 Found 121 hits with Last Name = 'sahin' and Initial = 'mf'
Found 121 hits with Last Name = 'sahin' and Initial = 'mf' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50368271
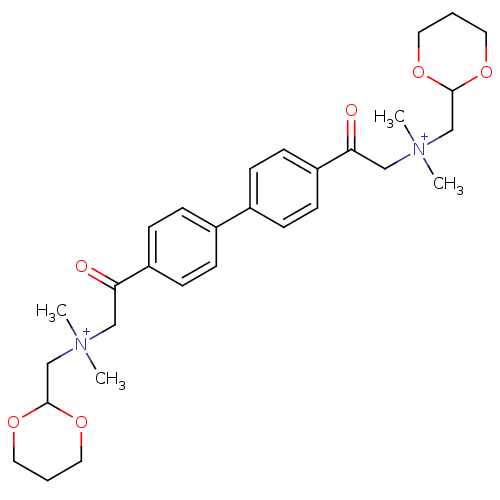
(CHEMBL1204112)Show SMILES C[N+](C)(CC1OCCCO1)CC(=O)c1ccc(cc1)-c1ccc(cc1)C(=O)C[N+](C)(C)CC1OCCCO1 Show InChI InChI=1S/C30H42N2O6/c1-31(2,21-29-35-15-5-16-36-29)19-27(33)25-11-7-23(8-12-25)24-9-13-26(14-10-24)28(34)20-32(3,4)22-30-37-17-6-18-38-30/h7-14,29-30H,5-6,15-22H2,1-4H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Concentration required to inhibit hydrolytic activity of bovine erythrocyte acetylcholinesterase by 50% |
J Med Chem 34: 1582-4 (1991)
BindingDB Entry DOI: 10.7270/Q26D5TK2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50325646
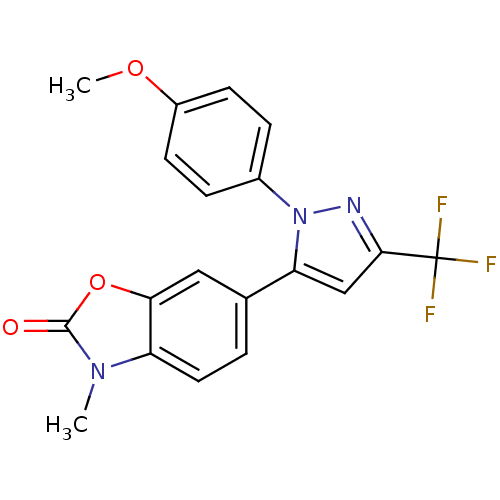
(1-(4-Methoxyphenyl)-3-trifluoromethyl-5-(3-methyl-...)Show SMILES COc1ccc(cc1)-n1nc(cc1-c1ccc2n(C)c(=O)oc2c1)C(F)(F)F Show InChI InChI=1S/C19H14F3N3O3/c1-24-14-8-3-11(9-16(14)28-18(24)26)15-10-17(19(20,21)22)23-25(15)12-4-6-13(27-2)7-5-12/h3-10H,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX1 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50325644
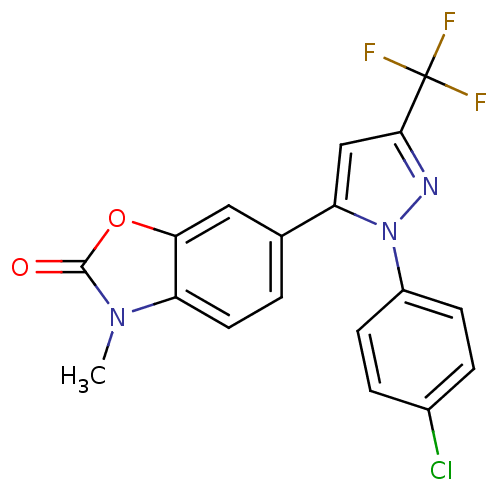
(1-(4-Chlorophenyl)-3-trifluoromethyl-5-(3-methyl-2...)Show SMILES Cn1c2ccc(cc2oc1=O)-c1cc(nn1-c1ccc(Cl)cc1)C(F)(F)F Show InChI InChI=1S/C18H11ClF3N3O2/c1-24-13-7-2-10(8-15(13)27-17(24)26)14-9-16(18(20,21)22)23-25(14)12-5-3-11(19)4-6-12/h2-9H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX2 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50325646

(1-(4-Methoxyphenyl)-3-trifluoromethyl-5-(3-methyl-...)Show SMILES COc1ccc(cc1)-n1nc(cc1-c1ccc2n(C)c(=O)oc2c1)C(F)(F)F Show InChI InChI=1S/C19H14F3N3O3/c1-24-14-8-3-11(9-16(14)28-18(24)26)15-10-17(19(20,21)22)23-25(15)12-4-6-13(27-2)7-5-12/h3-10H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX2 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50004000

