

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50098643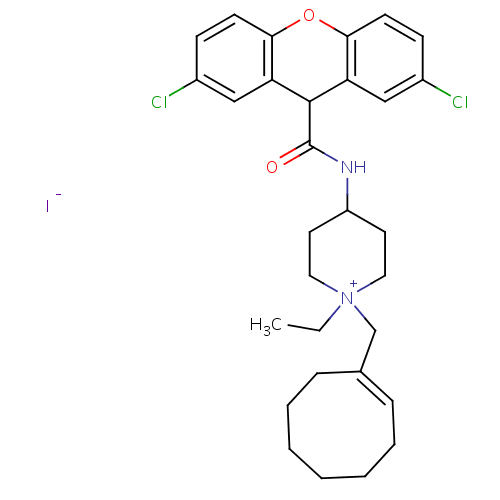 (1-((E)-1-Cyclooct-1-enyl)methyl-4-[(2,7-dichloro-9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of [125I]-Eotaxin binding to human CCR3 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 1219-23 (2001) BindingDB Entry DOI: 10.7270/Q22V2FDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50098624 (1-Cyclooct-1-enylmethyl-4-[(2,7-dichloro-9H-xanthe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against I-Eotaxin binding to human CCR3 receptors | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098624 (1-Cyclooct-1-enylmethyl-4-[(2,7-dichloro-9H-xanthe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098643 (1-((E)-1-Cyclooct-1-enyl)methyl-4-[(2,7-dichloro-9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of [125I]-Eotaxin binding to human CCR1 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 1219-23 (2001) BindingDB Entry DOI: 10.7270/Q22V2FDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098624 (1-Cyclooct-1-enylmethyl-4-[(2,7-dichloro-9H-xanthe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50029353 (CHEMBL414165 | [(5S,8S,11R,14R,16aR)-8-(1H-Indol-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory effect of the compound against Human ET-A receptor | J Med Chem 38: 4309-24 (1995) BindingDB Entry DOI: 10.7270/Q2D50M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098636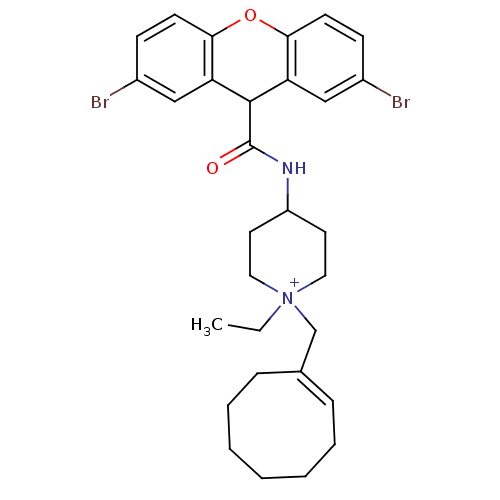 (1-Cyclooct-1-enylmethyl-4-[(2,7-dibromo-9H-xanthen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098641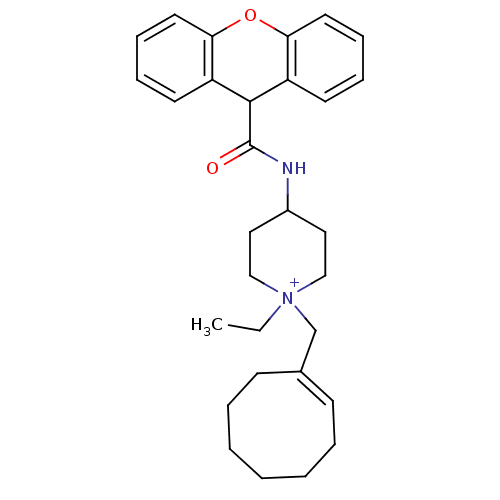 (1-Cyclooct-1-enylmethyl-1-ethyl-4-[(9H-xanthene-9-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50099482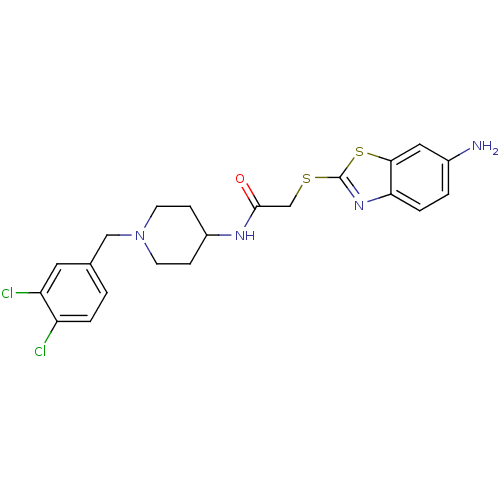 (2-(6-Amino-benzothiazol-2-ylsulfanyl)-N-[1-(3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of [125I]-Eotaxin binding to human CCR3 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 1219-23 (2001) BindingDB Entry DOI: 10.7270/Q22V2FDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098651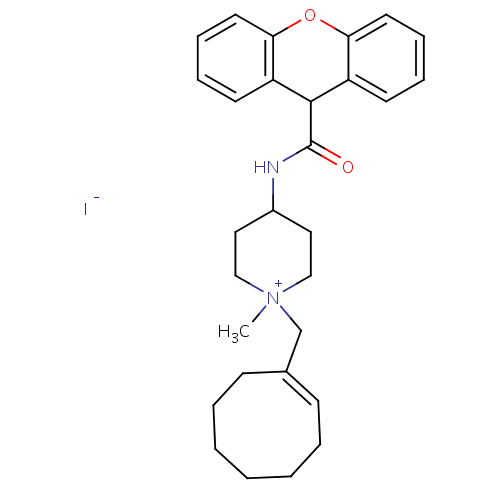 (1-Cyclooct-1-enylmethyl-1-methyl-4-[(9H-xanthene-9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098628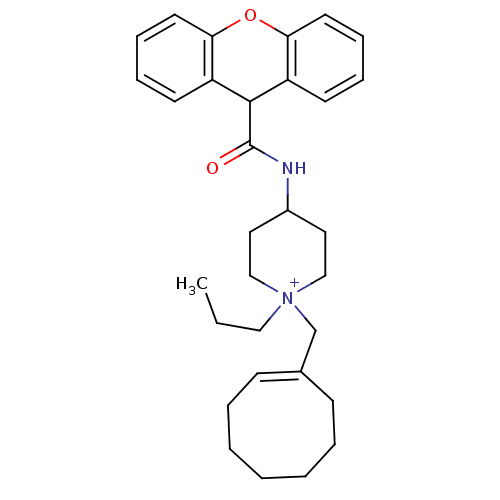 (1-Cyclooct-1-enylmethyl-1-propyl-4-[(9H-xanthene-9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50029353 (CHEMBL414165 | [(5S,8S,11R,14R,16aR)-8-(1H-Indol-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description The compound was tested for binding inhibitory activity against Endothelin A receptor from porcine aortic smooth muscle membranes. | J Med Chem 38: 4309-24 (1995) BindingDB Entry DOI: 10.7270/Q2D50M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50029353 (CHEMBL414165 | [(5S,8S,11R,14R,16aR)-8-(1H-Indol-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory effect of the compound against porcine ET-A receptor | J Med Chem 38: 4309-24 (1995) BindingDB Entry DOI: 10.7270/Q2D50M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098633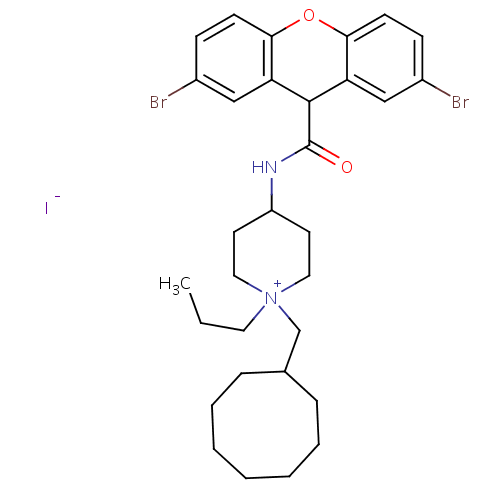 (1-Cyclooctylmethyl-4-[(2,7-dibromo-9H-xanthene-9-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098648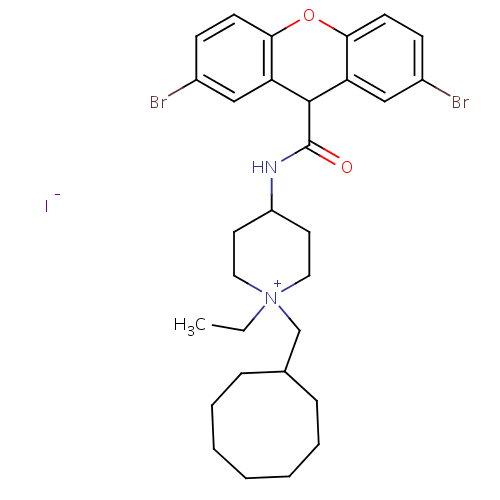 (1-Cyclooctylmethyl-4-[(2,7-dibromo-9H-xanthene-9-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50029354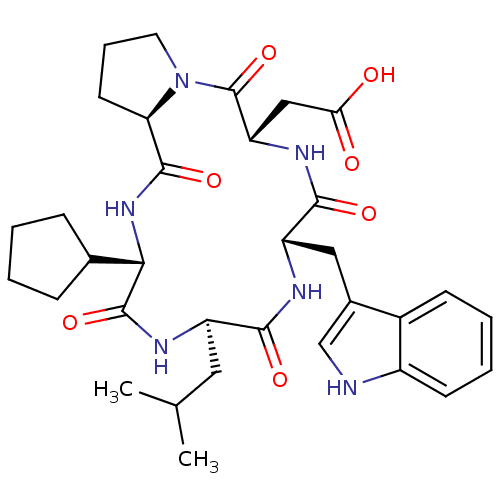 (CHEMBL133257 | [(5S,8S,11R,14S,16aR)-14-Cyclopenty...