

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Androgen receptor (Homo sapiens (Human)) | BDBM50367916 (METHYLTRIENOLONE | Metribolone | R-1881) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from androgen receptor (unknown origin) expressed in HEK293 cell lysate incubated overnight by microbeta scinti... | J Med Chem 59: 750-5 (2016) Article DOI: 10.1021/acs.jmedchem.5b01168 BindingDB Entry DOI: 10.7270/Q2H70HQ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50367916 (METHYLTRIENOLONE | Metribolone | R-1881) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from progesterone receptor (unknown origin) expressed in HEK293 cell lysate incubated overnight by microbeta sc... | J Med Chem 59: 750-5 (2016) Article DOI: 10.1021/acs.jmedchem.5b01168 BindingDB Entry DOI: 10.7270/Q2H70HQ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50367916 (METHYLTRIENOLONE | Metribolone | R-1881) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone from mineralocorticoid receptor (unknown origin) expressed in HEK293 cell lysate incubated overnight by microbeta sc... | J Med Chem 59: 750-5 (2016) Article DOI: 10.1021/acs.jmedchem.5b01168 BindingDB Entry DOI: 10.7270/Q2H70HQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50145862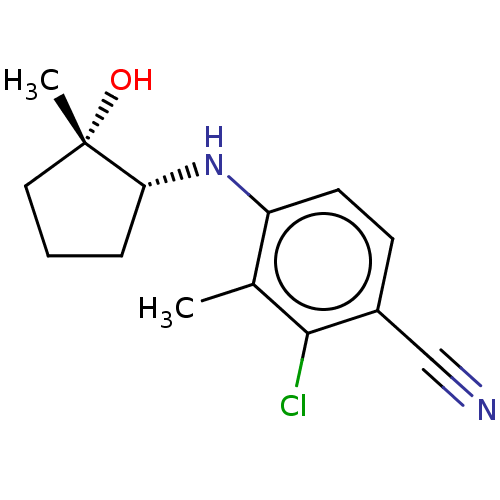 (CHEMBL3765171) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from androgen receptor (unknown origin) expressed in HEK293 cell lysate incubated overnight by microbeta scinti... | J Med Chem 59: 750-5 (2016) Article DOI: 10.1021/acs.jmedchem.5b01168 BindingDB Entry DOI: 10.7270/Q2H70HQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50145863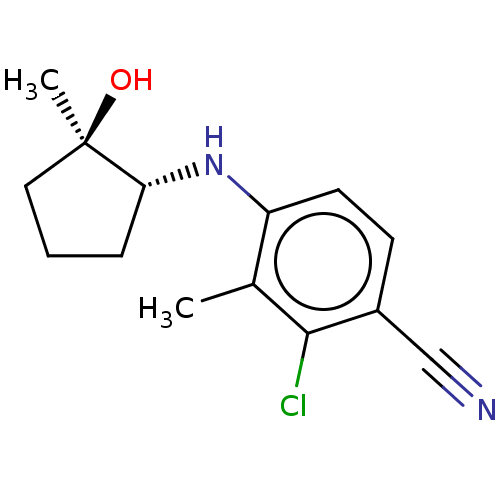 (CHEMBL3764185) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from androgen receptor (unknown origin) expressed in HEK293 cell lysate incubated overnight by microbeta scinti... | J Med Chem 59: 750-5 (2016) Article DOI: 10.1021/acs.jmedchem.5b01168 BindingDB Entry DOI: 10.7270/Q2H70HQ9 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50145860 (CHEMBL3764446) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from androgen receptor (unknown origin) expressed in HEK293 cell lysate incubated overnight by microbeta scinti... | J Med Chem 59: 750-5 (2016) Article DOI: 10.1021/acs.jmedchem.5b01168 BindingDB Entry DOI: 10.7270/Q2H70HQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50367916 (METHYLTRIENOLONE | Metribolone | R-1881) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from