 Found 184 hits with Last Name = 'salmas' and Initial = 're'
Found 184 hits with Last Name = 'salmas' and Initial = 're' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161477
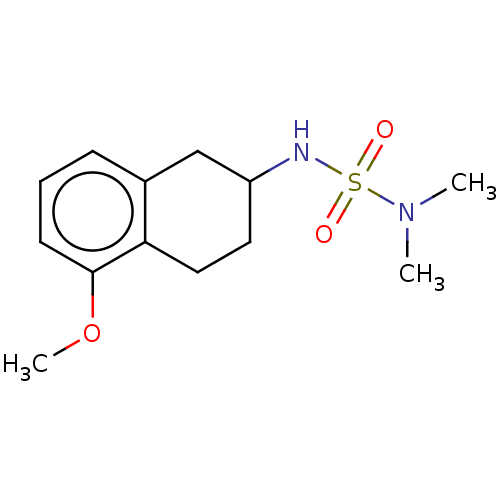
(CHEMBL3785269)Show InChI InChI=1S/C18H20N2O3S/c1-19(2)13-14-23-18-10-6-9-17-16(18)11-12-20(17)24(21,22)15-7-4-3-5-8-15/h3-12H,13-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161487
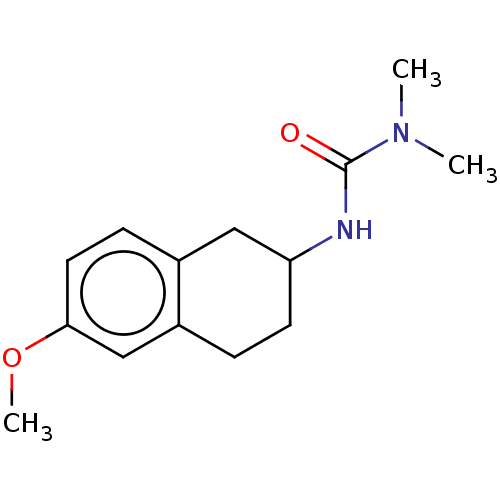
(CHEMBL3786516)Show InChI InChI=1S/C16H16N2O3S/c17-9-11-21-14-7-6-13-8-10-18(16(13)12-14)22(19,20)15-4-2-1-3-5-15/h1-8,10,12H,9,11,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161485
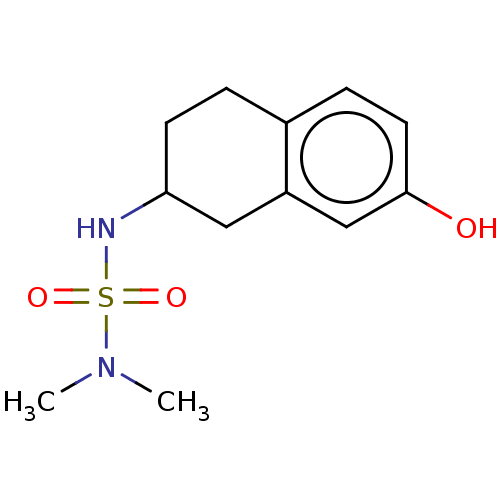
(CHEMBL3786719)Show InChI InChI=1S/C21H24N2O4S/c24-28(25,18-5-2-1-3-6-18)23-13-9-19-20(23)7-4-8-21(19)27-16-12-22-17-10-14-26-15-11-17/h1-9,13,17,22H,10-12,14-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161479
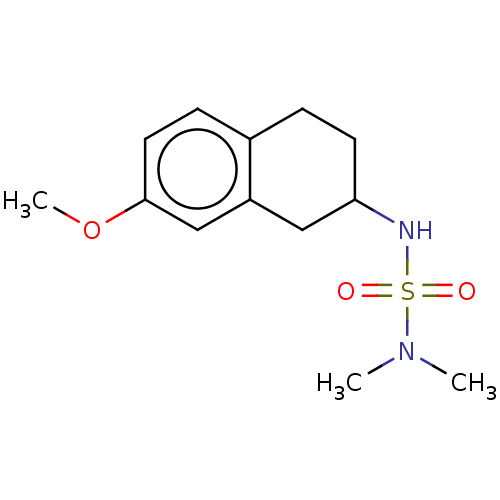
(CHEMBL3786448)Show InChI InChI=1S/C23H22N2O3S/c26-29(27,20-10-5-2-6-11-20)25-16-14-21-22(25)12-7-13-23(21)28-17-15-24-18-19-8-3-1-4-9-19/h1-14,16,24H,15,17-18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161491
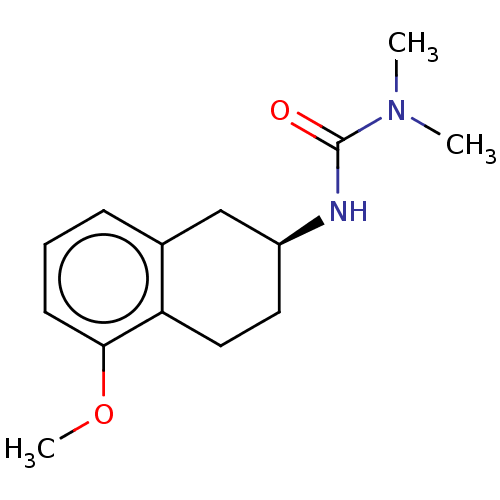
(CHEMBL3786651)Show InChI InChI=1S/C14H20N2O2/c1-16(2)14(17)15-11-7-8-12-10(9-11)5-4-6-13(12)18-3/h4-6,11H,7-9H2,1-3H3,(H,15,17)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161486