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory effect of the compound against Human ET-A receptor | J Med Chem 38: 4309-24 (1995) BindingDB Entry DOI: 10.7270/Q2D50M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098637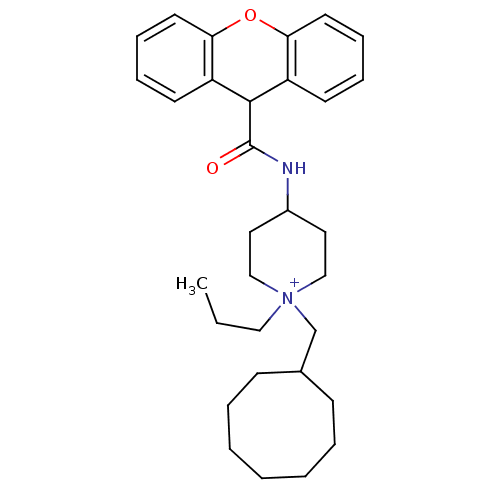 (1-Cyclooctylmethyl-1-propyl-4-[(9H-xanthene-9-carb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098627 (1-Cyclooctylmethyl-4-[(2,7-dibromo-9H-xanthene-9-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50029354 (CHEMBL133257 | [(5S,8S,11R,14S,16aR)-14-Cyclopenty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description The compound was tested for binding inhibitory activity against Endothelin A receptor from porcine aortic smooth muscle membranes. | J Med Chem 38: 4309-24 (1995) BindingDB Entry DOI: 10.7270/Q2D50M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50029354 (CHEMBL133257 | [(5S,8S,11R,14S,16aR)-14-Cyclopenty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory effect of the compound against porcine ET-A receptor | J Med Chem 38: 4309-24 (1995) BindingDB Entry DOI: 10.7270/Q2D50M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098642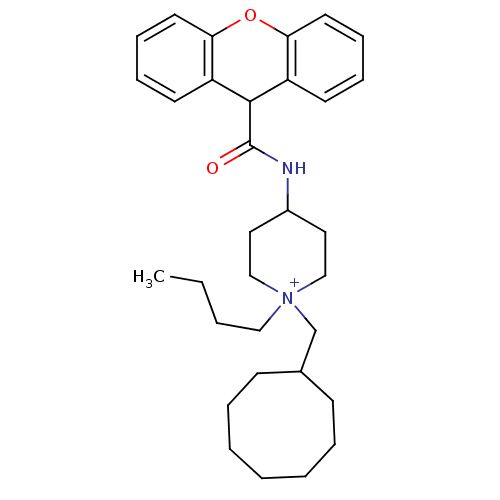 (1-Butyl-1-cyclooctylmethyl-4-[(9H-xanthene-9-carbo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098626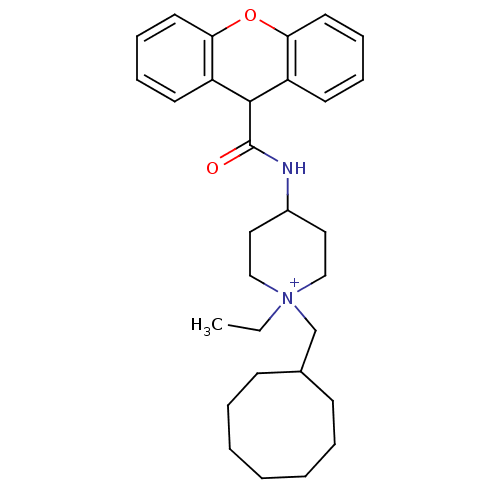 (1-Cyclooctylmethyl-1-ethyl-4-[(9H-xanthene-9-carbo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Mus musculus) | BDBM50098624 (1-Cyclooct-1-enylmethyl-4-[(2,7-dichloro-9H-xanthe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to mouse CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Mus musculus) | BDBM50098624 (1-Cyclooct-1-enylmethyl-4-[(2,7-dichloro-9H-xanthe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against 