glucocorticoid receptor (unknown origin) expressed in HEK293 cell lysate incubated overnight by microbeta sci... | J Med Chem 59: 750-5 (2016) Article DOI: 10.1021/acs.jmedchem.5b01168 BindingDB Entry DOI: 10.7270/Q2H70HQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50145861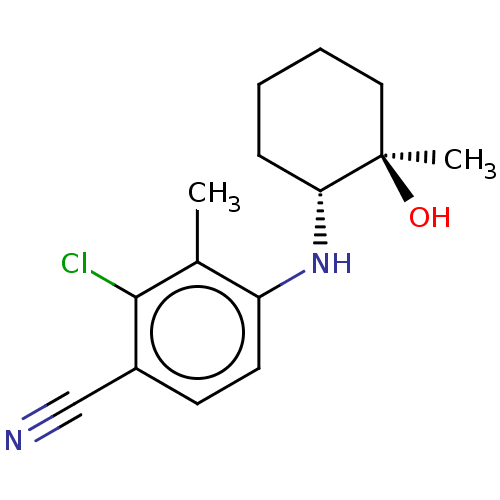 (CHEMBL3765823) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from androgen receptor (unknown origin) expressed in HEK293 cell lysate incubated overnight by microbeta scinti... | J Med Chem 59: 750-5 (2016) Article DOI: 10.1021/acs.jmedchem.5b01168 BindingDB Entry DOI: 10.7270/Q2H70HQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description Inhibition assay using AChE and BuChE. | Chem Biol Drug Des 80: 605-15 (2012) Article DOI: 10.1111/j.1747-0285.2012.01435.x BindingDB Entry DOI: 10.7270/Q28P5Z3T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM92543 (Coumarin analogue, 3a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description Inhibition assay using AChE and BuChE. | Chem Biol Drug Des 80: 605-15 (2012) Article DOI: 10.1111/j.1747-0285.2012.01435.x BindingDB Entry DOI: 10.7270/Q28P5Z3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM92554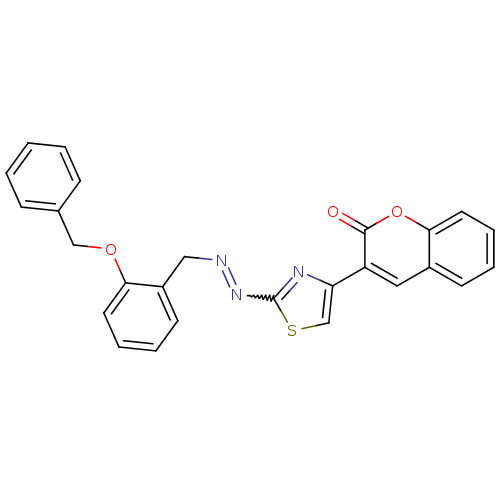 (Coumarin analogue, 3l) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description Inhibition assay using AChE and BuChE. | Chem Biol Drug Des 80: 605-15 (2012) Article DOI: 10.1111/j.1747-0285.2012.01435.x BindingDB Entry DOI: 10.7270/Q28P5Z3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM92545 (Coumarin analogue, 3c) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description Inhibition assay using AChE and BuChE. | Chem Biol Drug Des 80: 605-15 (2012) Article DOI: 10.1111/j.1747-0285.2012.01435.x BindingDB Entry DOI: 10.7270/Q28P5Z3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM92544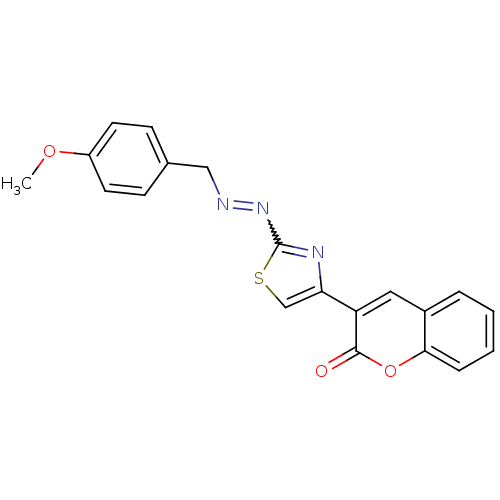 (Coumarin analogue, 3b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description Inhibition assay using AChE and BuChE. | Chem Biol Drug Des 80: 605-15 (2012) Article DOI: 10.1111/j.1747-0285.2012.01435.x BindingDB Entry DOI: 10.7270/Q28P5Z3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM92548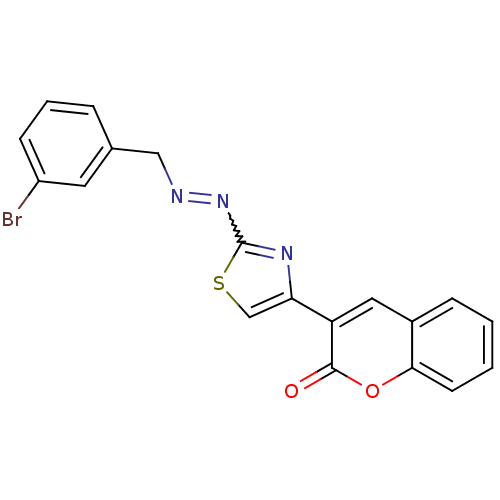 (Coumarin analogue, 3f) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description Inhibition assay using AChE and BuChE. | Chem Biol Drug Des 80: 605-15 (2012) Article DOI: 10.1111/j.1747-0285.2012.01435.x BindingDB Entry DOI: 