((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...)Show SMILES CNC(=O)Oc1ccc2N(C)[C@H]3N(C)CC[C@@]3(C)c2c1 |r| Show InChI InChI=1S/C15H21N3O2/c1-15-7-8-17(3)13(15)18(4)12-6-5-10(9-11(12)15)20-14(19)16-2/h5-6,9,13H,7-8H2,1-4H3,(H,16,19)/t13-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Concentration required to inhibit hydrolytic activity of Acetylcholinesterase by 50% |
J Med Chem 34: 1582-4 (1991)
BindingDB Entry DOI: 10.7270/Q26D5TK2 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50313079
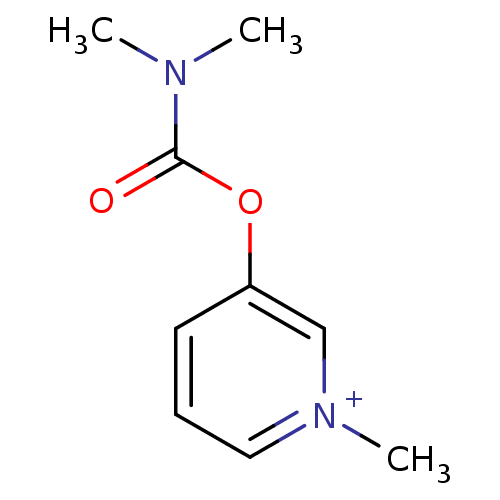
(3-Dimethylcarbamoyloxy-1-methyl-pyridinium; bromid...)Show InChI InChI=1S/C9H13N2O2/c1-10(2)9(12)13-8-5-4-6-11(3)7-8/h4-7H,1-3H3/q+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Concentration required to inhibit hydrolytic activity of bovine erythrocyte acetylcholinesterase by 50% |
J Med Chem 34: 1582-4 (1991)
BindingDB Entry DOI: 10.7270/Q26D5TK2 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50325645
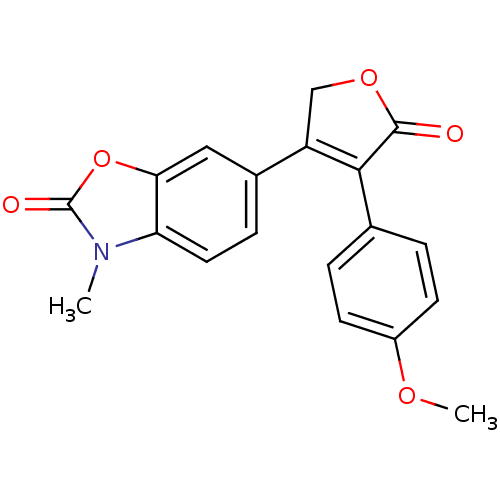
(3-(4-Methoxyphenyl)-4-(3-methyl-2-oxo-3H-benzoxazo...)Show SMILES COc1ccc(cc1)C1=C(COC1=O)c1ccc2n(C)c(=O)oc2c1 |t:9| Show InChI InChI=1S/C19H15NO5/c1-20-15-8-5-12(9-16(15)25-19(20)22)14-10-24-18(21)17(14)11-3-6-13(23-2)7-4-11/h3-9H,10H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX1 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 by enzyme immunoassay |
Eur J Med Chem 44: 1830-7 (2009)
Article DOI: 10.1016/j.ejmech.2008.10.039
BindingDB Entry DOI: 10.7270/Q27P8Z7F |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX1 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50325645

(3-(4-Methoxyphenyl)-4-(3-methyl-2-oxo-3H-benzoxazo...)Show SMILES COc1ccc(cc1)C1=C(COC1=O)c1ccc2n(C)c(=O)oc2c1 |t:9| Show InChI InChI=1S/C19H15NO5/c1-20-15-8-5-12(9-16(15)25-19(20)22)14-10-24-18(21)17(14)11-3-6-13(23-2)7-4-11/h3-9H,10H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 325 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX2 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by enzyme immunoassay |
Eur J Med Chem 44: 1830-7 (2009)
Article DOI: 10.1016/j.ejmech.2008.10.039