(CHEMBL3787502)Show InChI InChI=1S/C19H22N2O3S/c1-15(2)20-12-14-24-19-10-6-9-18-17(19)11-13-21(18)25(22,23)16-7-4-3-5-8-16/h3-11,13,15,20H,12,14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161478
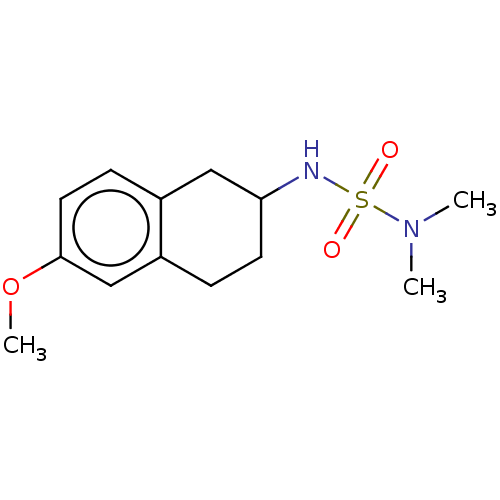
(CHEMBL3787223)Show InChI InChI=1S/C22H26N2O3S/c25-28(26,19-9-4-3-5-10-19)24-16-13-20-21(24)11-8-12-22(20)27-18-17-23-14-6-1-2-7-15-23/h3-5,8-13,16H,1-2,6-7,14-15,17-18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM26190

