

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244722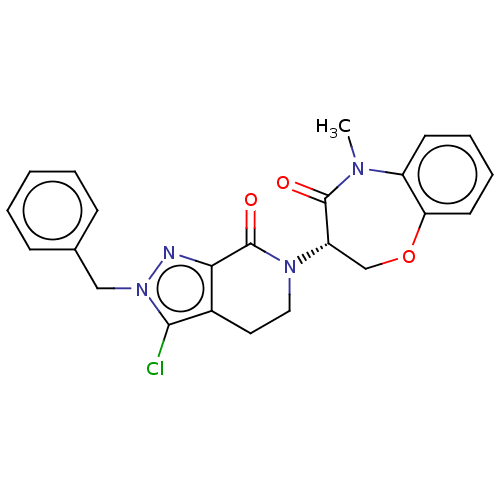 (CHEMBL4075976) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244729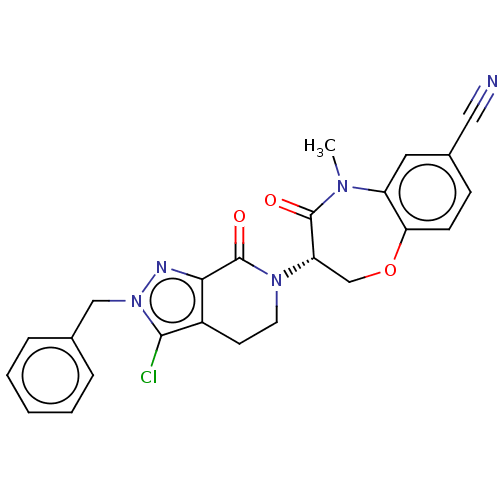 (CHEMBL4061975) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244730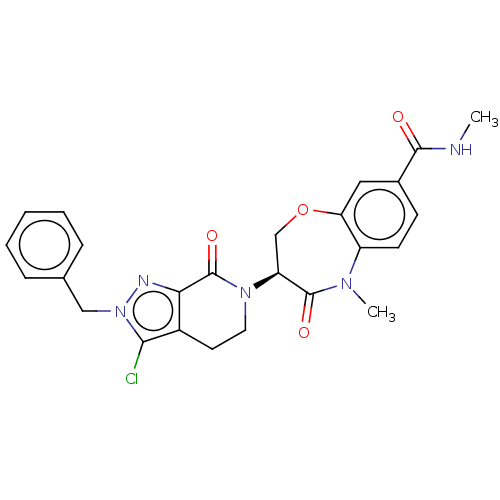 (CHEMBL4069537) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244721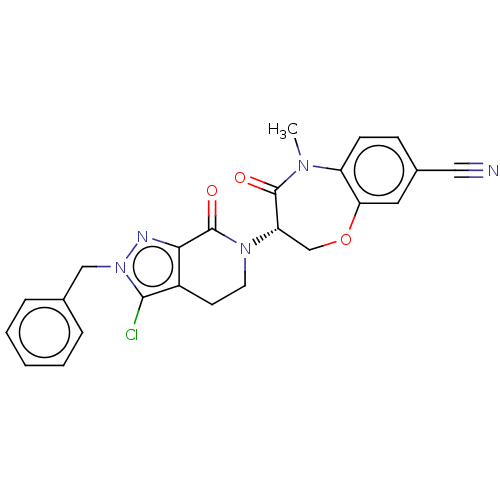 (CHEMBL4100398) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.912 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50233225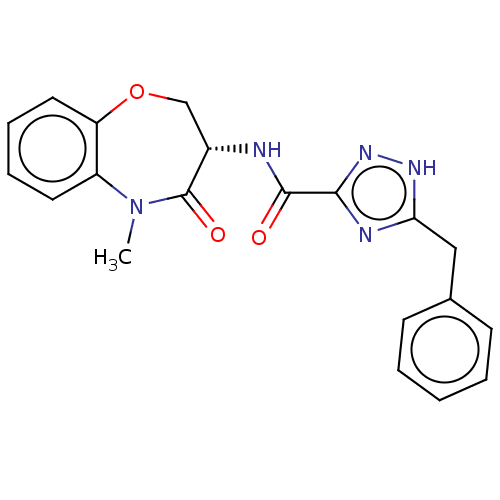 (CHEMBL4071864) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.977 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244765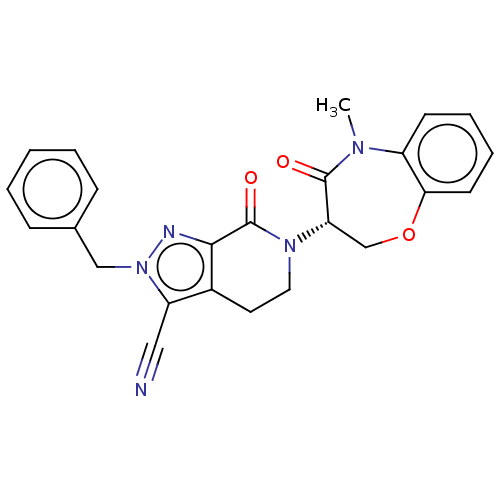 (CHEMBL4084681) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244763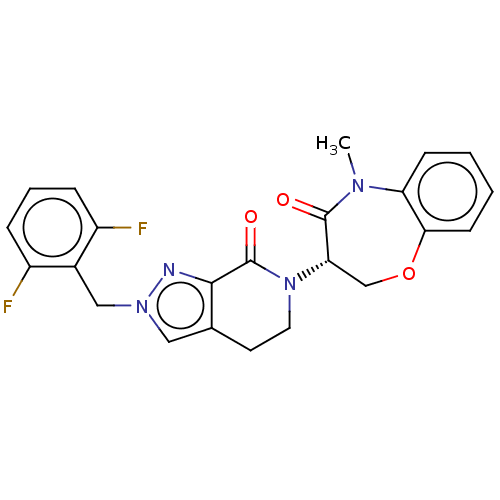 (CHEMBL4082215) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244679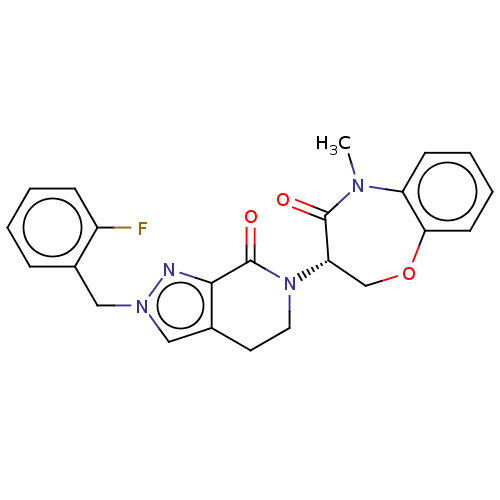 (CHEMBL4090975) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244761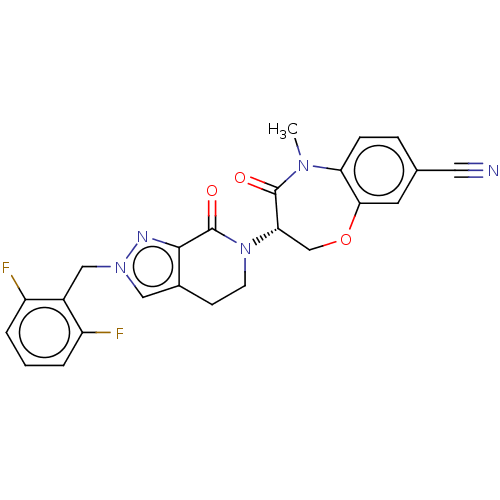 (CHEMBL4075705) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM15003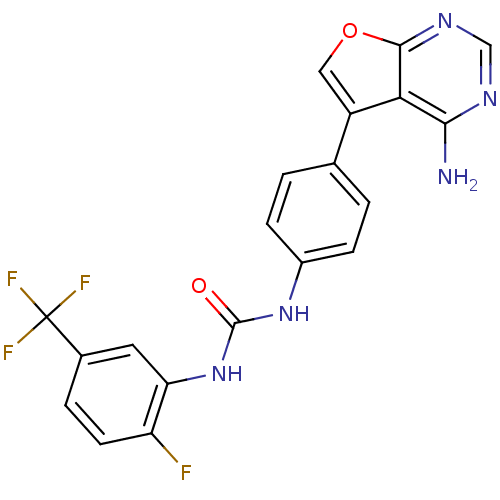 (3-(4-{4-aminofuro[2,3-d]pyrimidin-5-yl}phenyl)-1-[...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244728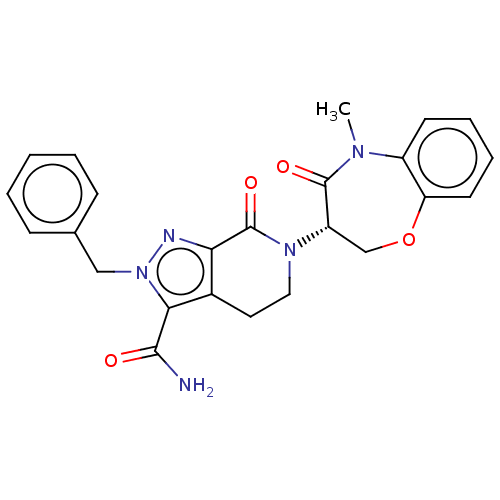 (CHEMBL4102622) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244720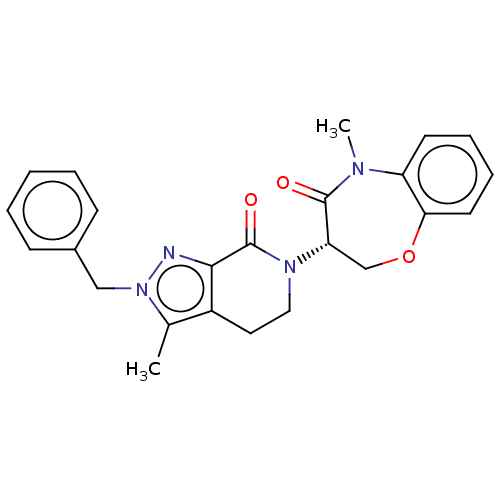 (CHEMBL4064701) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244731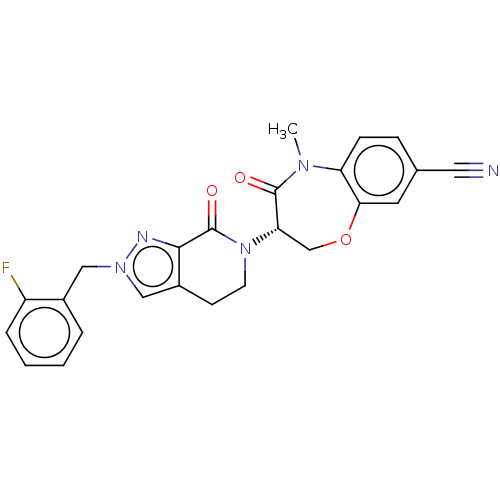 (CHEMBL4085728) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244766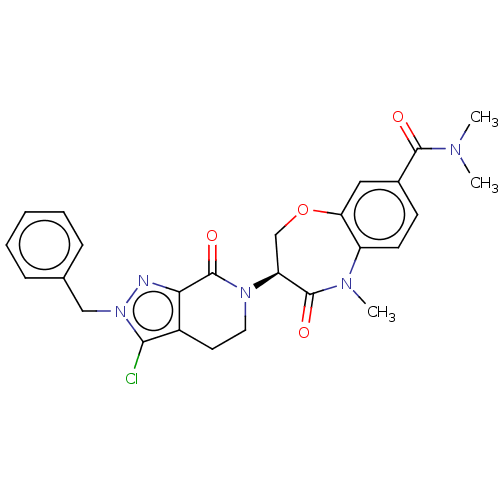 (CHEMBL4073321) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244678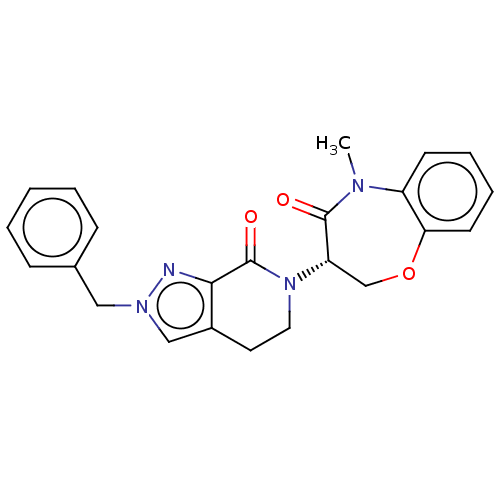 (CHEMBL4088216) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244710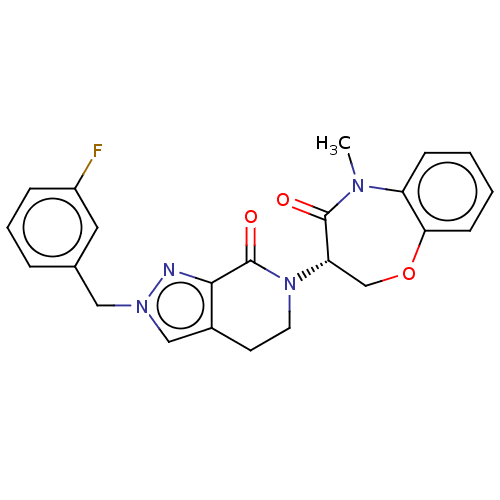 (CHEMBL4071690) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244711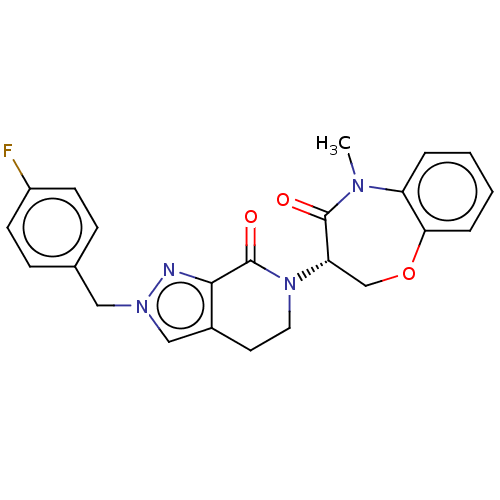 (CHEMBL4098708) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244714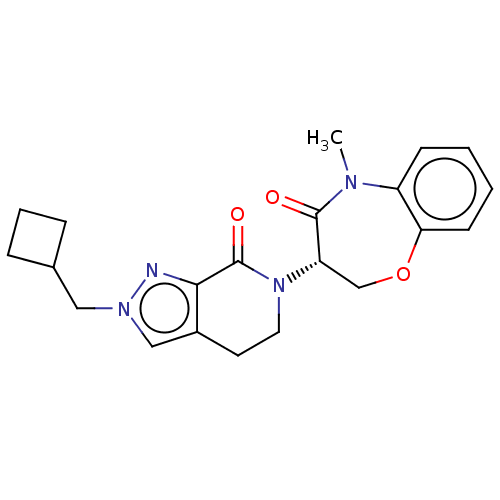 (CHEMBL4060308) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244716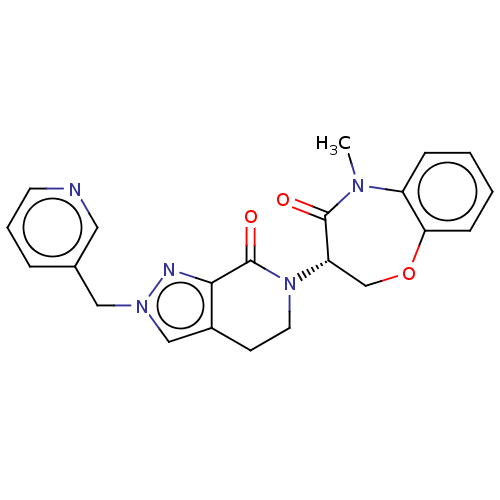 (CHEMBL4090065) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244717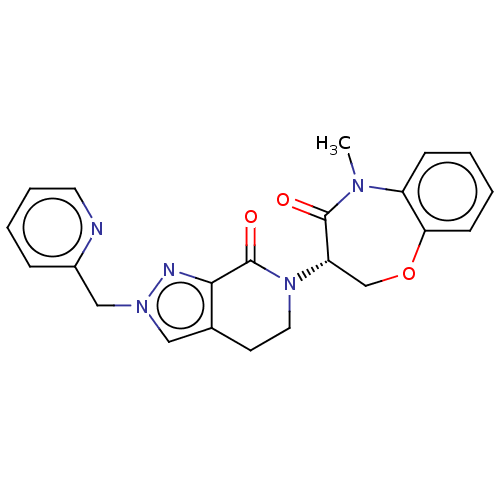 (CHEMBL4076592) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244676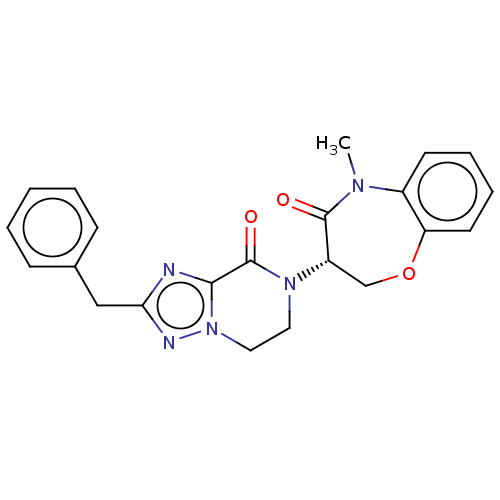 (CHEMBL4100309) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244670 (CHEMBL4077039) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Mus musculus) | BDBM50244721 (CHEMBL4100398) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244677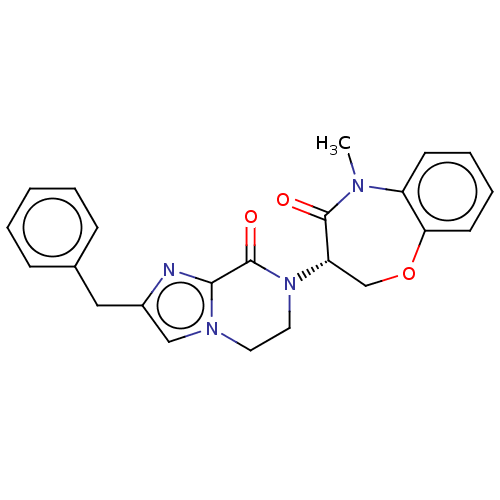 (CHEMBL4066941) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244777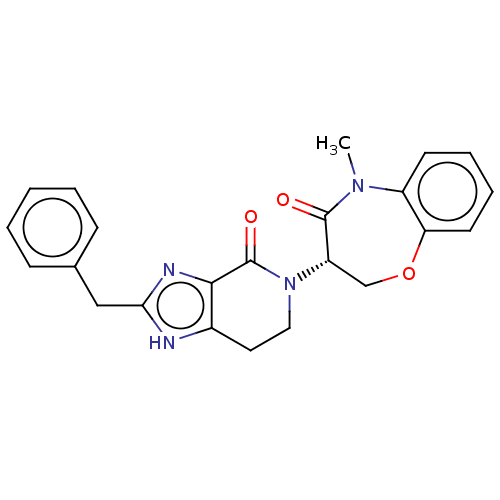 (CHEMBL4082291) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244762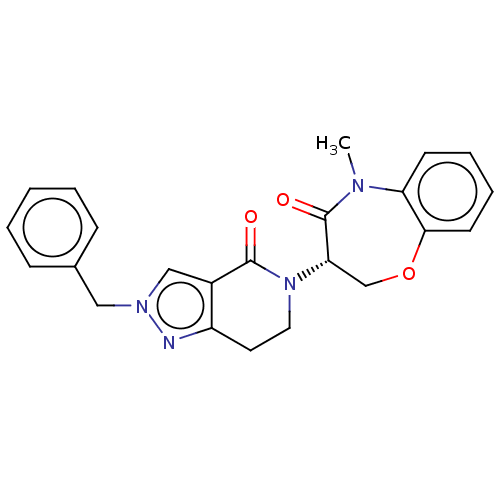 (CHEMBL4063075) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244719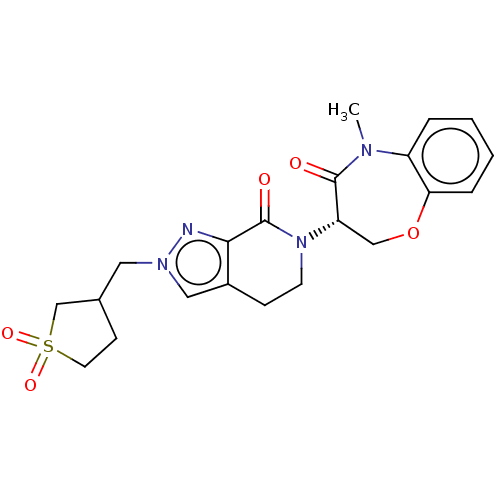 (CHEMBL4079755) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244764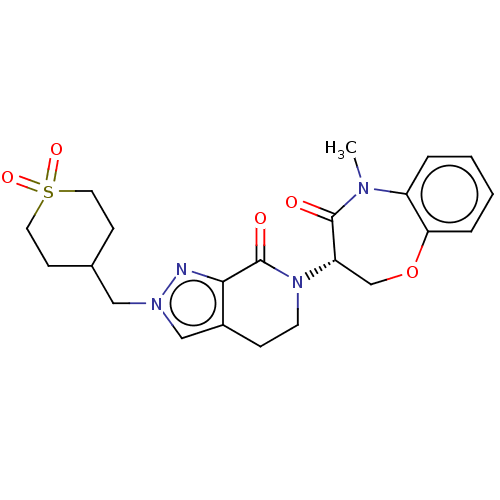 (CHEMBL4097778) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244721 (CHEMBL4100398) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of RIPK1 in human HT-29 cells assessed as decrease in TNFalpha/AT-406/zVAD-FMK-induced MLKL phosphorylation at S358 residue preincubated f... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM31587 (oxadiazole derivative, 20x) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Takeda Pharmaceutical Company Ltd. | Assay Description Human GSK-3beta was expressed as the N-terminal FLAG-tagged protein using a baculovirus expression system. The kinase assay was was conducted in a 96... | Bioorg Med Chem 17: 2017-29 (2009) Article DOI: 10.1016/j.bmc.2009.01.019 BindingDB Entry DOI: 10.7270/Q2DR2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM31586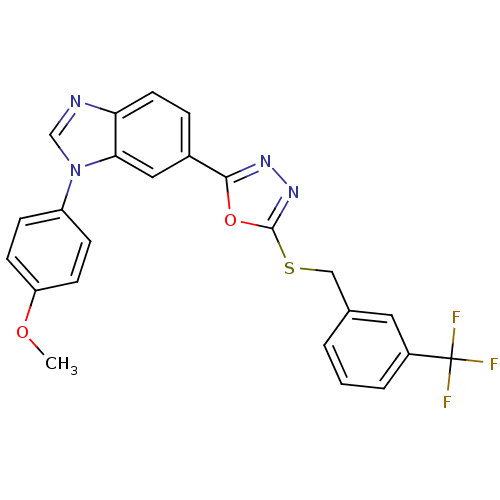 (oxadiazole derivative, 20w) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Takeda Pharmaceutical Company Ltd. | Assay Description Human GSK-3beta was expressed as the N-terminal FLAG-tagged protein using a baculovirus expression system. The kinase assay was was conducted in a 96... | Bioorg Med Chem 17: 2017-29 (2009) Article DOI: 10.1016/j.bmc.2009.01.019 BindingDB Entry DOI: 10.7270/Q2DR2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Mus musculus) | BDBM50244721 (CHEMBL4100398) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of RIPK1 in mouse L929 cells assessed as decrease in TNFalpha/zVAD-FMK-induced MLKL phosphorylation at S358 residue preincubated for 30 mi... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM31578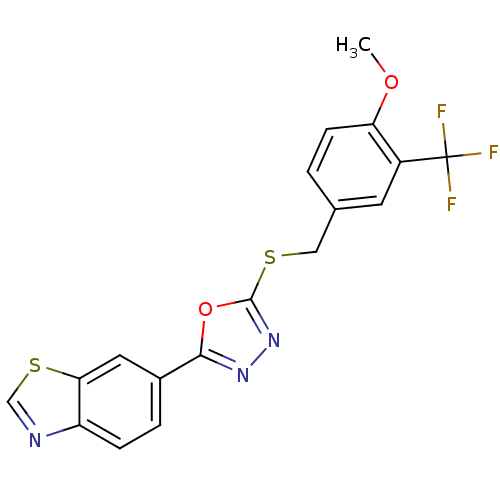 (oxadiazole derivative, 20o) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Takeda Pharmaceutical Company Ltd. | Assay Description Human GSK-3beta was expressed as the N-terminal FLAG-tagged protein using a baculovirus expression system. The kinase assay was was conducted in a 96... | Bioorg Med Chem 17: 2017-29 (2009) Article DOI: 10.1016/j.bmc.2009.01.019 BindingDB Entry DOI: 10.7270/Q2DR2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM31584 (oxadiazole derivative, 20u) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Takeda Pharmaceutical Company Ltd. | Assay Description Human GSK-3beta was expressed as the N-terminal FLAG-tagged protein using a baculovirus expression system. The kinase assay was was conducted in a 96... | Bioorg Med Chem 17: 2017-29 (2009) Article DOI: 10.1016/j.bmc.2009.01.019 BindingDB Entry DOI: 10.7270/Q2DR2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity mitogen-activated protein kinase kinase 3 (Homo sapiens (Human)) | BDBM50134587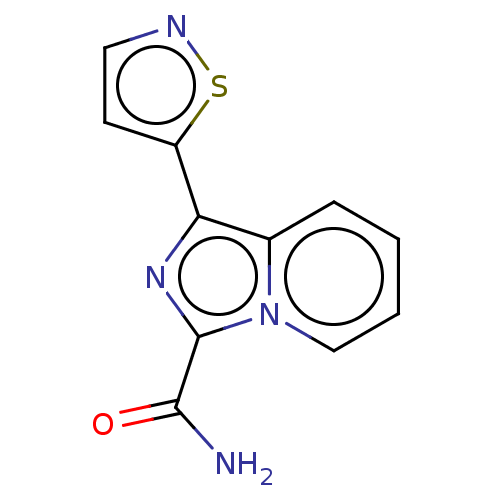 (CHEMBL3746157) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Inc. Curated by ChEMBL | Assay Description Inhibition of MKK3 (unknown origin) using [gamma-33P]-ATP after 20 mins by radiometric assay | Bioorg Med Chem Lett 26: 1086-9 (2016) Article DOI: 10.1016/j.bmcl.2015.11.054 BindingDB Entry DOI: 10.7270/Q28K7BXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity mitogen-activated protein kinase kinase 3 (Homo sapiens (Human)) | BDBM50134589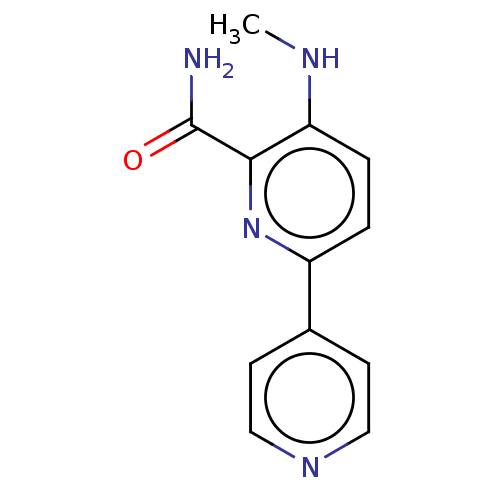 (CHEMBL3747095) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California Inc. Curated by ChEMBL | Assay Description Inhibition of MKK3 (unknown origin) using [gamma-33P]-ATP after 20 mins by radiometric assay | Bioorg Med Chem Lett 26: 1086-9 (2016) Article DOI: 10.1016/j.bmcl.2015.11.054 BindingDB Entry DOI: 10.7270/Q28K7BXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM31577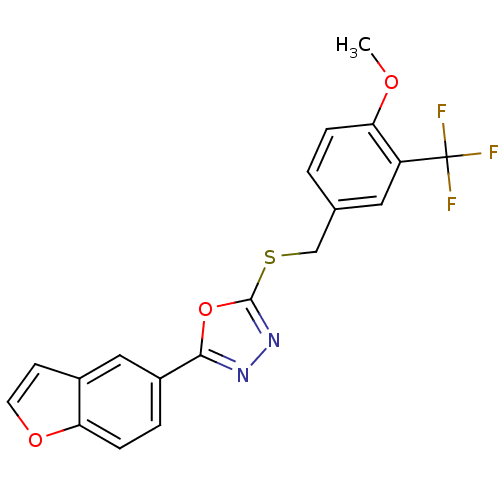 (oxadiazole derivative, 20n) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Takeda Pharmaceutical Company Ltd. | Assay Description Human GSK-3beta was expressed as the N-terminal FLAG-tagged protein using a baculovirus expression system. The kinase assay was was conducted in a 96... | Bioorg Med Chem 17: 2017-29 (2009) Article DOI: 10.1016/j.bmc.2009.01.019 BindingDB Entry DOI: 10.7270/Q2DR2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM31572 (oxadiazole derivative, 20m) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Takeda Pharmaceutical Company Ltd. | Assay Description Human GSK-3beta was expressed as the N-terminal FLAG-tagged protein using a baculovirus expression system. The kinase assay was was conducted in a 96... | Bioorg Med Chem 17: 2017-29 (2009) Article DOI: 10.1016/j.bmc.2009.01.019 BindingDB Entry DOI: 10.7270/Q2DR2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM31580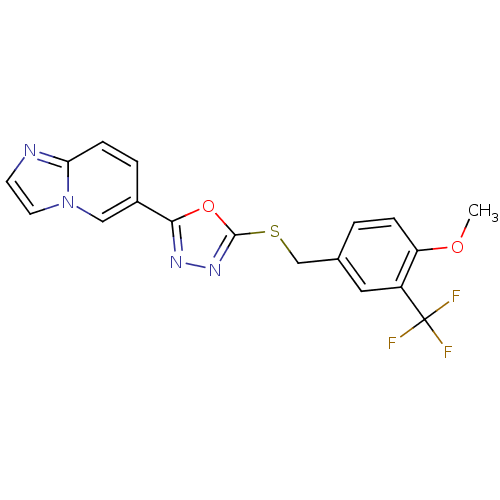 (oxadiazole derivative, 20q) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Takeda Pharmaceutical Company Ltd. | Assay Description Human GSK-3beta was expressed as the N-terminal FLAG-tagged protein using a baculovirus expression system. The kinase