BindingDB Entry DOI: 10.7270/Q27P8Z7F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX2 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 537 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX2 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 537 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by enzyme immunoassay |
Eur J Med Chem 44: 1830-7 (2009)
Article DOI: 10.1016/j.ejmech.2008.10.039
BindingDB Entry DOI: 10.7270/Q27P8Z7F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024874

(CHEMBL3335066)Show SMILES O=c1oc2cc(OCCCN3CCN(Cc4ccccc4)CC3)ccc2c2ccccc12 Show InChI InChI=1S/C27H28N2O3/c30-27-25-10-5-4-9-23(25)24-12-11-22(19-26(24)32-27)31-18-6-13-28-14-16-29(17-15-28)20-21-7-2-1-3-8-21/h1-5,7-12,19H,6,13-18,20H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024881
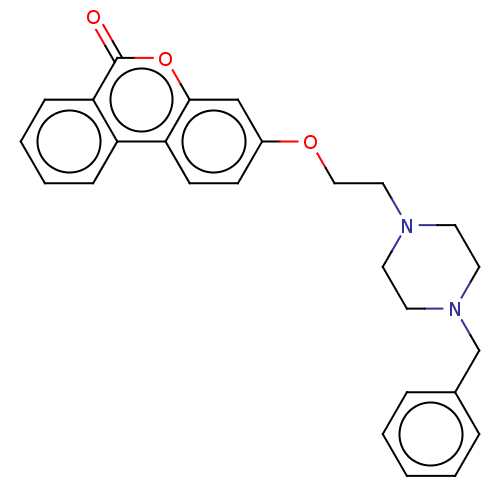
(CHEMBL3335060)Show SMILES O=c1oc2cc(OCCN3CCN(Cc4ccccc4)CC3)ccc2c2ccccc12 Show InChI InChI=1S/C26H26N2O3/c29-26-24-9-5-4-8-22(24)23-11-10-21(18-25(23)31-26)30-17-16-27-12-14-28(15-13-27)19-20-6-2-1-3-7-20/h1-11,18H,12-17,19H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024893
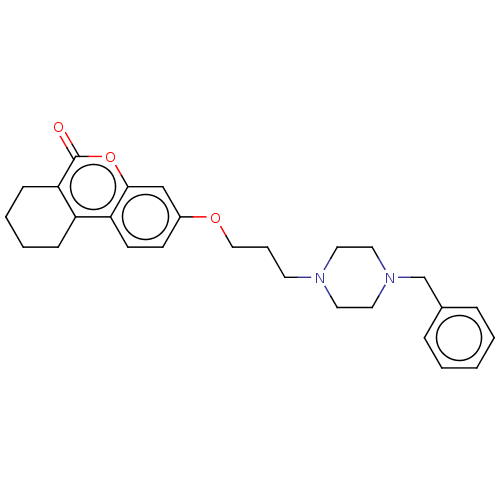
(CHEMBL3335048)Show SMILES O=c1oc2cc(OCCCN3CCN(Cc4ccccc4)CC3)ccc2c2CCCCc12 Show InChI InChI=1S/C27H32N2O3/c30-27-25-10-5-4-9-23(25)24-12-11-22(19-26(24)32-27)31-18-6-13-28-14-16-29(17-15-28)20-21-7-2-1-3-8-21/h1-3,7-8,11-12,19H,4-6,9-10,13-18,20H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50325644

(1-(4-Chlorophenyl)-3-trifluoromethyl-5-(3-methyl-2...)Show SMILES Cn1c2ccc(cc2oc1=O)-c1cc(nn1-c1ccc(Cl)cc1)C(F)(F)F Show InChI InChI=1S/C18H11ClF3N3O2/c1-24-13-7-2-10(8-15(13)27-17(24)26)14-9-16(18(20,21)22)23-25(14)12-5-3-11(19)4-6-12/h2-9H,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX1 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50325643

(1-(4-Fluorophenyl)-3-trifluoromethyl-5-(3-methyl-2...)Show SMILES Cn1c2ccc(cc2oc1=O)-c1cc(nn1-c1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H11F4N3O2/c1-24-13-7-2-10(8-15(13)27-17(24)26)14-9-16(18(20,21)22)23-25(14)12-5-3-11(19)4-6-12/h2-9H,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX2 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024891