(1,4-Dihydroxybenzene, XIII | 1,4-dihydroxybenzene ...)Show InChI InChI=1S/C6H6O2/c7-5-1-2-6(8)4-3-5/h1-4,7-8H | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161484
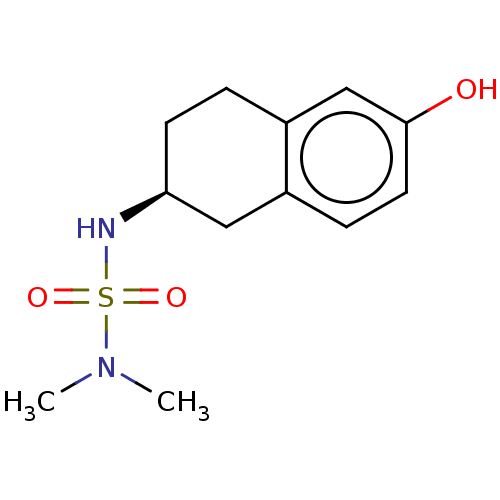
(CHEMBL3786442)Show InChI InChI=1S/C12H18N2O3S/c1-14(2)18(16,17)13-11-5-3-10-8-12(15)6-4-9(10)7-11/h4,6,8,11,13,15H,3,5,7H2,1-2H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161483
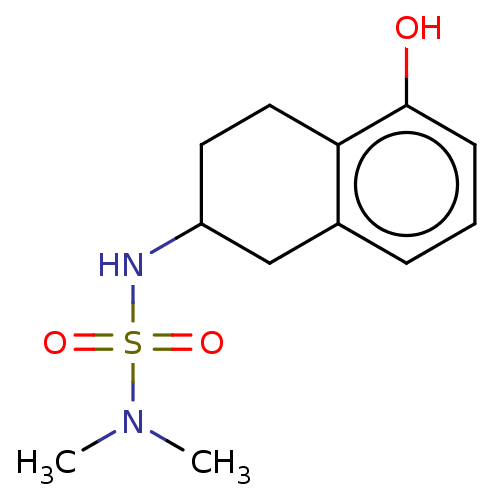
(CHEMBL3786873)Show InChI InChI=1S/C20H22N2O3S/c23-26(24,17-7-2-1-3-8-17)22-14-11-18-19(22)9-6-10-20(18)25-16-15-21-12-4-5-13-21/h1-3,6-11,14H,4-5,12-13,15-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161481
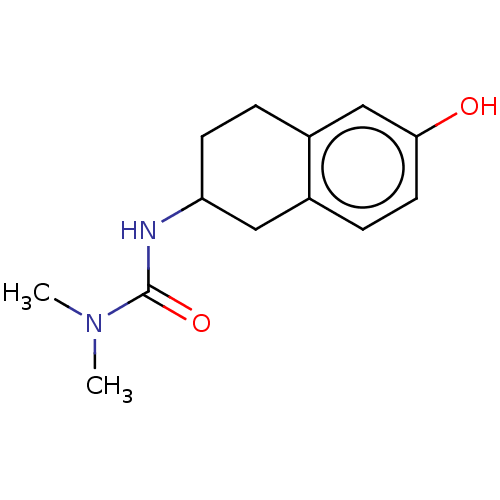
(CHEMBL3785391)Show InChI InChI=1S/C21H24N2O3S/c24-27(25,18-8-3-1-4-9-18)23-15-12-19-20(23)10-7-11-21(19)26-17-16-22-13-5-2-6-14-22/h1,3-4,7-12,15H,2,5-6,13-14,16-17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50291668

(CHEMBL4160691)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H](N)CO)C(C)C)[C@@H](C)CC)C(O)=O |r| Show InChI InChI=1S/C46H73N13O11/c1-7-25(5)36(43(67)55-33(20-28-21-50-23-52-28)44(68)59-18-10-12-34(59)41(65)58-37(45(69)70)26(6)8-2)57-40(64)32(19-27-13-15-29(61)16-14-27)54-42(66)35(24(3)4)56-39(63)31(11-9-17-51-46(48)49)53-38(62)30(47)22-60/h13-16,21,23-26,30-37,60-61H,7-12,17-20,22,47H2,1-6H3,(H,50,52)(H,53,62)(H,54,66)(H,55,67)(H,56,63)(H,57,64)(H,58,65)(H,69,70)(H4,48,49,51)/t25-,26-,30-,31-,32-,33-,34-,35-,36-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bahcesehir University (BAU)
Curated by ChEMBL
| Assay Description
Displacement of [125I-Sar1-Ile8]-Ang2 from human angiotensin 2 receptor type 1 receptor expressed in HEK293 cells after 1 hr by gamma counting analys... |
Eur J Med Chem 145: 273-290 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.021
BindingDB Entry DOI: 10.7270/Q2639S81 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161480
(CHEMBL3786937)Show InChI InChI=1S/C24H24N2O4S/c1-29-21-7-5-6-19(16-21)18-25-13-15-30-22-11-10-20-12-14-26(24(20)17-22)31(27,28)23-8-3-2-4-9-23/h2-12,14,16-17,25H,13,15,18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161477