125 I -MIP-1 alpha binding to mouse CCR1 receptors | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50098624 (1-Cyclooct-1-enylmethyl-4-[(2,7-dichloro-9H-xanthe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against I-Eotaxin induced [Ca2+] response in human CCR3 receptor | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50029352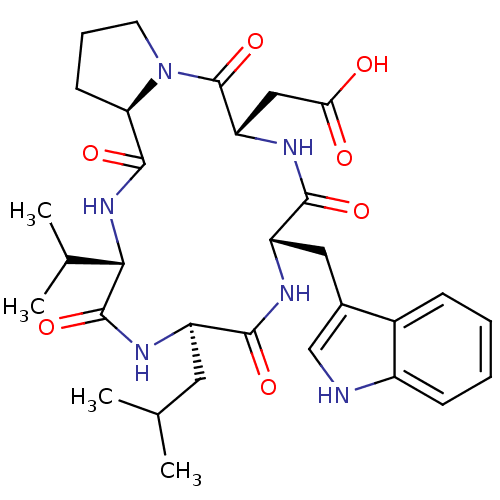 (CHEMBL336033 | [(5S,8S,11R,14S,16aR)-8-(1H-Indol-3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory effect of the compound against Human Endothelin A receptor | J Med Chem 38: 4309-24 (1995) BindingDB Entry DOI: 10.7270/Q2D50M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098620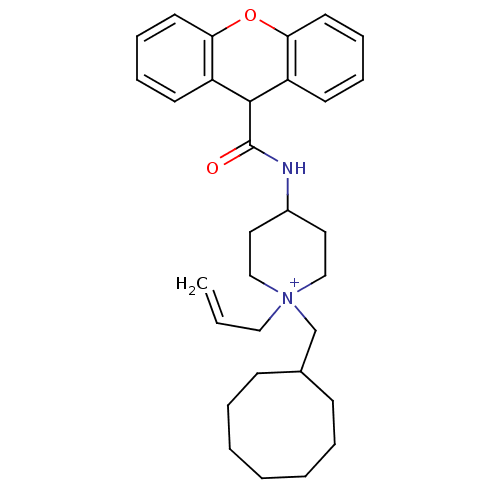 (1-Allyl-1-cyclooctylmethyl-4-[(9H-xanthene-9-carbo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50407614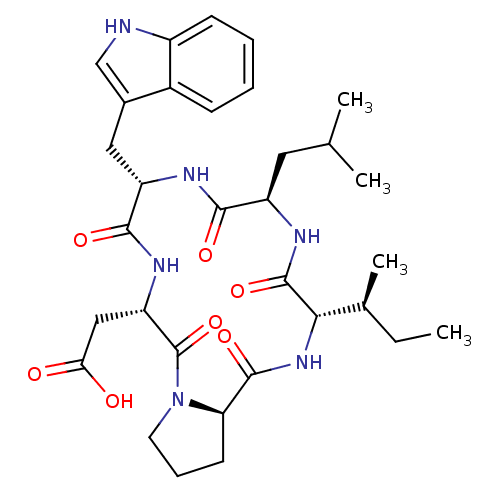 (CHEMBL137659) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description The compound was tested for binding inhibitory activity against Endothelin A receptor from porcine aortic smooth muscle membranes. | J Med Chem 38: 4309-24 (1995) BindingDB Entry DOI: 10.7270/Q2D50M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50047595 (1-Butyl-3-{2-[3-(5-isopropyl-4-phenyl-imidazol-1-y...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Inhibition against acyl coenzyme A:cholesterol acyltransferase derived from rabbit aorta homogenate | J Med Chem 36: 1641-53 (1993) BindingDB Entry DOI: 10.7270/Q2XP7406 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Mus musculus) | BDBM50098636 (1-Cyclooct-1-enylmethyl-4-[(2,7-dibromo-9H-xanthen...