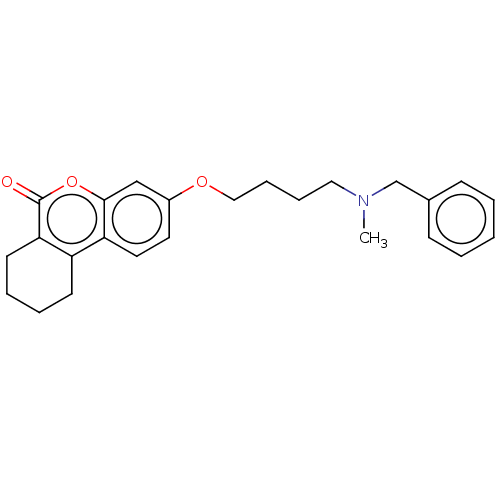
(CHEMBL3335050)Show InChI InChI=1S/C25H29NO3/c1-26(18-19-9-3-2-4-10-19)15-7-8-16-28-20-13-14-22-21-11-5-6-12-23(21)25(27)29-24(22)17-20/h2-4,9-10,13-14,17H,5-8,11-12,15-16,18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024872
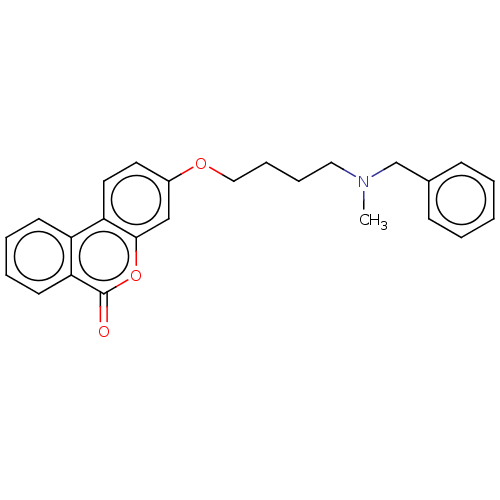
(CHEMBL3335068)Show SMILES CN(CCCCOc1ccc2c(c1)oc(=O)c1ccccc21)Cc1ccccc1 Show InChI InChI=1S/C25H25NO3/c1-26(18-19-9-3-2-4-10-19)15-7-8-16-28-20-13-14-22-21-11-5-6-12-23(21)25(27)29-24(22)17-20/h2-6,9-14,17H,7-8,15-16,18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024900
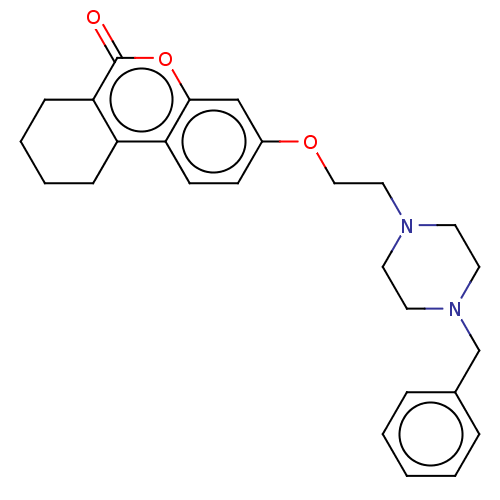
(CHEMBL3335029)Show SMILES O=c1oc2cc(OCCN3CCN(Cc4ccccc4)CC3)ccc2c2CCCCc12 Show InChI InChI=1S/C26H30N2O3/c29-26-24-9-5-4-8-22(24)23-11-10-21(18-25(23)31-26)30-17-16-27-12-14-28(15-13-27)19-20-6-2-1-3-7-20/h1-3,6-7,10-11,18H,4-5,8-9,12-17,19H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024890
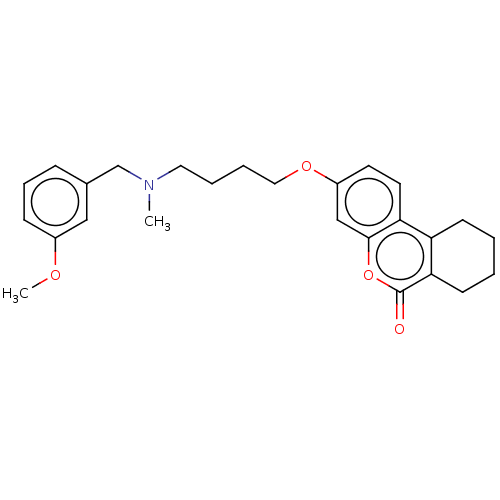
(CHEMBL3335051)Show SMILES COc1cccc(CN(C)CCCCOc2ccc3c4CCCCc4c(=O)oc3c2)c1 Show InChI InChI=1S/C26H31NO4/c1-27(18-19-8-7-9-20(16-19)29-2)14-5-6-15-30-21-12-13-23-22-10-3-4-11-24(22)26(28)31-25(23)17-21/h7-9,12-13,16-17H,3-6,10-11,14-15,18H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Ovis aries (Sheep)) | BDBM50279625
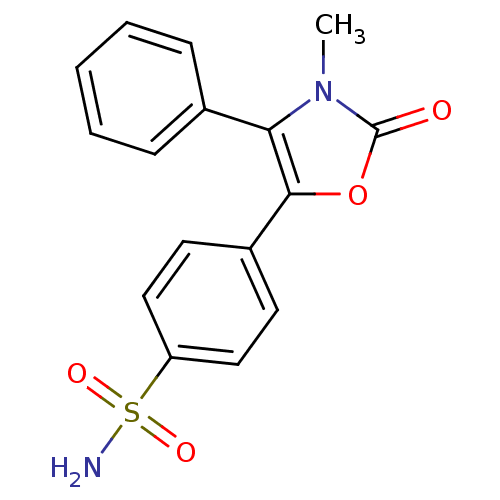
(4-(4-Phenyl-3-methyl-2-oxo-3H-1,3-oxazol-5-yl)benz...)Show SMILES Cn1c(c(oc1=O)-c1ccc(cc1)S(N)(=O)=O)-c1ccccc1 Show InChI InChI=1S/C16H14N2O4S/c1-18-14(11-5-3-2-4-6-11)15(22-16(18)19)12-7-9-13(10-8-12)23(17,20)21/h2-10H,1H3,(H2,17,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX2 by enzyme immunoassay |
Eur J Med Chem 44: 1830-7 (2009)
Article DOI: 10.1016/j.ejmech.2008.10.039
BindingDB Entry DOI: 10.7270/Q27P8Z7F |
More data for this
Ligand-Target Pair | |
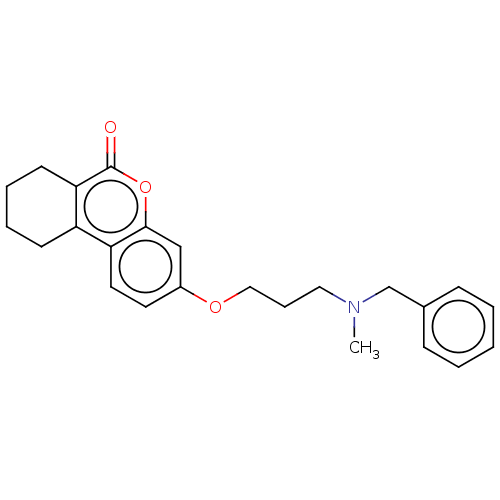
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024898
(CHEMBL3335031)Show InChI InChI=1S/C24H27NO3/c1-25(17-18-8-3-2-4-9-18)14-7-15-27-19-12-13-21-20-10-5-6-11-22(20)24(26)28-23(21)16-19/h2-4,8-9,12-13,16H,5-7,10-11,14-15,17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024897