10.7270/Q28P5Z3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM92551 (Coumarin analogue, 3i) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description Inhibition assay using AChE and BuChE. | Chem Biol Drug Des 80: 605-15 (2012) Article DOI: 10.1111/j.1747-0285.2012.01435.x BindingDB Entry DOI: 10.7270/Q28P5Z3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM92553 (Coumarin analogue, 3k) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description Inhibition assay using AChE and BuChE. | Chem Biol Drug Des 80: 605-15 (2012) Article DOI: 10.1111/j.1747-0285.2012.01435.x BindingDB Entry DOI: 10.7270/Q28P5Z3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM92549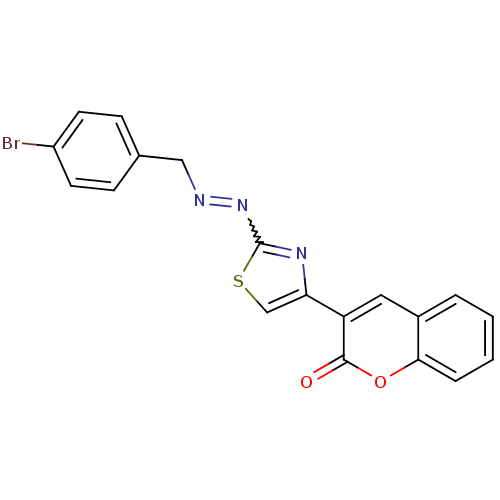 (Coumarin analogue, 3g) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description Inhibition assay using AChE and BuChE. | Chem Biol Drug Des 80: 605-15 (2012) Article DOI: 10.1111/j.1747-0285.2012.01435.x BindingDB Entry DOI: 10.7270/Q28P5Z3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM92550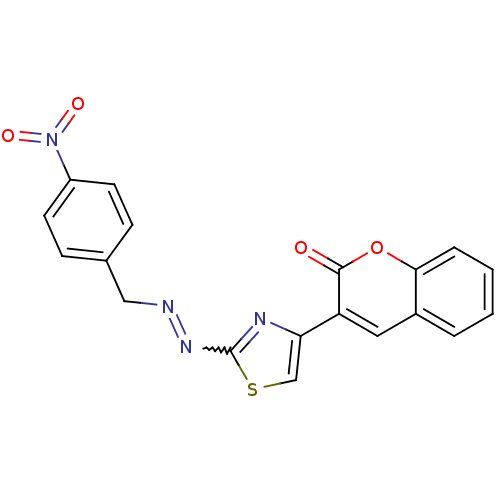 (Coumarin analogue, 3h) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description Inhibition assay using AChE and BuChE. | Chem Biol Drug Des 80: 605-15 (2012) Article DOI: 10.1111/j.1747-0285.2012.01435.x BindingDB Entry DOI: 10.7270/Q28P5Z3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50356190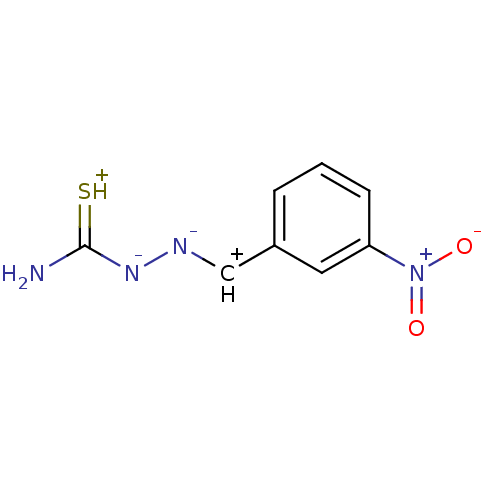 (CHEMBL193474) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology Curated by ChEMBL | Assay Description Inhibition of jack bean urease assessed as ammonia production after 30 mins by indophenol method | Eur J Med Chem 46: 5473-9 (2011) Article DOI: 10.1016/j.ejmech.2011.09.009 BindingDB Entry DOI: 10.7270/Q2GM87QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50275870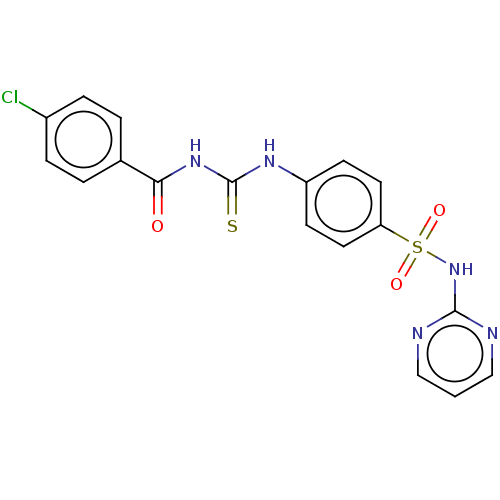 (CHEMBL4129331) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-i-Azam University Curated by ChEMBL | Assay Description Mixed-type inhibition of calf intestinal alkaline phosphatase assessed as enzyme-inhibitor dissociation constant using varying levels of p-NPP as sub... | Bioorg Med Chem 26: 3707-3715 (2018) Article DOI: 10.1016/j.bmc.2018.06.002 BindingDB Entry DOI: 10.7270/Q2C53PBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50356195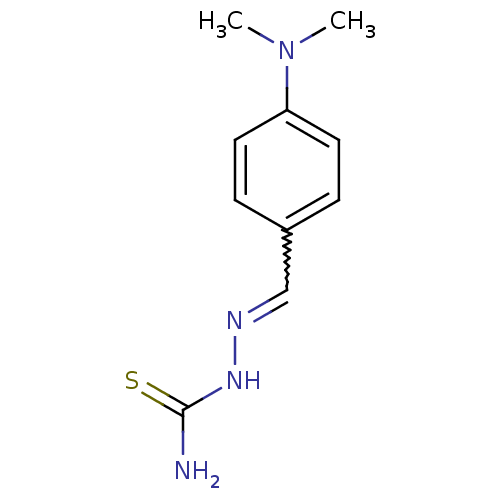 (CHEMBL241902) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology Curated by ChEMBL | Assay Description Inhibition of jack bean urease assessed as ammonia production after 