assay was was conducted in a 96... | Bioorg Med Chem 17: 2017-29 (2009) Article DOI: 10.1016/j.bmc.2009.01.019 BindingDB Entry DOI: 10.7270/Q2DR2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50346122 (2-(4-(2-(4-bromo-5-methylthiophen-2-yl)-5-ethyl-6-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates | Bioorg Med Chem Lett 19: 3174-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.121 BindingDB Entry DOI: 10.7270/Q26110NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM31583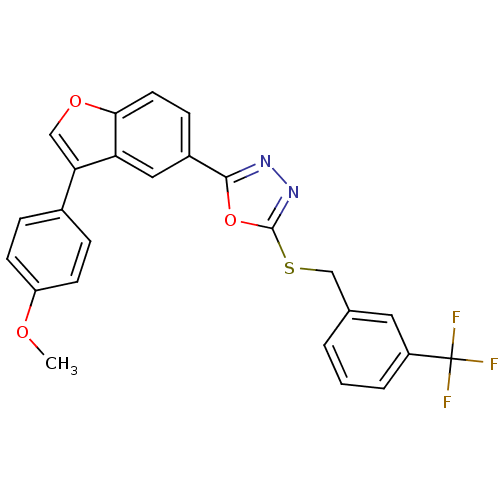 (oxadiazole derivative, 20t) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Takeda Pharmaceutical Company Ltd. | Assay Description Human GSK-3beta was expressed as the N-terminal FLAG-tagged protein using a baculovirus expression system. The kinase assay was was conducted in a 96... | Bioorg Med Chem 17: 2017-29 (2009) Article DOI: 10.1016/j.bmc.2009.01.019 BindingDB Entry DOI: 10.7270/Q2DR2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM31585 (oxadiazole derivative, 20v) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Takeda Pharmaceutical Company Ltd. | Assay Description Human GSK-3beta was expressed as the N-terminal FLAG-tagged protein using a baculovirus expression system. The kinase assay was was conducted in a 96... | Bioorg Med Chem 17: 2017-29 (2009) Article DOI: 10.1016/j.bmc.2009.01.019 BindingDB Entry DOI: 10.7270/Q2DR2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM31588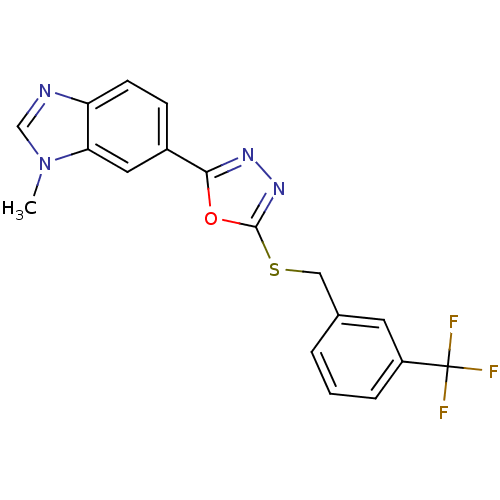 (oxadiazole derivative, 20y) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Takeda Pharmaceutical Company Ltd. | Assay