(CHEMBL3335032)Show SMILES COc1cccc(CN(C)CCCOc2ccc3c4CCCCc4c(=O)oc3c2)c1 Show InChI InChI=1S/C25H29NO4/c1-26(17-18-7-5-8-19(15-18)28-2)13-6-14-29-20-11-12-22-21-9-3-4-10-23(21)25(27)30-24(22)16-20/h5,7-8,11-12,15-16H,3-4,6,9-10,13-14,17H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024862

(CHEMBL3335019)Show InChI InChI=1S/C18H19NO3.ClH/c1-11(19(2)3)12-9-15(21-4)17-13-7-5-6-8-14(13)18(20)22-16(17)10-12;/h5-11H,1-4H3;1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024953

(CHEMBL3335025)Show InChI InChI=1S/C23H25NO3/c1-24(16-17-7-3-2-4-8-17)13-14-26-18-11-12-20-19-9-5-6-10-21(19)23(25)27-22(20)15-18/h2-4,7-8,11-12,15H,5-6,9-10,13-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50024893

(CHEMBL3335048)Show SMILES O=c1oc2cc(OCCCN3CCN(Cc4ccccc4)CC3)ccc2c2CCCCc12 Show InChI InChI=1S/C27H32N2O3/c30-27-25-10-5-4-9-23(25)24-12-11-22(19-26(24)32-27)31-18-6-13-28-14-16-29(17-15-28)20-21-7-2-1-3-8-21/h1-3,7-8,11-12,19H,4-6,9-10,13-18,20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BuChE using butyrylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50024874

(CHEMBL3335066)Show SMILES O=c1oc2cc(OCCCN3CCN(Cc4ccccc4)CC3)ccc2c2ccccc12 Show InChI InChI=1S/C27H28N2O3/c30-27-25-10-5-4-9-23(25)24-12-11-22(19-26(24)32-27)31-18-6-13-28-14-16-29(17-15-28)20-21-7-2-1-3-8-21/h1-5,7-12,19H,6,13-18,20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BuChE using butyrylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
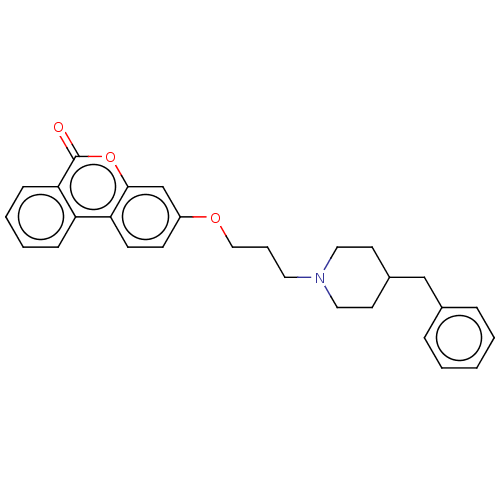
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024876
(CHEMBL3335064)Show SMILES O=c1oc2cc(OCCCN3CCC(Cc4ccccc4)CC3)ccc2c2ccccc12 Show InChI InChI=1S/C28H29NO3/c30-28-26-10-5-4-9-24(26)25-12-11-23(20-27(25)32-28)31-18-6-15-29-16-13-22(14-17-29)19-21-7-2-1-3-8-21/h1-5,7-12,20,22H,6,13-19H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
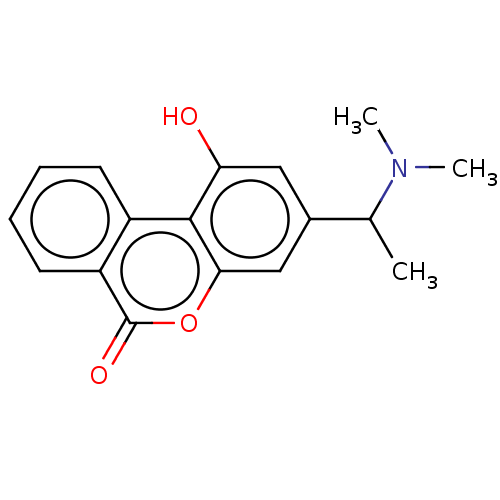
Cholinesterase
(Homo sapiens (Human)) | BDBM50024957