30 mins by indophenol method | Eur J Med Chem 46: 5473-9 (2011) Article DOI: 10.1016/j.ejmech.2011.09.009 BindingDB Entry DOI: 10.7270/Q2GM87QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM53627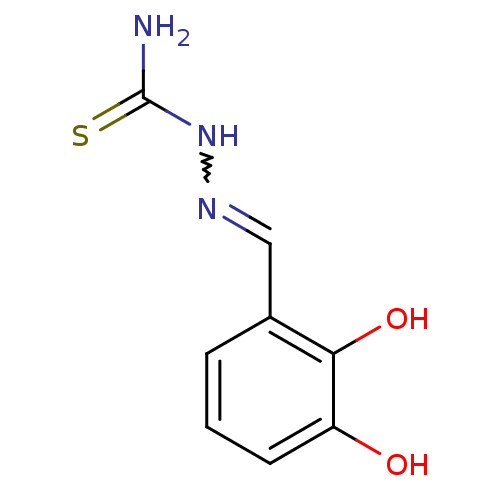 (1-[[(E)-(5-oxidanyl-6-oxidanylidene-cyclohexa-2,4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology Curated by ChEMBL | Assay Description Inhibition of jack bean urease assessed as ammonia production after 30 mins by indophenol method | Eur J Med Chem 46: 5473-9 (2011) Article DOI: 10.1016/j.ejmech.2011.09.009 BindingDB Entry DOI: 10.7270/Q2GM87QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50356192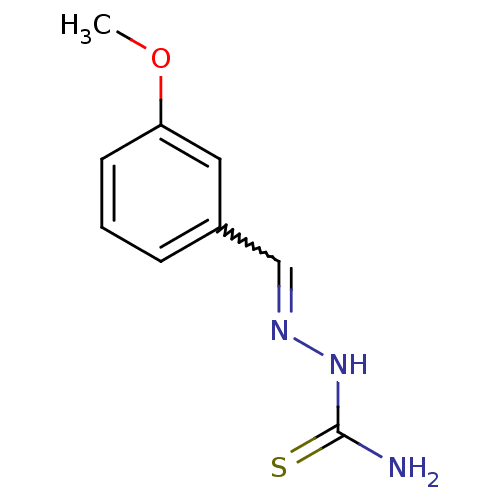 (CHEMBL241894) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology Curated by ChEMBL | Assay Description Inhibition of jack bean urease assessed as ammonia production after 30 mins by indophenol method | Eur J Med Chem 46: 5473-9 (2011) Article DOI: 10.1016/j.ejmech.2011.09.009 BindingDB Entry DOI: 10.7270/Q2GM87QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM92546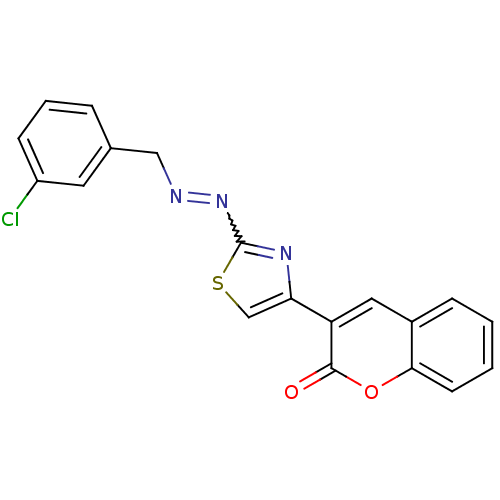 (Coumarin analogue, 3d) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description Inhibition assay using AChE and BuChE. | Chem Biol Drug Des 80: 605-15 (2012) Article DOI: 10.1111/j.1747-0285.2012.01435.x BindingDB Entry DOI: 10.7270/Q28P5Z3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50356201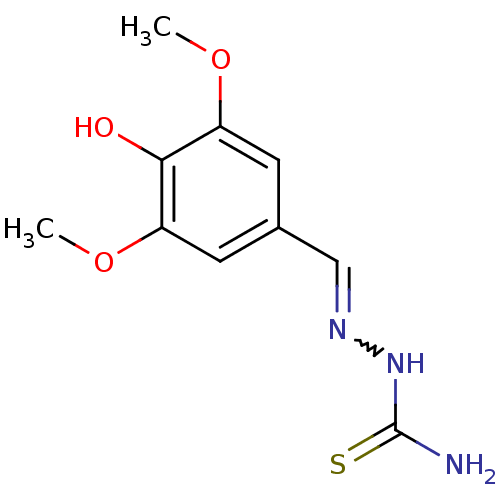 (CHEMBL1910224) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology Curated by ChEMBL | Assay Description Inhibition of jack bean urease assessed as ammonia production after 30 mins by indophenol method | Eur J Med Chem 46: 5473-9 (2011) Article DOI: 10.1016/j.ejmech.2011.09.009 BindingDB Entry DOI: 10.7270/Q2GM87QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50356197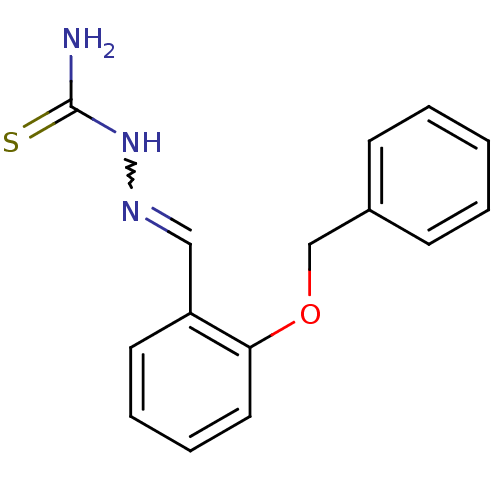 (CHEMBL1910221) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology Curated by ChEMBL | Assay Description Inhibition of jack bean urease assessed as ammonia production after 30 mins by indophenol method | Eur J Med Chem 46: 5473-9 (2011) Article DOI: 10.1016/j.ejmech.2011.09.009 BindingDB Entry DOI: 10.7270/Q2GM87QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50356186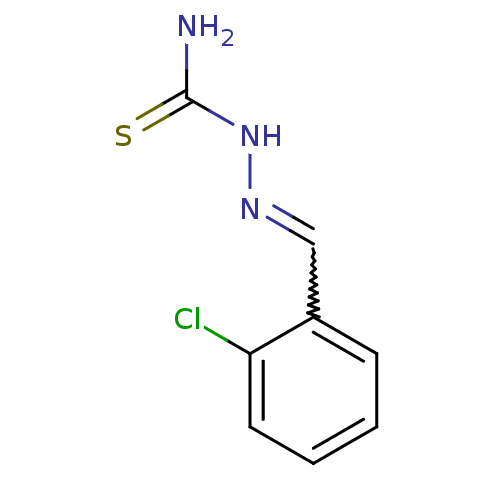 (CHEMBL239452) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology Curated by ChEMBL | Assay Description Inhibition of jack bean urease assessed as ammonia production after 