Description Human GSK-3beta was expressed as the N-terminal FLAG-tagged protein using a baculovirus expression system. The kinase assay was was conducted in a 96... | Bioorg Med Chem 17: 2017-29 (2009) Article DOI: 10.1016/j.bmc.2009.01.019 BindingDB Entry DOI: 10.7270/Q2DR2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM31570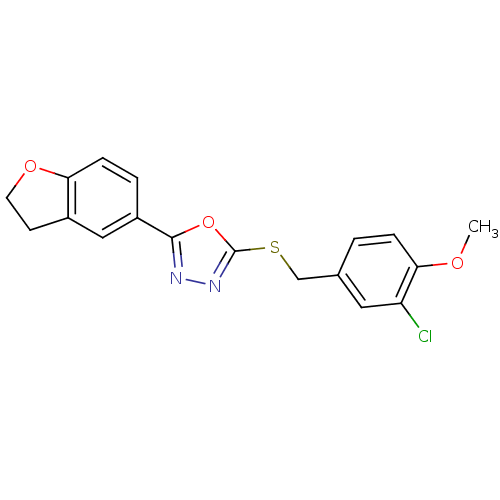 (oxadiazole derivative, 20k) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Takeda Pharmaceutical Company Ltd. | Assay Description Human GSK-3beta was expressed as the N-terminal FLAG-tagged protein using a baculovirus expression system. The kinase assay was was conducted in a 96... | Bioorg Med Chem 17: 2017-29 (2009) Article DOI: 10.1016/j.bmc.2009.01.019 BindingDB Entry DOI: 10.7270/Q2DR2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50299079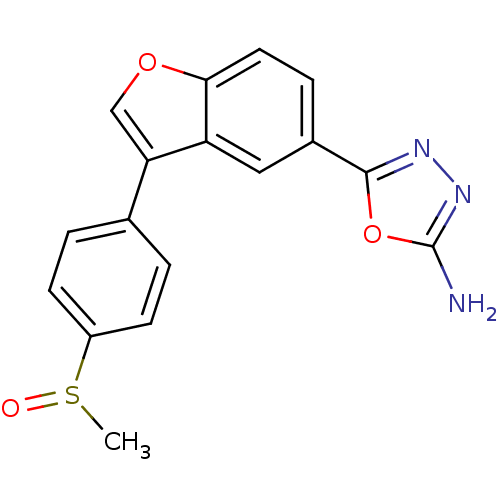 (5-{3-[4-(Methylsulfinyl)phenyl]-1-benzofuran-5-yl}...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3-beta | J Med Chem 52: 6270-86 (2009) Article DOI: 10.1021/jm900647e BindingDB Entry DOI: 10.7270/Q2CZ377V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50346123 (2-(4-(5-ethyl-2-(5-fluorothiophen-2-yl)-6-methylpy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates | Bioorg Med Chem Lett 19: 3174-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.121 BindingDB Entry DOI: 10.7270/Q26110NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4B (Homo sapiens (Human)) | BDBM50346121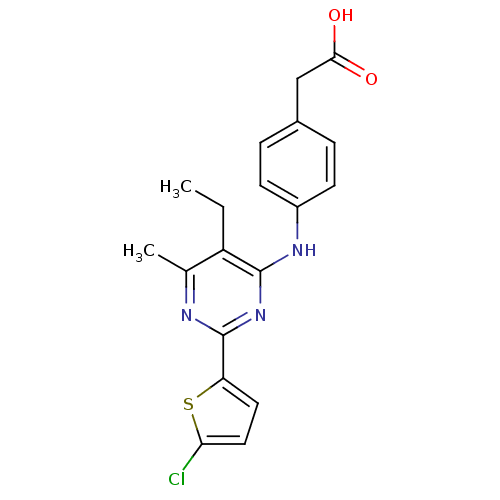 (2-(4-(2-(5-chlorothiophen-2-yl)-5-ethyl-6-methylpy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates | Bioorg Med Chem Lett 19: 3174-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.121 BindingDB Entry DOI: 10.7270/Q26110NQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM31579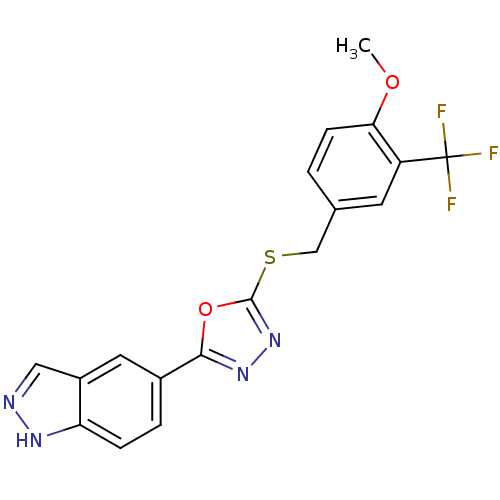 (oxadiazole derivative, 20p) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Takeda Pharmaceutical Company Ltd. | Assay Description Human GSK-3beta was expressed as the N-terminal FLAG-tagged protein using a baculovirus expression system. The kinase assay was was conducted in a 96... | Bioorg Med Chem 17: 2017-29 (2009) Article DOI: 10.1016/j.bmc.2009.01.019 BindingDB Entry DOI: 10.7270/Q2DR2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50058973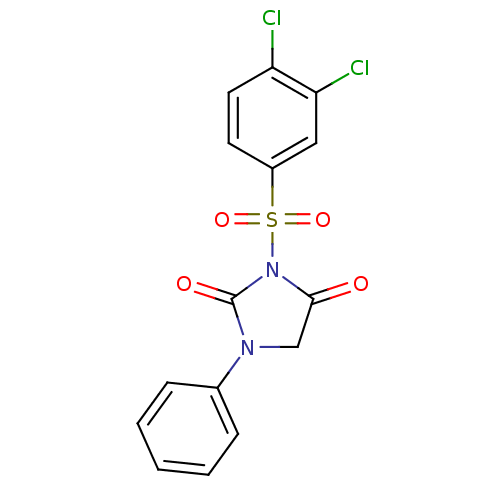 (3-(3,4-Dichloro-benzenesulfonyl)-1-phenyl-imidazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Suntory Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against human heart chymase in vitro. | J Med Chem 40: 2156-63 (1997) Article DOI: 10.1021/jm960793t BindingDB Entry DOI: 10.7270/Q2QJ7GDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM31581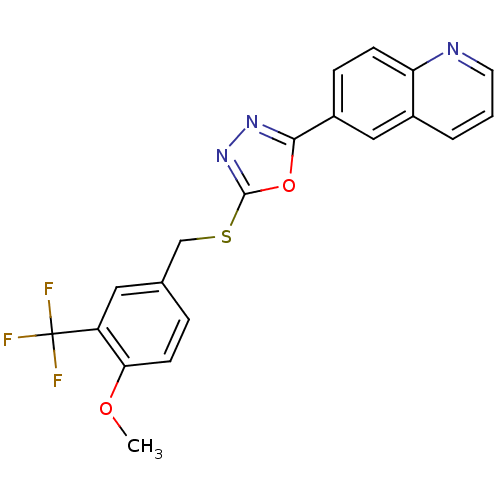 (oxadiazole derivative, 20r) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Takeda Pharmaceutical Company Ltd. | Assay Description Human GSK-3beta was expressed as the N-terminal FLAG-tagged protein using a baculovirus expression system. The kinase assay was was conducted in a 96... | Bioorg Med Chem 17: 2017-29 (2009) Article DOI: 10.1016/j.bmc.2009.01.019 BindingDB Entry DOI: 10.7270/Q2DR2STZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 402 total ) | Next | Last >> |