(CHEMBL3335021)Show InChI InChI=1S/C17H17NO3.ClH/c1-10(18(2)3)11-8-14(19)16-12-6-4-5-7-13(12)17(20)21-15(16)9-11;/h4-10,19H,1-3H3;1H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BuChE using butyrylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
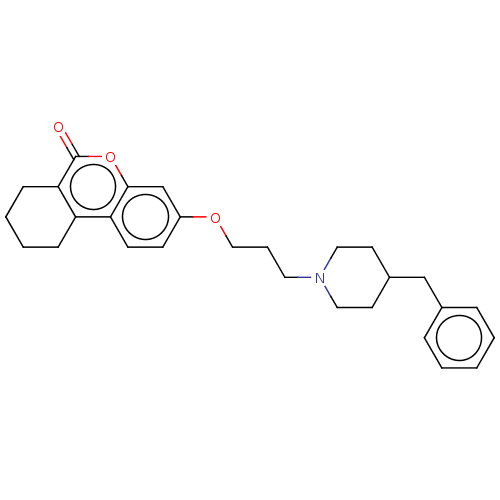
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024896
(CHEMBL3335046)Show SMILES O=c1oc2cc(OCCCN3CCC(Cc4ccccc4)CC3)ccc2c2CCCCc12 Show InChI InChI=1S/C28H33NO3/c30-28-26-10-5-4-9-24(26)25-12-11-23(20-27(25)32-28)31-18-6-15-29-16-13-22(14-17-29)19-21-7-2-1-3-8-21/h1-3,7-8,11-12,20,22H,4-6,9-10,13-19H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024895
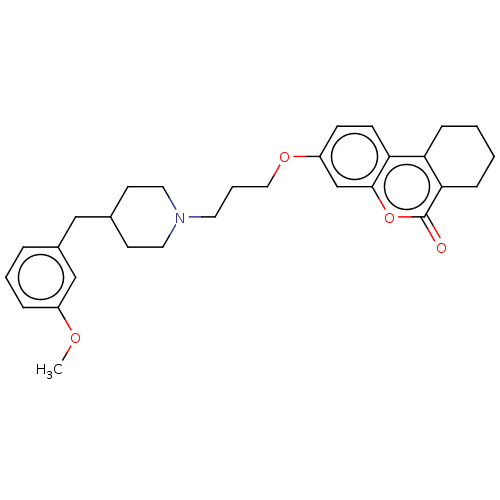
(CHEMBL3335047)Show SMILES COc1cccc(CC2CCN(CCCOc3ccc4c5CCCCc5c(=O)oc4c3)CC2)c1 Show InChI InChI=1S/C29H35NO4/c1-32-23-7-4-6-22(19-23)18-21-12-15-30(16-13-21)14-5-17-33-24-10-11-26-25-8-2-3-9-27(25)29(31)34-28(26)20-24/h4,6-7,10-11,19-21H,2-3,5,8-9,12-18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM50325643

(1-(4-Fluorophenyl)-3-trifluoromethyl-5-(3-methyl-2...)Show SMILES Cn1c2ccc(cc2oc1=O)-c1cc(nn1-c1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C18H11F4N3O2/c1-24-13-7-2-10(8-15(13)27-17(24)26)14-9-16(18(20,21)22)23-25(14)12-5-3-11(19)4-6-12/h2-9H,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gazi University
Curated by ChEMBL
| Assay Description
Inhibition of sheep COX1 by enzyme immunoassay |
Bioorg Med Chem 18: 6367-76 (2010)
Article DOI: 10.1016/j.bmc.2010.07.009
BindingDB Entry DOI: 10.7270/Q2VH5P15 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024933
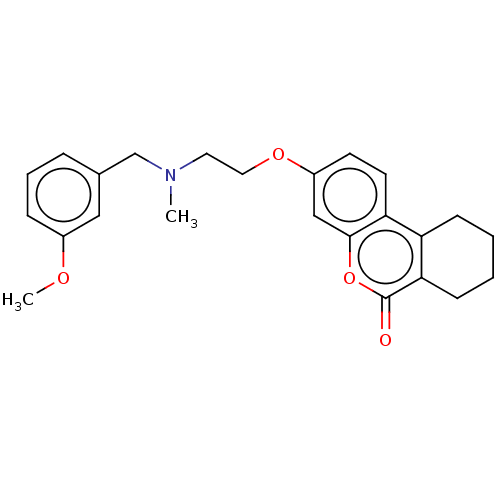
(CHEMBL3335026)Show SMILES COc1cccc(CN(C)CCOc2ccc3c4CCCCc4c(=O)oc3c2)c1 Show InChI InChI=1S/C24H27NO4/c1-25(16-17-6-5-7-18(14-17)27-2)12-13-28-19-10-11-21-20-8-3-4-9-22(20)24(26)29-23(21)15-19/h5-7,10-11,14-15H,3-4,8-9,12-13,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024892
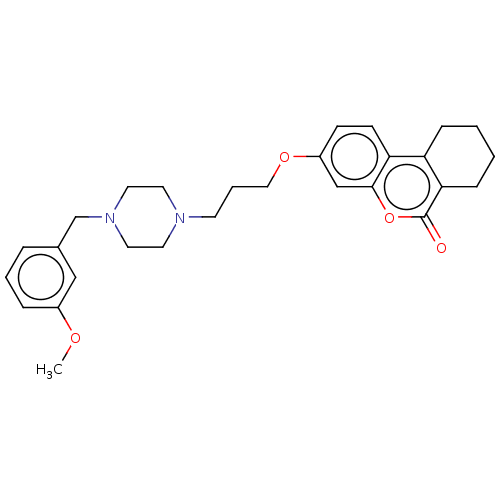
