

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50217448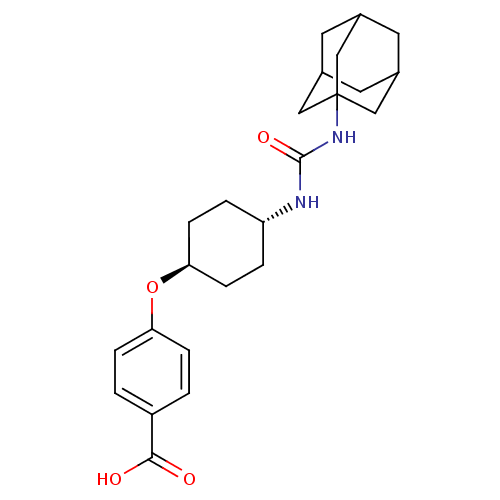 (CHEMBL242459 | US9029401, 1471 (t-AUCB) | trans-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50560757 (CHEMBL4784283) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant rat soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measured ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Rattus norvegicus) | BDBM50560743 (CHEMBL4759592) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant rat soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measured ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50351247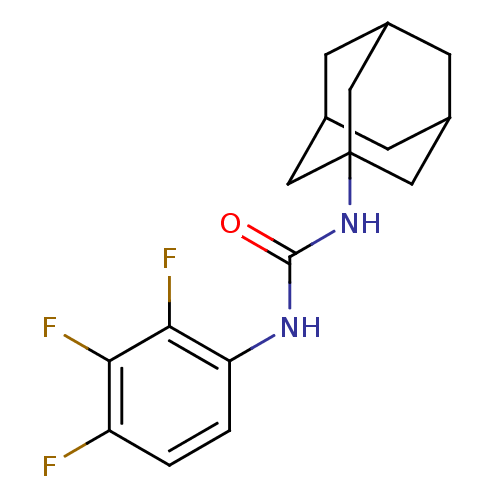 (CHEMBL1818385) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50191854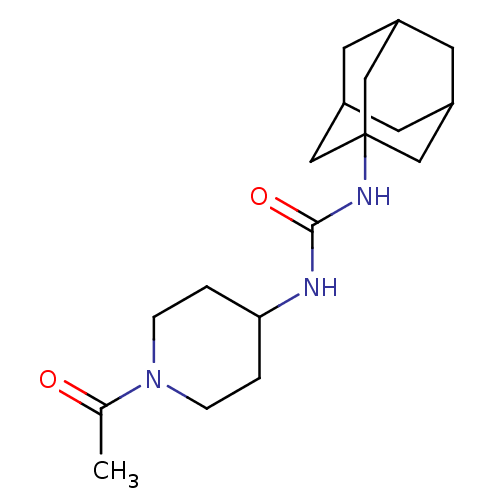 (CHEMBL436774 | N-(1-acetyl-piperidin-4-yl)-N'-(ada...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50560744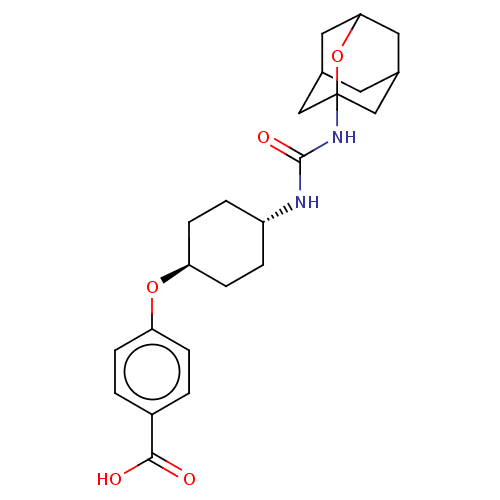 (CHEMBL4779073) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50560750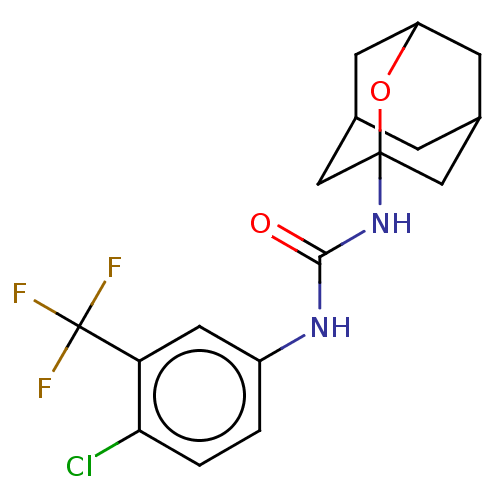 (CHEMBL4778324) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50560745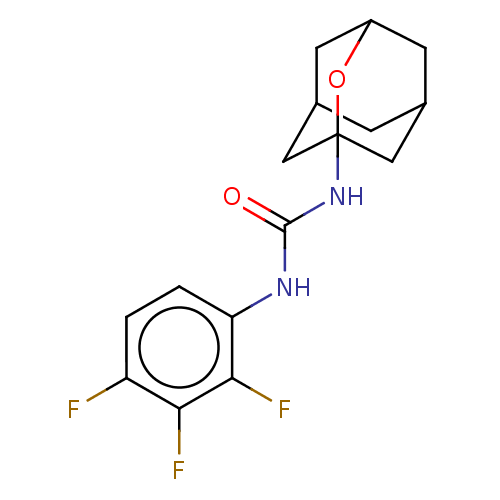 (CHEMBL4797443) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM19992 (2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50560743 (CHEMBL4759592) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50560751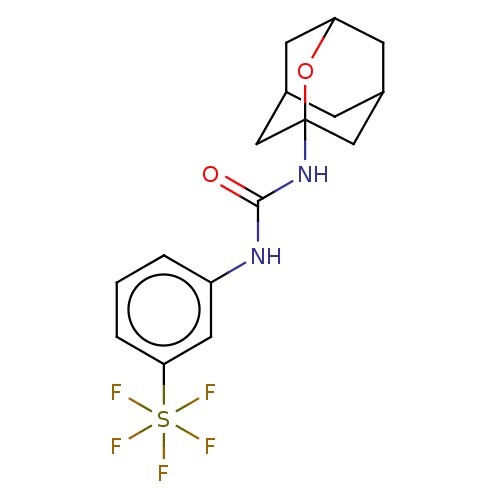 (CHEMBL4750570) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50560756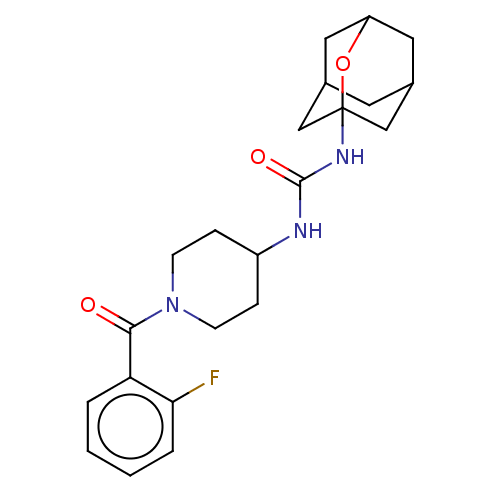 (CHEMBL4784603) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50560749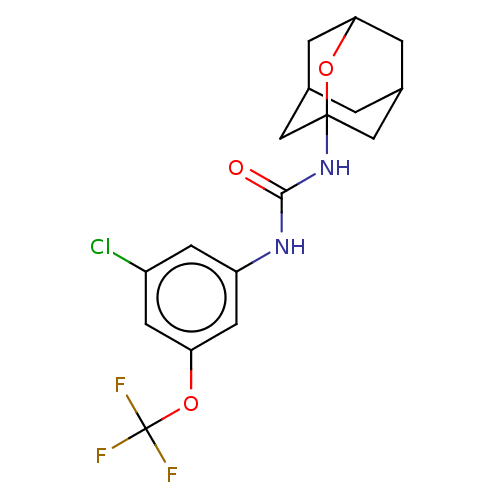 (CHEMBL4762265) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20132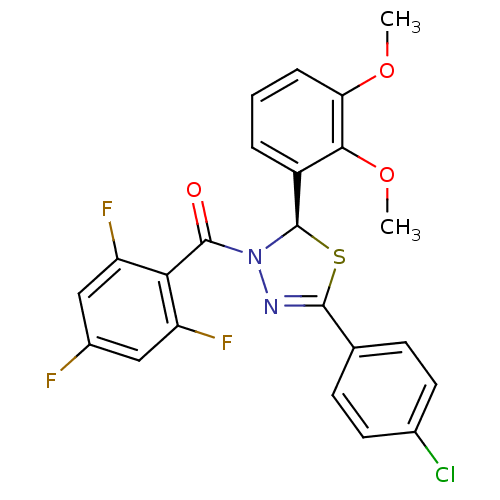 ((2R)-5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50560756 (CHEMBL4784603) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50560757 (CHEMBL4784283) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50560760 (CHEMBL4753341) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50560743 (CHEMBL4759592) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50560748 (CHEMBL4751517) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50560760 (CHEMBL4753341) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM19992 (2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50560758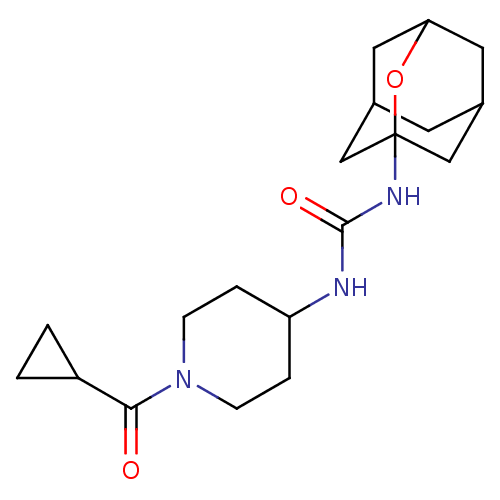 (CHEMBL4800149) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50560755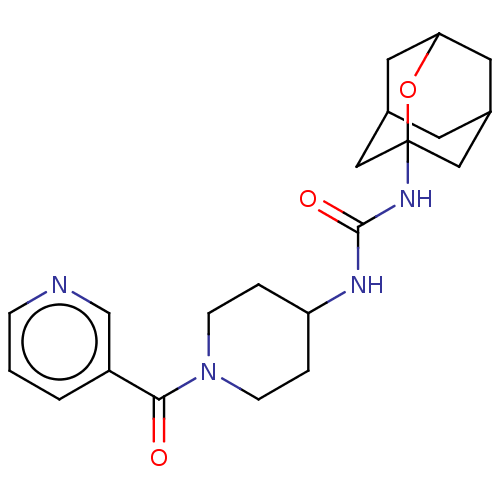 (CHEMBL4785423) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen-induced gene 1 protein (Homo sapiens (Human)) | BDBM195603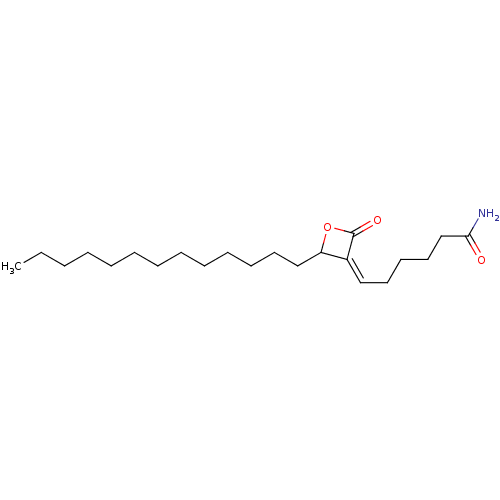 (KC01) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Tissue and cell proteomes (50 μL) were treated with either FP-rhodamine (1 μM) or WHP01 (2 μM) for 30 min at 37 °C. The reactions were... | Nat Chem Biol 12: 367-72 (2016) Article DOI: 10.1038/nchembio.2051 BindingDB Entry DOI: 10.7270/Q2N015CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50560761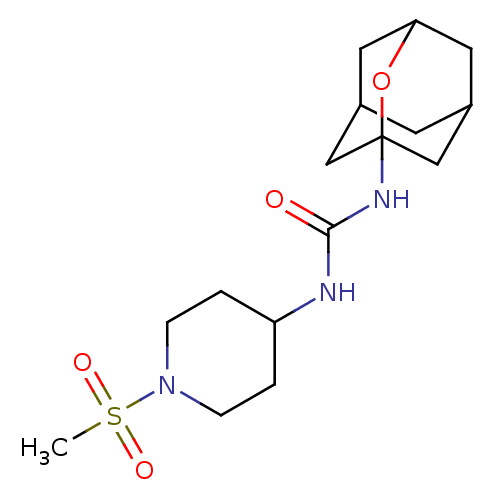 (CHEMBL4758083) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50560757 (CHEMBL4784283) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 197 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50560758 (CHEMBL4800149) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 213 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50560759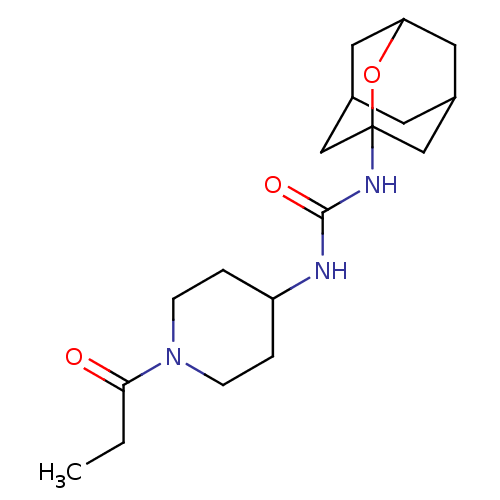 (CHEMBL4764423) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 234 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50560761 (CHEMBL4758083) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 247 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50560755 (CHEMBL4785423) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 291 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM20131 (5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-[(2,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50560750 (CHEMBL4778324) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 344 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50560759 (CHEMBL4764423) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 356 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50560753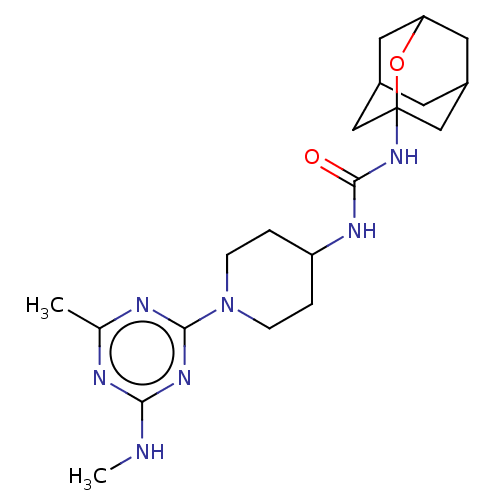 (CHEMBL4746168) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 365 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50560754 (CHEMBL4762846) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 491 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50560753 (CHEMBL4746168) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 496 