(CHEMBL3785269)Show InChI InChI=1S/C18H20N2O3S/c1-19(2)13-14-23-18-10-6-9-17-16(18)11-12-20(17)24(21,22)15-7-4-3-5-8-15/h3-12H,13-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
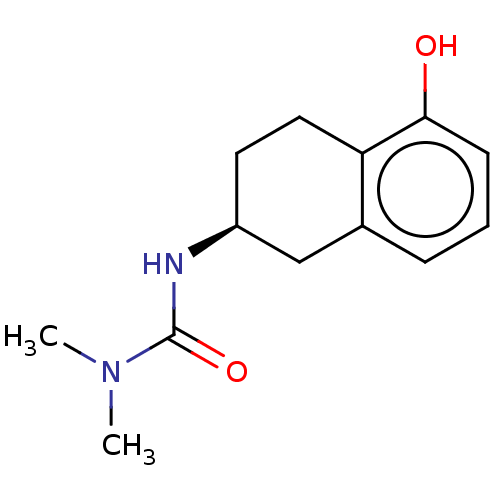
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50161482
(CHEMBL3785207)Show InChI InChI=1S/C18H20N2O3S/c1-2-19-12-14-23-18-10-6-9-17-16(18)11-13-20(17)24(21,22)15-7-4-3-5-8-15/h3-11,13,19H,2,12,14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161485

(CHEMBL3786719)Show InChI InChI=1S/C21H24N2O4S/c24-28(25,18-5-2-1-3-6-18)23-13-9-19-20(23)7-4-8-21(19)27-16-12-22-17-10-14-26-15-11-17/h1-9,13,17,22H,10-12,14-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161486

(CHEMBL3787502)Show InChI InChI=1S/C19H22N2O3S/c1-15(2)20-12-14-24-19-10-6-9-18-17(19)11-13-21(18)25(22,23)16-7-4-3-5-8-16/h3-11,13,15,20H,12,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161479

(CHEMBL3786448)Show InChI InChI=1S/C23H22N2O3S/c26-29(27,20-10-5-2-6-11-20)25-16-14-21-22(25)12-7-13-23(21)28-17-15-24-18-19-8-3-1-4-9-19/h1-14,16,24H,15,17-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161478

(CHEMBL3787223)Show InChI InChI=1S/C22H26N2O3S/c25-28(26,19-9-4-3-5-10-19)24-16-13-20-21(24)11-8-12-22(20)27-18-17-23-14-6-1-2-7-15-23/h3-5,8-13,16H,1-2,6-7,14-15,17-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161487

(CHEMBL3786516)Show InChI InChI=1S/C16H16N2O3S/c17-9-11-21-14-7-6-13-8-10-18(16(13)12-14)22(19,20)15-4-2-1-3-5-15/h1-8,10,12H,9,11,17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161484

(CHEMBL3786442)Show InChI InChI=1S/C12H18N2O3S/c1-14(2)18(16,17)13-11-5-3-10-8-12(15)6-4-9(10)7-11/h4,6,8,11,13,15H,3,5,7H2,1-2H3/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161483

(CHEMBL3786873)Show InChI InChI=1S/C20H22N2O3S/c23-26(24,17-7-2-1-3-8-17)22-14-11-18-19(22)9-6-10-20(18)25-16-15-21-12-4-5-13-21/h1-3,6-11,14H,4-5,12-13,15-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161483

(CHEMBL3786873)Show InChI InChI=1S/C20H22N2O3S/c23-26(24,17-7-2-1-3-8-17)22-14-11-18-19(22)9-6-10-20(18)25-16-15-21-12-4-5-13-21/h1-3,6-11,14H,4-5,12-13,15-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161491

(CHEMBL3786651)Show InChI InChI=1S/C14H20N2O2/c1-16(2)14(17)15-11-7-8-12-10(9-11)5-4-6-13(12)18-3/h4-6,11H,7-9H2,1-3H3,(H,15,17)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161481

(CHEMBL3785391)Show InChI InChI=1S/C21H24N2O3S/c24-27(25,18-8-3-1-4-9-18)23-15-12-19-20(23)10-7-11-21(19)26-17-16-22-13-5-2-6-14-22/h1,3-4,7-12,15H,2,5-6,13-14,16-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161478

(CHEMBL3787223)Show InChI InChI=1S/C22H26N2O3S/c25-28(26,19-9-4-3-5-10-19)24-16-13-20-21(24)11-8-12-22(20)27-18-17-23-14-6-1-2-7-15-23/h3-5,8-13,16H,1-2,6-7,14-15,17-18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161477