(CHEMBL3335049)Show SMILES COc1cccc(CN2CCN(CCCOc3ccc4c5CCCCc5c(=O)oc4c3)CC2)c1 Show InChI InChI=1S/C28H34N2O4/c1-32-22-7-4-6-21(18-22)20-30-15-13-29(14-16-30)12-5-17-33-23-10-11-25-24-8-2-3-9-26(24)28(31)34-27(25)19-23/h4,6-7,10-11,18-19H,2-3,5,8-9,12-17,20H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024887
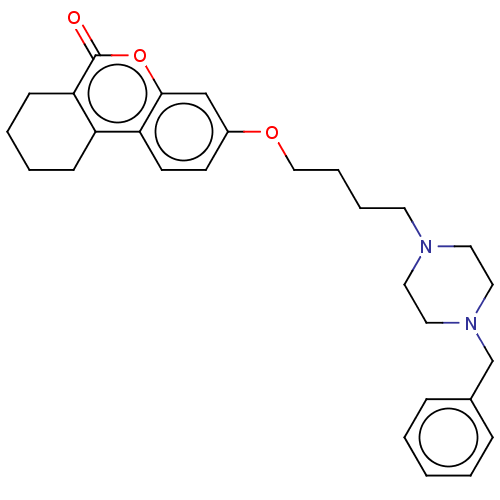
(CHEMBL3335054)Show SMILES O=c1oc2cc(OCCCCN3CCN(Cc4ccccc4)CC3)ccc2c2CCCCc12 Show InChI InChI=1S/C28H34N2O3/c31-28-26-11-5-4-10-24(26)25-13-12-23(20-27(25)33-28)32-19-7-6-14-29-15-17-30(18-16-29)21-22-8-2-1-3-9-22/h1-3,8-9,12-13,20H,4-7,10-11,14-19,21H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50024872

(CHEMBL3335068)Show SMILES CN(CCCCOc1ccc2c(c1)oc(=O)c1ccccc21)Cc1ccccc1 Show InChI InChI=1S/C25H25NO3/c1-26(18-19-9-3-2-4-10-19)15-7-8-16-28-20-13-14-22-21-11-5-6-12-23(21)25(27)29-24(22)17-20/h2-6,9-14,17H,7-8,15-16,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BuChE using butyrylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024885
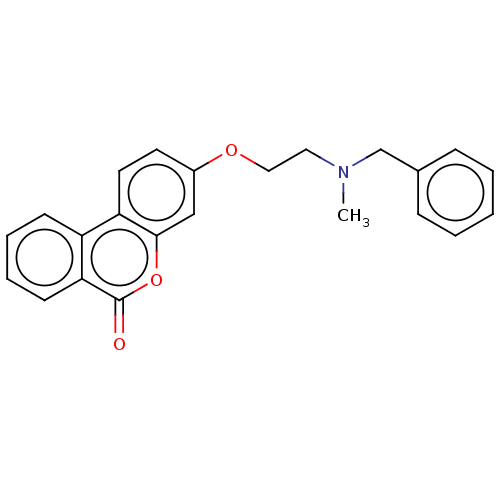
(CHEMBL3335056)Show InChI InChI=1S/C23H21NO3/c1-24(16-17-7-3-2-4-8-17)13-14-26-18-11-12-20-19-9-5-6-10-21(19)23(25)27-22(20)15-18/h2-12,15H,13-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50368269
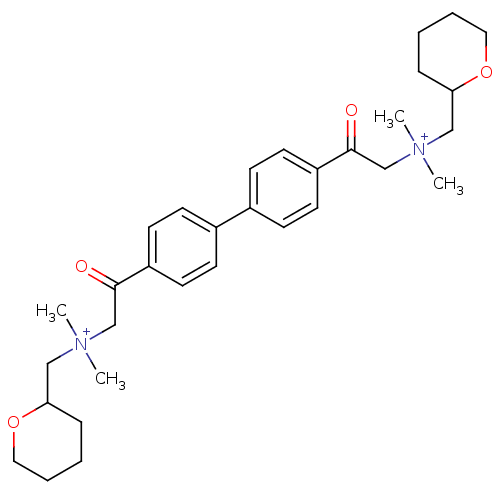
(CHEMBL1202156)Show SMILES C[N+](C)(CC1CCCCO1)CC(=O)c1ccc(cc1)-c1ccc(cc1)C(=O)C[N+](C)(C)CC1CCCCO1 Show InChI InChI=1S/C32H46N2O4/c1-33(2,21-29-9-5-7-19-37-29)23-31(35)27-15-11-25(12-16-27)26-13-17-28(18-14-26)32(36)24-34(3,4)22-30-10-6-8-20-38-30/h11-18,29-30H,5-10,19-24H2,1-4H3/q+2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Iowa
Curated by ChEMBL
| Assay Description
Concentration required to inhibit hydrolytic activity of bovine erythrocyte acetylcholinesterase by 50% |
J Med Chem 34: 1582-4 (1991)
BindingDB Entry DOI: 10.7270/Q26D5TK2 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BuChE using butyrylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024899
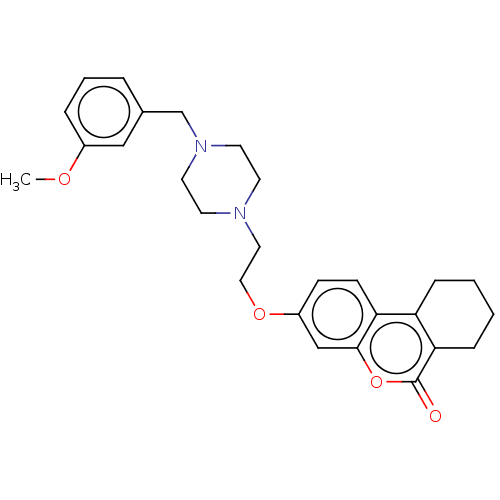
(CHEMBL3335030)Show SMILES COc1cccc(CN2CCN(CCOc3ccc4c5CCCCc5c(=O)oc4c3)CC2)c1 Show InChI InChI=1S/C27H32N2O4/c1-31-21-6-4-5-20(17-21)19-29-13-11-28(12-14-29)15-16-32-22-9-10-24-23-7-2-3-8-25(23)27(30)33-26(24)18-22/h4-6,9-10,17-18H,2-3,7-8,11-16,19H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024864