30 mins by indophenol method | Eur J Med Chem 46: 5473-9 (2011) Article DOI: 10.1016/j.ejmech.2011.09.009 BindingDB Entry DOI: 10.7270/Q2GM87QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM92547 (Coumarin analogue, 3e) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 181 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description Inhibition assay using AChE and BuChE. | Chem Biol Drug Des 80: 605-15 (2012) Article DOI: 10.1111/j.1747-0285.2012.01435.x BindingDB Entry DOI: 10.7270/Q28P5Z3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50356198 (CHEMBL1910222) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology Curated by ChEMBL | Assay Description Inhibition of jack bean urease assessed as ammonia production after 30 mins by indophenol method | Eur J Med Chem 46: 5473-9 (2011) Article DOI: 10.1016/j.ejmech.2011.09.009 BindingDB Entry DOI: 10.7270/Q2GM87QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM92552 (Coumarin analogue, 3j) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 212 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description Inhibition assay using AChE and BuChE. | Chem Biol Drug Des 80: 605-15 (2012) Article DOI: 10.1111/j.1747-0285.2012.01435.x BindingDB Entry DOI: 10.7270/Q28P5Z3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50356199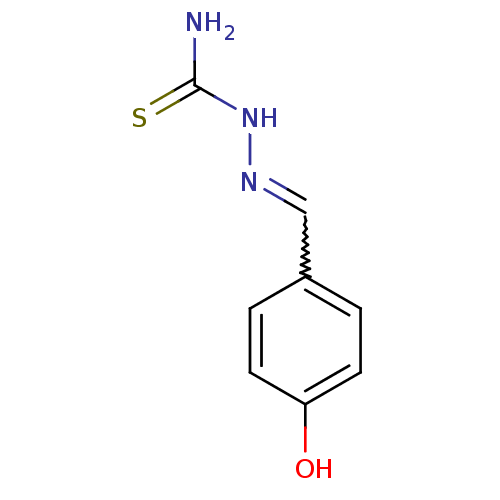 (CHEMBL242113) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 391 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology Curated by ChEMBL | Assay Description Inhibition of jack bean urease assessed as ammonia production after 30 mins by indophenol method | Eur J Med Chem 46: 5473-9 (2011) Article DOI: 10.1016/j.ejmech.2011.09.009 BindingDB Entry DOI: 10.7270/Q2GM87QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50356188 (CHEMBL391982) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology Curated by ChEMBL | Assay Description Inhibition of jack bean urease assessed as ammonia production after 30 mins by indophenol method | Eur J Med Chem 46: 5473-9 (2011) Article DOI: 10.1016/j.ejmech.2011.09.009 BindingDB Entry DOI: 10.7270/Q2GM87QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50356196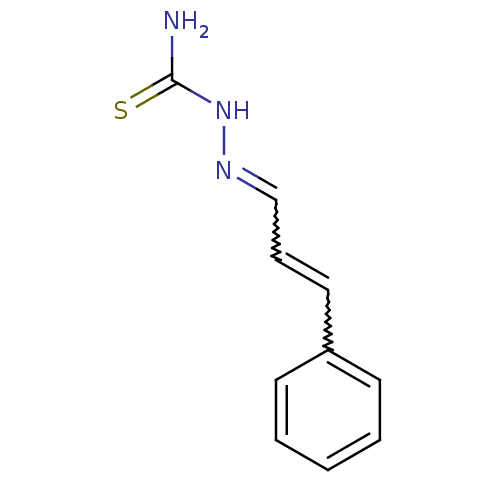 (CHEMBL1276230) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology Curated by ChEMBL | Assay Description Inhibition of jack bean urease assessed as ammonia production after 30 mins by indophenol method | Eur J Med Chem 46: 5473-9 (2011) Article DOI: 10.1016/j.ejmech.2011.09.009 