(CHEMBL3785269)Show InChI InChI=1S/C18H20N2O3S/c1-19(2)13-14-23-18-10-6-9-17-16(18)11-12-20(17)24(21,22)15-7-4-3-5-8-15/h3-12H,13-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
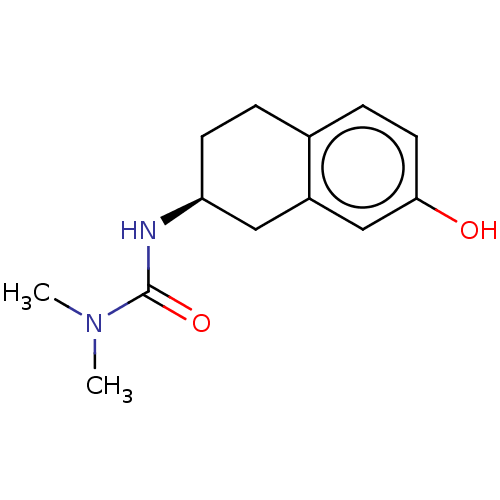
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161482

(CHEMBL3785207)Show InChI InChI=1S/C18H20N2O3S/c1-2-19-12-14-23-18-10-6-9-17-16(18)11-13-20(17)24(21,22)15-7-4-3-5-8-15/h3-11,13,19H,2,12,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50161480

(CHEMBL3786937)Show InChI InChI=1S/C24H24N2O4S/c1-29-21-7-5-6-19(16-21)18-25-13-15-30-22-11-10-20-12-14-26(24(20)17-22)31(27,28)23-8-3-2-4-9-23/h2-12,14,16-17,25H,13,15,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161479

(CHEMBL3786448)Show InChI InChI=1S/C23H22N2O3S/c26-29(27,20-10-5-2-6-11-20)25-16-14-21-22(25)12-7-13-23(21)28-17-15-24-18-19-8-3-1-4-9-19/h1-14,16,24H,15,17-18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50049186
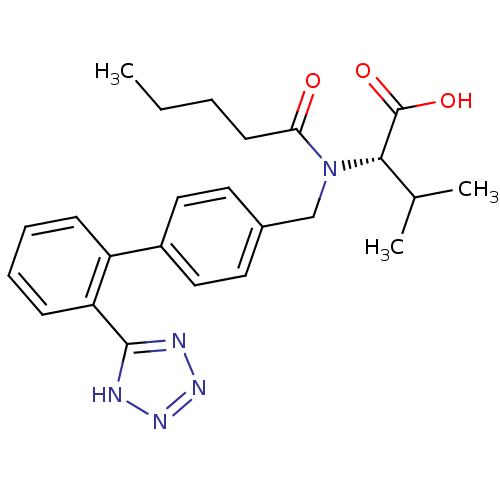
((S)-3-Methyl-2-{pentanoyl-[2'-(1H-tetrazol-5-yl)-b...)Show SMILES CCCCC(=O)N(Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1)[C@@H](C(C)C)C(O)=O Show InChI InChI=1S/C24H29N5O3/c1-4-5-10-21(30)29(22(16(2)3)24(31)32)15-17-11-13-18(14-12-17)19-8-6-7-9-20(19)23-25-27-28-26-23/h6-9,11-14,16,22H,4-5,10,15H2,1-3H3,(H,31,32)(H,25,26,27,28)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bahcesehir University (BAU)
Curated by ChEMBL
| Assay Description
Displacement of [125I-Sar1-Ile8]-Ang2 from human angiotensin 2 receptor type 1 receptor expressed in HEK293 cells after 1 hr by gamma counting analys... |
Eur J Med Chem 145: 273-290 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.021
BindingDB Entry DOI: 10.7270/Q2639S81 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161485

(CHEMBL3786719)Show InChI InChI=1S/C21H24N2O4S/c24-28(25,18-5-2-1-3-6-18)23-13-9-19-20(23)7-4-8-21(19)27-16-12-22-17-10-14-26-15-11-17/h1-9,13,17,22H,10-12,14-16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161487

(CHEMBL3786516)Show InChI InChI=1S/C16H16N2O3S/c17-9-11-21-14-7-6-13-8-10-18(16(13)12-14)22(19,20)15-4-2-1-3-5-15/h1-8,10,12H,9,11,17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161484