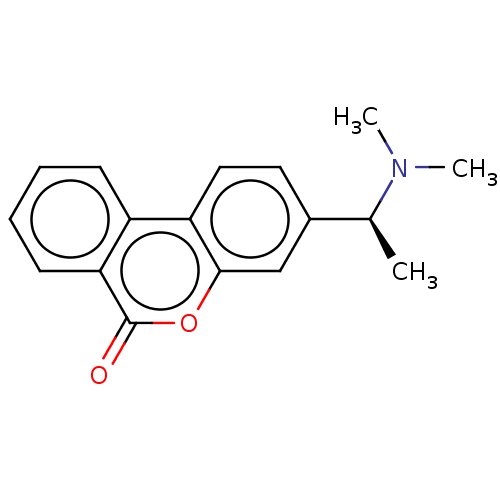
(CHEMBL3335018)Show SMILES Cl.C[C@H](N(C)C)c1ccc2c(c1)oc(=O)c1ccccc21 |r| Show InChI InChI=1S/C17H17NO2.ClH/c1-11(18(2)3)12-8-9-14-13-6-4-5-7-15(13)17(19)20-16(14)10-12;/h4-11H,1-3H3;1H/t11-;/m0./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024924
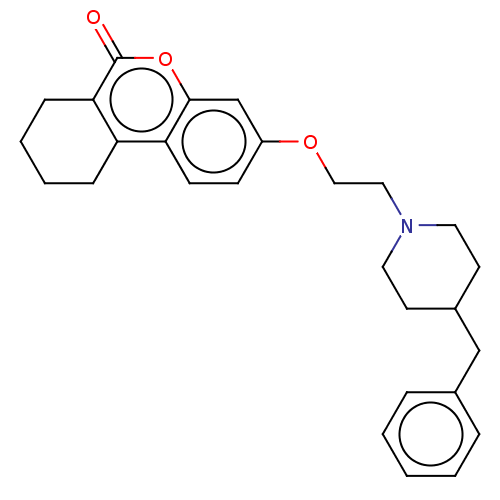
(CHEMBL3335027)Show SMILES O=c1oc2cc(OCCN3CCC(Cc4ccccc4)CC3)ccc2c2CCCCc12 Show InChI InChI=1S/C27H31NO3/c29-27-25-9-5-4-8-23(25)24-11-10-22(19-26(24)31-27)30-17-16-28-14-12-21(13-15-28)18-20-6-2-1-3-7-20/h1-3,6-7,10-11,19,21H,4-5,8-9,12-18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024868
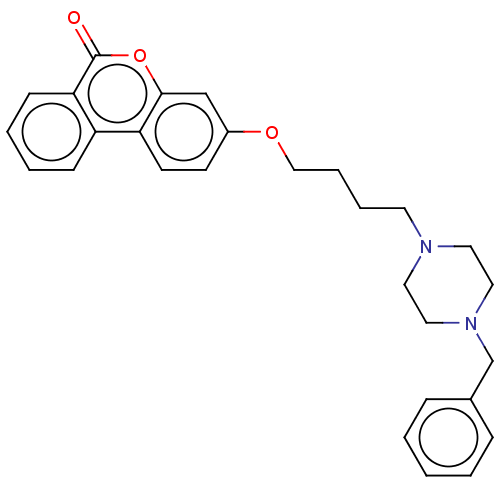
(CHEMBL3335071)Show SMILES O=c1oc2cc(OCCCCN3CCN(Cc4ccccc4)CC3)ccc2c2ccccc12 Show InChI InChI=1S/C28H30N2O3/c31-28-26-11-5-4-10-24(26)25-13-12-23(20-27(25)33-28)32-19-7-6-14-29-15-17-30(18-16-29)21-22-8-2-1-3-9-22/h1-5,8-13,20H,6-7,14-19,21H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024871
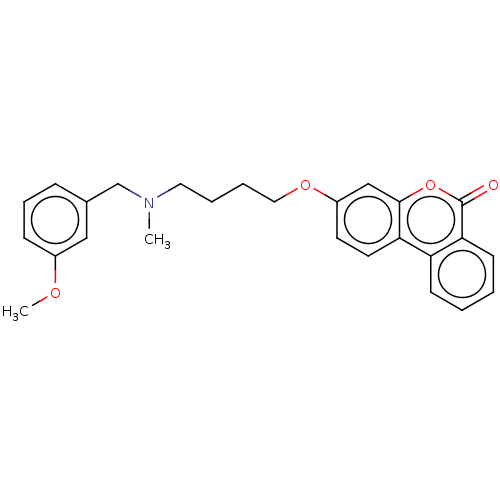
(CHEMBL3335069)Show SMILES COc1cccc(CN(C)CCCCOc2ccc3c(c2)oc(=O)c2ccccc32)c1 Show InChI InChI=1S/C26H27NO4/c1-27(18-19-8-7-9-20(16-19)29-2)14-5-6-15-30-21-12-13-23-22-10-3-4-11-24(22)26(28)31-25(23)17-21/h3-4,7-13,16-17H,5-6,14-15,18H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024879
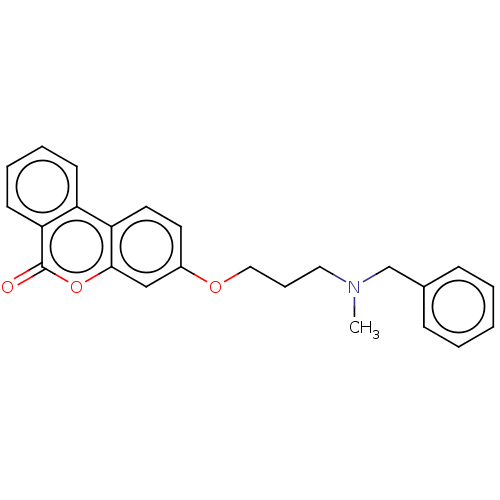
(CHEMBL3335062)Show SMILES CN(CCCOc1ccc2c(c1)oc(=O)c1ccccc21)Cc1ccccc1 Show InChI InChI=1S/C24H23NO3/c1-25(17-18-8-3-2-4-9-18)14-7-15-27-19-12-13-21-20-10-5-6-11-22(20)24(26)28-23(21)16-19/h2-6,8-13,16H,7,14-15,17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Mediterranean University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AChE using acetylthiocholine iodide substrate incubated for 15 mins by spectrophotometry based Ellman's method |
Bioorg Med Chem 22: 5141-54 (2014)
Article DOI: 10.1016/j.bmc.2014.08.016
BindingDB Entry DOI: 10.7270/Q2WH2RKB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data