activity | Bioorg Med Chem Lett 15: 5247-52 (2005) Article DOI: 10.1016/j.bmcl.2005.08.044 BindingDB Entry DOI: 10.7270/Q2NC60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty acid synthase (Homo sapiens (Human)) | BDBM50242243 (CHEMBL4059718) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Baylor University Curated by ChEMBL | Assay Description Inhibition of FAS thioster domain (unknown origin) | Bioorg Med Chem 25: 2901-2916 (2017) Article DOI: 10.1016/j.bmc.2017.01.020 BindingDB Entry DOI: 10.7270/Q26W9DG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyketide synthase Pks13 (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50582183 (CHEMBL5080207) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Mycobacterium tuberculosis Pks13-TE domain using 4-methylumbelliferyl heptanoate as a fluorogenic substrate measured for 110 mins at 10... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01586 BindingDB Entry DOI: 10.7270/Q24T6P88 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM50174776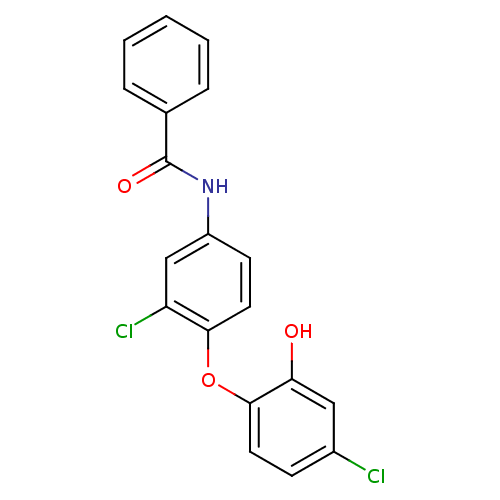 (CHEMBL200871 | N-(3-chloro-4-(4-chloro-2-hydroxyph...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of PfENR enzymatic activity | Bioorg Med Chem Lett 15: 5247-52 (2005) Article DOI: 10.1016/j.bmcl.2005.08.044 BindingDB Entry DOI: 10.7270/Q2NC60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM25410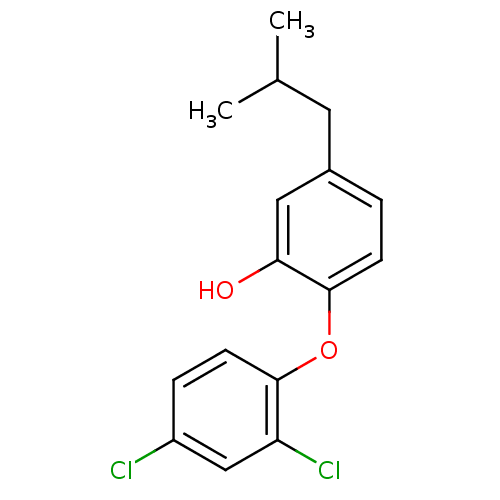 (2-(2,4-dichlorophenoxy)-5-(2-methylpropyl)phenol |...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Jacobus Pharmaceutical Company | Assay Description PfENR inhibition assays were carried out on a Cary 100 Bio Spectrophotometer or POLARstar Optima by monitoring oxidation of NADH at 340 nm. The IC50 ... | J Biol Chem 282: 25436-44 (2007) Article DOI: 10.1074/jbc.M701813200 BindingDB Entry DOI: 10.7270/Q22V2DFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Displayed 1 to 50 (of 276 total ) | Next | Last >> |