(CHEMBL3786442)Show InChI InChI=1S/C12H18N2O3S/c1-14(2)18(16,17)13-11-5-3-10-8-12(15)6-4-9(10)7-11/h4,6,8,11,13,15H,3,5,7H2,1-2H3/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161486

(CHEMBL3787502)Show InChI InChI=1S/C19H22N2O3S/c1-15(2)20-12-14-24-19-10-6-9-18-17(19)11-13-21(18)25(22,23)16-7-4-3-5-8-16/h3-11,13,15,20H,12,14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161482

(CHEMBL3785207)Show InChI InChI=1S/C18H20N2O3S/c1-2-19-12-14-23-18-10-6-9-17-16(18)11-13-20(17)24(21,22)15-7-4-3-5-8-15/h3-11,13,19H,2,12,14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161480

(CHEMBL3786937)Show InChI InChI=1S/C24H24N2O4S/c1-29-21-7-5-6-19(16-21)18-25-13-15-30-22-11-10-20-12-14-26(24(20)17-22)31(27,28)23-8-3-2-4-9-23/h2-12,14,16-17,25H,13,15,18H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161481

(CHEMBL3785391)Show InChI InChI=1S/C21H24N2O3S/c24-27(25,18-8-3-1-4-9-18)23-15-12-19-20(23)10-7-11-21(19)26-17-16-22-13-5-2-6-14-22/h1,3-4,7-12,15H,2,5-6,13-14,16-17H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50161491