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen-induced gene 1 protein (Homo sapiens (Human)) | BDBM195604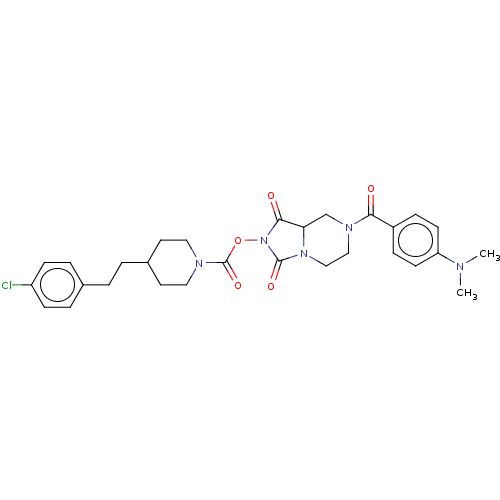 (JJH260) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Tissue and cell proteomes (50 μL) were treated with either FP-rhodamine (1 μM) or WHP01 (2 μM) for 30 min at 37 °C. The reactions were... | Nat Chem Biol 12: 367-72 (2016) Article DOI: 10.1038/nchembio.2051 BindingDB Entry DOI: 10.7270/Q2N015CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50560754 (CHEMBL4762846) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 512 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50560745 (CHEMBL4797443) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 553 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50560751 (CHEMBL4750570) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 723 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50560746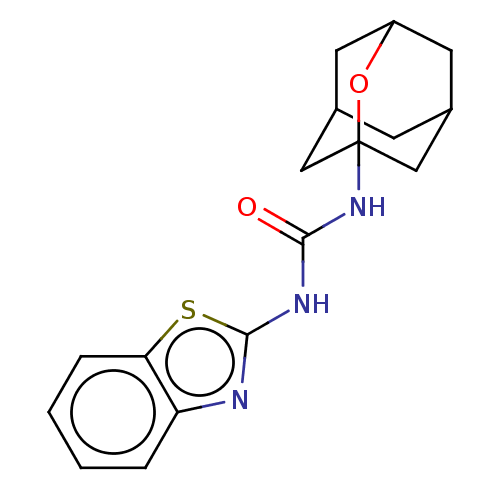 (CHEMBL4764141) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 782 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50560747 (CHEMBL4790388) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 911 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50560752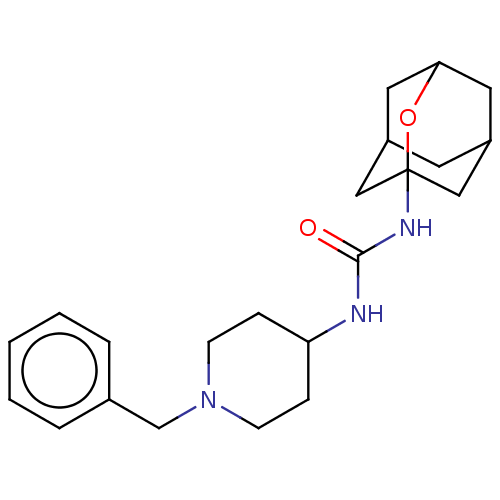 (CHEMBL4748933) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen-dependent TFPI-regulating protein (Homo sapiens (Human)) | BDBM195603 (KC01) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Tissue and cell proteomes (50 μL) were treated with either FP-rhodamine (1 μM) or WHP01 (2 