BindingDB Entry DOI: 10.7270/Q2GM87QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Intestinal-type alkaline phosphatase (Bos taurus (Cattle)) | BDBM50275870 (CHEMBL4129331) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 415 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-i-Azam University Curated by ChEMBL | Assay Description Mixed-type inhibition of calf intestinal alkaline phosphatase assessed as enzyme-substrate-inhibitor dissociation constant using varying levels of p-... | Bioorg Med Chem 26: 3707-3715 (2018) Article DOI: 10.1016/j.bmc.2018.06.002 BindingDB Entry DOI: 10.7270/Q2C53PBN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50145861 (CHEMBL3765823) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 415 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone from mineralocorticoid receptor (unknown origin) expressed in HEK293 cell lysate incubated overnight by microbeta sc... | J Med Chem 59: 750-5 (2016) Article DOI: 10.1021/acs.jmedchem.5b01168 BindingDB Entry DOI: 10.7270/Q2H70HQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50356202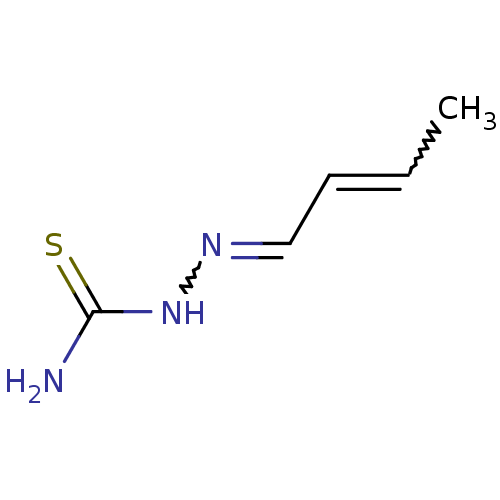 (CHEMBL1910225) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology Curated by ChEMBL | Assay Description Inhibition of jack bean urease assessed as ammonia production after 30 mins by indophenol method | Eur J Med Chem 46: 5473-9 (2011) Article DOI: 10.1016/j.ejmech.2011.09.009 BindingDB Entry DOI: 10.7270/Q2GM87QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50145862 (CHEMBL3765171) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 448 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from progesterone receptor (unknown origin) expressed in HEK293 cell lysate incubated overnight by microbeta sc... | J Med Chem 59: 750-5 (2016) Article DOI: 10.1021/acs.jmedchem.5b01168 BindingDB Entry DOI: 10.7270/Q2H70HQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50145862 (CHEMBL3765171) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 462 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-dexamethasone from glucocorticoid receptor (unknown origin) expressed in HEK293 cell lysate incubated overnight by microbeta sci... | J Med Chem 59: 750-5 (2016) Article DOI: 10.1021/acs.jmedchem.5b01168 BindingDB Entry DOI: 10.7270/Q2H70HQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50356191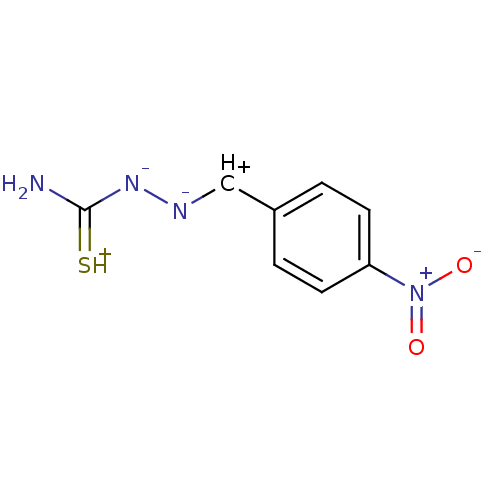 (CHEMBL193804) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 497 