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to mouse CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50407622 (CHEMBL2370830) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description The compound was tested for binding inhibitory activity against Endothelin A receptor from porcine aortic smooth muscle membranes. | J Med Chem 38: 4309-24 (1995) BindingDB Entry DOI: 10.7270/Q2D50M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098634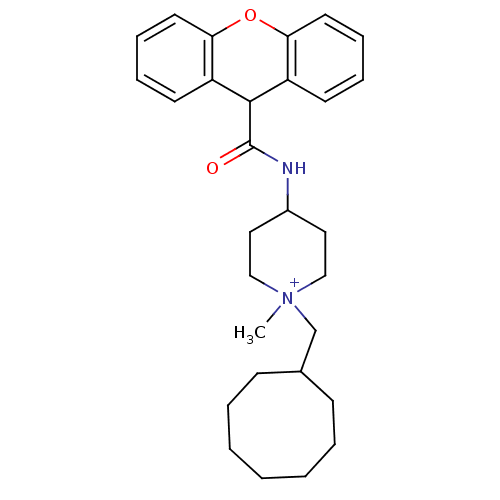 (1-Cyclooctylmethyl-1-methyl-4-[(9H-xanthene-9-carb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50407565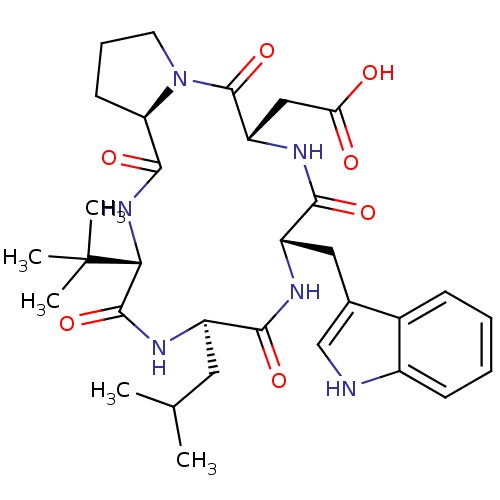 (CHEMBL336563) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description The compound was tested for binding inhibitory activity against Endothelin A receptor from porcine aortic smooth muscle membranes. | J Med Chem 38: 4309-24 (1995) BindingDB Entry DOI: 10.7270/Q2D50M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50047525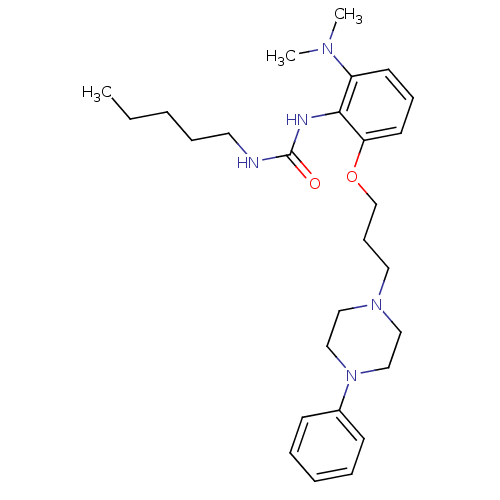 (1-{2-Dimethylamino-6-[3-(4-phenyl-piperazin-1-yl)-...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Tested for inhibition against acyl coenzyme A:cholesterol acyltransferase from rabbit aorta homogenate. | J Med Chem 36: 1630-40 (1993) BindingDB Entry DOI: 10.7270/Q22B8X4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50098630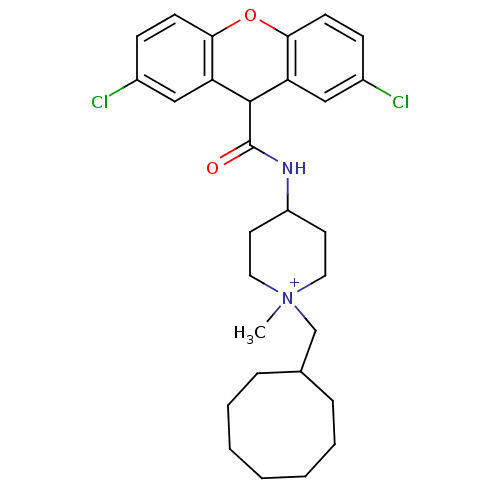 (1-Cyclooctylmethyl-4-[(2,7-dichloro-9H-xanthene-9-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-MIP-1 alpha binding to human CCR1 receptors. | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50407570 (CHEMBL2370825) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description The compound was tested for binding inhibitory activity against Endothelin A receptor from