μM) for 30 min at 37 °C. The reactions were... | Nat Chem Biol 12: 367-72 (2016) Article DOI: 10.1038/nchembio.2051 BindingDB Entry DOI: 10.7270/Q2N015CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50560748 (CHEMBL4751517) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50560749 (CHEMBL4762265) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Mus musculus (Mouse)) | BDBM50560747 (CHEMBL4790388) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant mouse soluble epoxide hydrolase using CMNPC as substrate preincubated for 5 mins followed by substrate addition and measure... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00310 BindingDB Entry DOI: 10.7270/Q23F4TCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20132 ((2R)-5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen-dependent TFPI-regulating protein (Homo sapiens (Human)) | BDBM195604 (JJH260) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute | Assay Description Tissue and cell proteomes (50 μL) were treated with either FP-rhodamine (1 μM) or WHP01 (2 μM) for 30 min at 37 °C. The reactions were... | Nat Chem Biol 12: 367-72 (2016) Article DOI: 10.1038/nchembio.2051 BindingDB Entry DOI: 10.7270/Q2N015CW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM20131 (5-(4-chlorophenyl)-2-(2,3-dimethoxyphenyl)-3-[(2,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values... | J Med Chem 50: 4255-9 (2007) Article DOI: 10.1021/jm070453f BindingDB Entry DOI: 10.7270/Q2BK19N6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 609 total ) | Next | Last >> |