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology Curated by ChEMBL | Assay Description Inhibition of jack bean urease assessed as ammonia production after 30 mins by indophenol method | Eur J Med Chem 46: 5473-9 (2011) Article DOI: 10.1016/j.ejmech.2011.09.009 BindingDB Entry DOI: 10.7270/Q2GM87QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50145861 (CHEMBL3765823) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from progesterone receptor (unknown origin) expressed in HEK293 cell lysate incubated overnight by microbeta sc... | J Med Chem 59: 750-5 (2016) Article DOI: 10.1021/acs.jmedchem.5b01168 BindingDB Entry DOI: 10.7270/Q2H70HQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50356187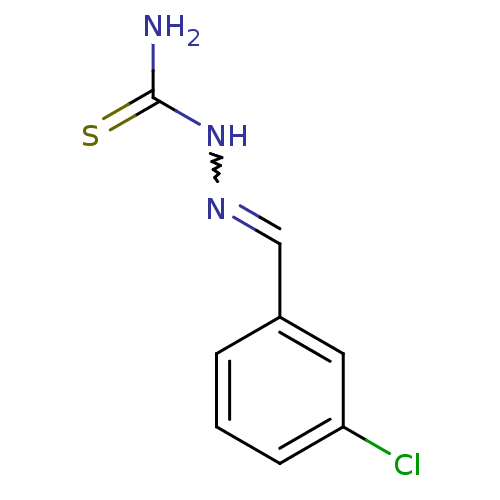 (CHEMBL1269725) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 625 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology Curated by ChEMBL | Assay Description Inhibition of jack bean urease assessed as ammonia production after 30 mins by indophenol method | Eur J Med Chem 46: 5473-9 (2011) Article DOI: 10.1016/j.ejmech.2011.09.009 BindingDB Entry DOI: 10.7270/Q2GM87QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50114665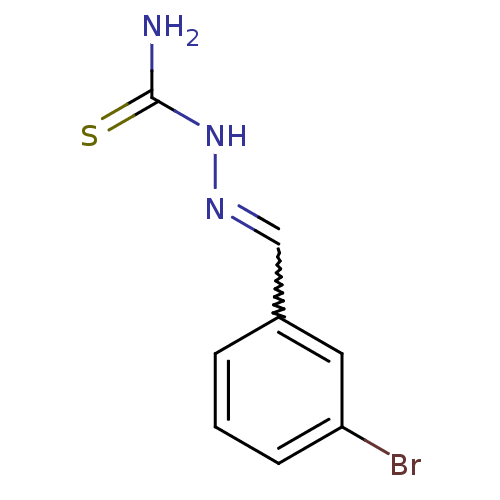 (2-(trifluoromethyl)benzaldehyde thiosemicarbazone ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology Curated by ChEMBL | Assay Description Inhibition of jack bean urease assessed as ammonia production after 30 mins by indophenol method | Eur J Med Chem 46: 5473-9 (2011) Article DOI: 10.1016/j.ejmech.2011.09.009 BindingDB Entry DOI: 10.7270/Q2GM87QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50356189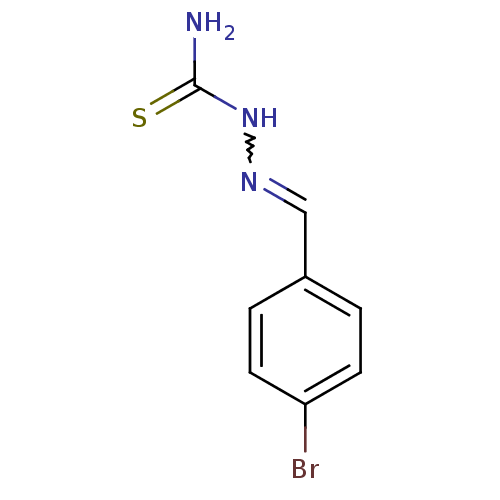 (CHEMBL1910220) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology Curated by ChEMBL | Assay Description Inhibition of jack bean urease assessed as ammonia production after 30 mins by indophenol method | Eur J Med Chem 46: 5473-9 (2011) Article DOI: 10.1016/j.ejmech.2011.09.009 BindingDB Entry DOI: 10.7270/Q2GM87QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50356193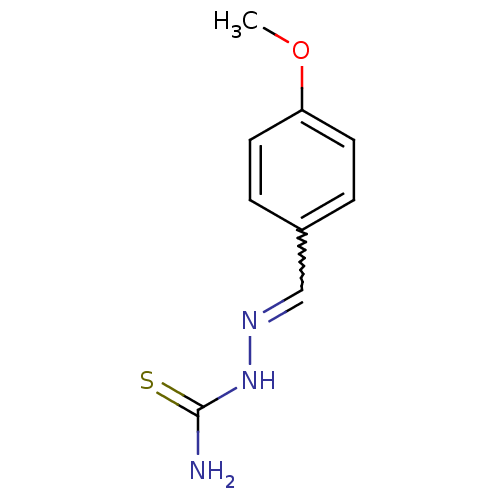 (CHEMBL398206) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 807 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology Curated by ChEMBL | Assay Description Inhibition of jack bean urease assessed as ammonia production after 30 mins by indophenol method | Eur J Med Chem 46: 5473-9 (2011) Article DOI: 10.1016/j.ejmech.2011.09.009 BindingDB Entry DOI: 