porcine aortic smooth muscle membranes. | J Med Chem 38: 4309-24 (1995) BindingDB Entry DOI: 10.7270/Q2D50M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50407615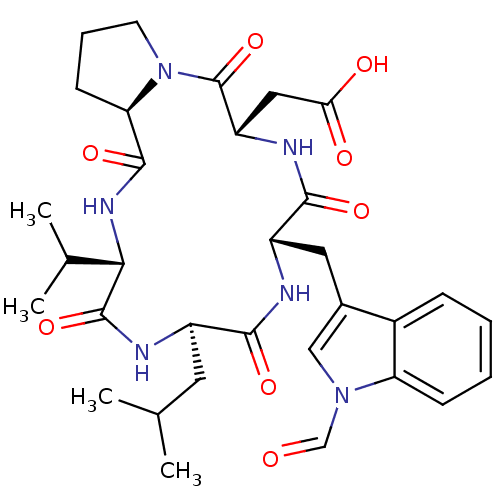 (CHEMBL343516) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description The compound was tested for binding inhibitory activity against Endothelin A receptor from porcine aortic smooth muscle membranes. | J Med Chem 38: 4309-24 (1995) BindingDB Entry DOI: 10.7270/Q2D50M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50407612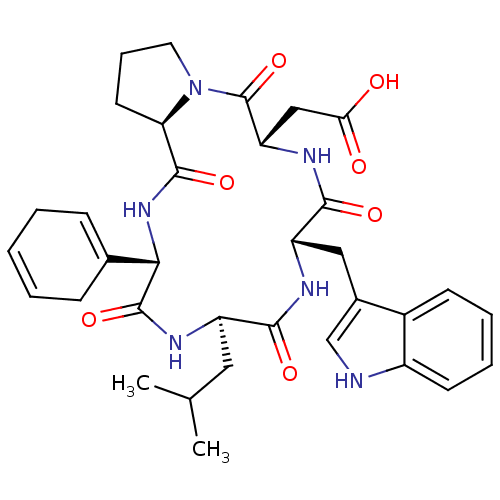 (CHEMBL341994) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description The compound was tested for binding inhibitory activity against Endothelin A receptor from porcine aortic smooth muscle membranes. | J Med Chem 38: 4309-24 (1995) BindingDB Entry DOI: 10.7270/Q2D50M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50047534 (1-{2-[3-(5-Isopropyl-4-phenyl-imidazol-1-yl)-propo...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Inhibition against acyl coenzyme A:cholesterol acyltransferase derived from rabbit aorta homogenate | J Med Chem 36: 1641-53 (1993) BindingDB Entry DOI: 10.7270/Q2XP7406 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50047599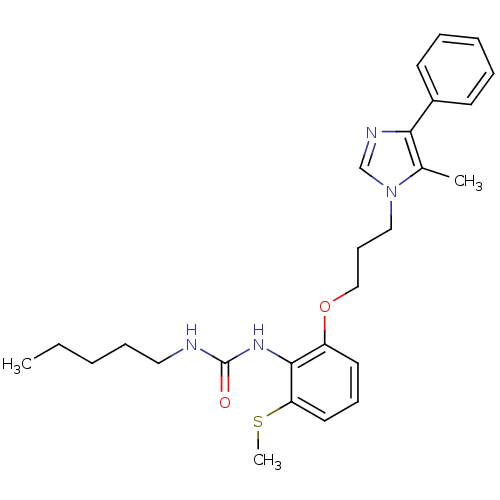 (1-{2-[3-(5-Methyl-4-phenyl-imidazol-1-yl)-propoxy]...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Inhibition against acyl coenzyme A:cholesterol acyltransferase derived from rabbit aorta homogenate | J Med Chem 36: 1641-53 (1993) BindingDB Entry DOI: 10.7270/Q2XP7406 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50038991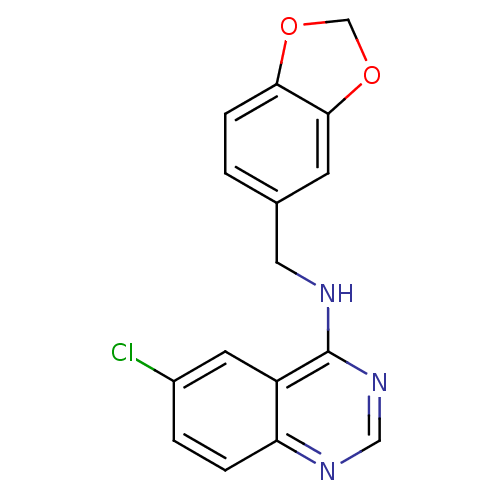 (Benzo[1,3]dioxol-5-ylmethyl-(6-chloro-quinazolin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against cGMP-phosphodiesterase from porcine aorta | J Med Chem 37: 2106-11 (1994) BindingDB Entry DOI: 10.7270/Q2Q52NPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50099479 (CHEMBL21143 | N-[1-(3,4-Dichloro-benzyl)-piperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of [125I]-Eotaxin binding to human CCR3 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 1219-23 (2001) BindingDB Entry DOI: 10.7270/Q22V2FDC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50407600 (CHEMBL343062) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description The compound was tested for binding inhibitory activity against Endothelin A receptor from porcine aortic smooth muscle membranes. | J Med Chem 38: 4309-24 (1995) BindingDB Entry DOI: 10.7270/Q2D50M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50029351 (CHEMBL342615 | [(5R,8S,11R,14S,16aR)-8-(1H-Indol-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory effect of the compound against porcine Endothelin A receptor | J Med Chem 38: 4309-24 (1995) BindingDB Entry DOI: 10.7270/Q2D50M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50029351 (CHEMBL342615 | [(5R,8S,11R,14S,16aR)-8-(1H-Indol-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description The compound was tested for binding inhibitory activity against Endothelin A receptor from porcine aortic smooth muscle membranes. | J Med Chem 38: 4309-24 (1995) BindingDB Entry DOI: 10.7270/Q2D50M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Mus musculus) | BDBM50098624 (1-Cyclooct-1-enylmethyl-4-[(2,7-dichloro-9H-xanthe...