10.7270/Q2GM87QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50145863 (CHEMBL3764185) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 872 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from progesterone receptor (unknown origin) expressed in HEK293 cell lysate incubated overnight by microbeta sc... | J Med Chem 59: 750-5 (2016) Article DOI: 10.1021/acs.jmedchem.5b01168 BindingDB Entry DOI: 10.7270/Q2H70HQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease (Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50356194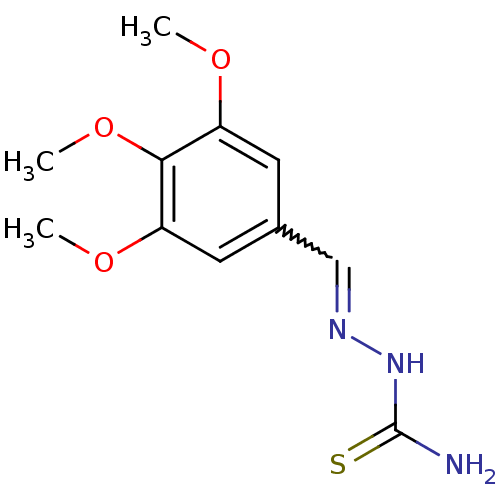 (CHEMBL499789) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology Curated by ChEMBL | Assay Description Inhibition of jack bean urease assessed as ammonia production after 30 mins by indophenol method | Eur J Med Chem 46: 5473-9 (2011) Article DOI: 10.1016/j.ejmech.2011.09.009 BindingDB Entry DOI: 10.7270/Q2GM87QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM92548 (Coumarin analogue, 3f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description Inhibition assay using AChE and BuChE. | Chem Biol Drug Des 80: 605-15 (2012) Article DOI: 10.1111/j.1747-0285.2012.01435.x BindingDB Entry DOI: 10.7270/Q28P5Z3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50283374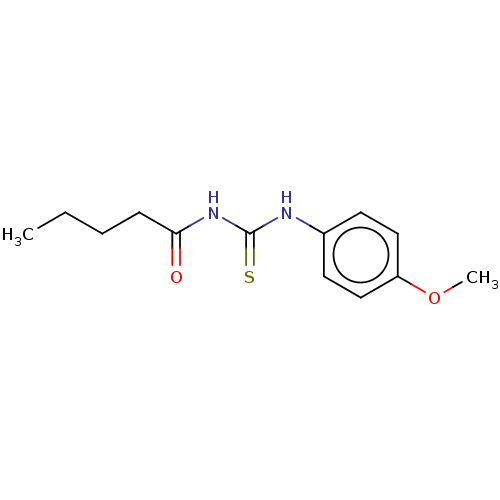 (CHEMBL4169273) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Quaid-i-Azam University Curated by ChEMBL | Assay Description Non-competitive inhibition of mushroom tyrosinase using L-DOPA as substrate preincubated for 10 mins followed by substrate addition measured up to 5 ... | Eur J Med Chem 141: 273-281 (2017) Article DOI: 10.1016/j.ejmech.2017.09.059 BindingDB Entry DOI: 10.7270/Q28G8P6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50145860 (CHEMBL3764446) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-methyltrienolone from progesterone receptor (unknown origin) expressed in HEK293 cell lysate incubated overnight by microbeta sc... | J Med Chem 59: 750-5 (2016) Article DOI: 10.1021/acs.jmedchem.5b01168 BindingDB Entry DOI: 10.7270/Q2H70HQ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM92544 (Coumarin analogue, 3b) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
COMSATS Institute of Information Technology | Assay Description Inhibition assay using AChE and BuChE. | Chem Biol Drug Des 80: 605-15 (2012) Article DOI: 10.1111/j.1747-0285.2012.01435.x BindingDB Entry DOI: 10.7270/Q28P5Z3T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 614 total ) | Next | Last >> |