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description inhibitory activity against MIP-1 alpha- induced [Ca2+] response in U937 cells expressing mouse CCR1 receptor | J Med Chem 44: 1429-35 (2001) BindingDB Entry DOI: 10.7270/Q2G73D0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50029352 (CHEMBL336033 | [(5S,8S,11R,14S,16aR)-8-(1H-Indol-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description The compound was tested for binding inhibitory activity against Endothelin A receptor from porcine aortic smooth muscle membranes. | J Med Chem 38: 4309-24 (1995) BindingDB Entry DOI: 10.7270/Q2D50M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50029352 (CHEMBL336033 | [(5S,8S,11R,14S,16aR)-8-(1H-Indol-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory effect of the compound against porcine Endothelin A receptor | J Med Chem 38: 4309-24 (1995) BindingDB Entry DOI: 10.7270/Q2D50M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA:cholesterol acyltransferase (Oryctolagus cuniculus) | BDBM50047509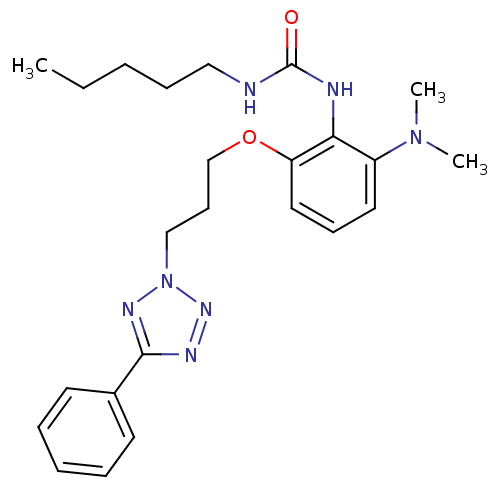 (1-{2-Dimethylamino-6-[3-(5-phenyl-tetrazol-2-yl)-p...) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Company, Ltd. Curated by ChEMBL | Assay Description Tested for inhibition against acyl coenzyme A:cholesterol acyltransferase from rabbit aorta homogenate. | J Med Chem 36: 1630-40 (1993) BindingDB Entry DOI: 10.7270/Q22B8X4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Sus scrofa) | BDBM50407611 (CHEMBL343582) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description The compound was tested for binding inhibitory activity against Endothelin A receptor from porcine aortic smooth muscle membranes. | J Med Chem 38: 4309-24 (1995) BindingDB Entry DOI: 10.7270/Q2D50M0H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 512 total ) | Next | Last >> |