(CHEMBL3786651)Show InChI InChI=1S/C14H20N2O2/c1-16(2)14(17)15-11-7-8-12-10(9-11)5-4-6-13(12)18-3/h4-6,11H,7-9H2,1-3H3,(H,15,17)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) using acetylcholine iodate as substrate preincubated for 10 mins followed by substrate addition by Lineweaver-Bur... |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50240609
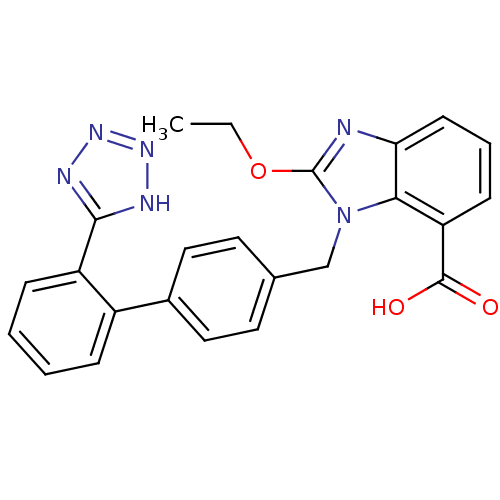
(2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmet...)Show SMILES CCOc1nc2cccc(C(O)=O)c2n1Cc1ccc(cc1)-c1ccccc1-c1nnn[nH]1 Show InChI InChI=1S/C24H20N6O3/c1-2-33-24-25-20-9-5-8-19(23(31)32)21(20)30(24)14-15-10-12-16(13-11-15)17-6-3-4-7-18(17)22-26-28-29-27-22/h3-13H,2,14H2,1H3,(H,31,32)(H,26,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bahcesehir University (BAU)
Curated by ChEMBL
| Assay Description
Displacement of [125I-Sar1-Ile8]-Ang2 from human angiotensin 2 receptor type 1 receptor expressed in HEK293 cells after 1 hr by gamma counting analys... |
Eur J Med Chem 145: 273-290 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.021
BindingDB Entry DOI: 10.7270/Q2639S81 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 2 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 204 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Erzurum Technical University
Curated by ChEMBL
| Assay Description
Inhibition of human carbonic anhydrase 1 using 4-nitrophenylacetate as substrate by Lineweaver-Burk plot analysis |
Bioorg Med Chem 24: 2318-29 (2016)
Article DOI: 10.1016/j.bmc.2016.04.002
BindingDB Entry DOI: 10.7270/Q2930W3J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50291670
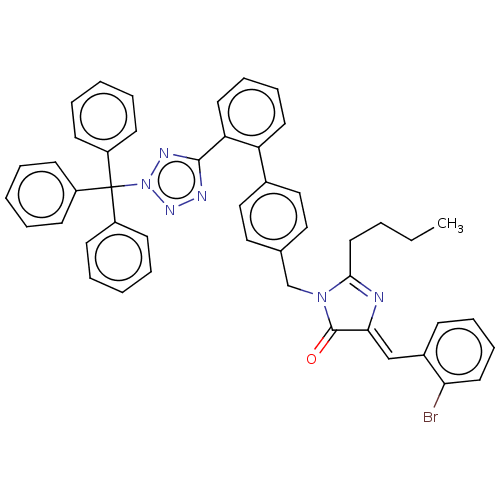
(CHEMBL4175147)Show SMILES CCCCC1=N\C(=C/c2ccccc2Br)C(=O)N1Cc1ccc(cc1)-c1ccccc1-c1nnn(n1)C(c1ccccc1)(c1ccccc1)c1ccccc1 |t:4| Show InChI InChI=1S/C47H39BrN6O/c1-2-3-27-44-49-43(32-36-17-13-16-26-42(36)48)46(55)53(44)33-34-28-30-35(31-29-34)40-24-14-15-25-41(40)45-50-52-54(51-45)47(37-18-7-4-8-19-37,38-20-9-5-10-21-38)39-22-11-6-12-23-39/h4-26,28-32H,2-3,27,33H2,1H3/b43-32- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 317 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bahcesehir University (BAU)
Curated by ChEMBL
| Assay Description
Displacement of [125I-Sar1-Ile8]-Ang2 from human angiotensin 2 receptor type 1 receptor expressed in HEK293 cells after 1 hr by gamma counting analys... |
Eur J Med Chem 145: 273-290 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.021
BindingDB Entry DOI: 10.7270/Q2639S81 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10860
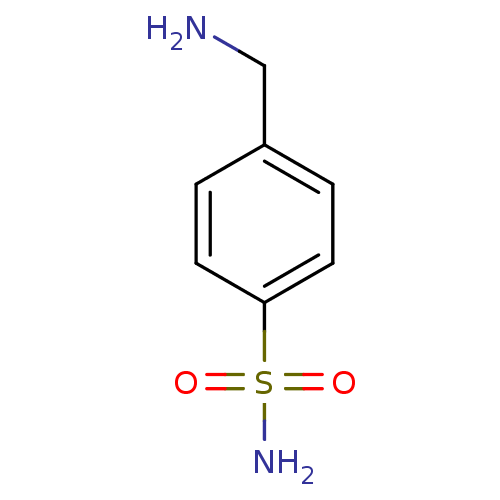
(4-(aminomethyl)benzene-1-sulfonamide | CHEMBL419 |...)Show InChI InChI=1S/C7H10N2O2S/c8-5-6-1-3-7(4-2-6)12(9,10)11/h1-4H,5,8H2,(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 612 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gebze Technical University
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 2 esterase activity isolated from human serum using 4-nitrophenylacetate as substrate measured over 3 mins... |
Bioorg Med Chem 23: 7353-8 (2015)
Article DOI: 10.1016/j.bmc.2015.10.009
BindingDB Entry DOI: 10.7270/Q2ZS2ZB2 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10857
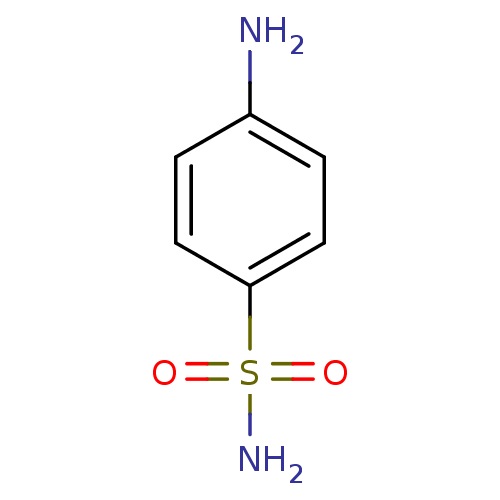
(4-aminobenzene-1-sulfonamide | CHEMBL21 | Sulfanil...)Show InChI InChI=1S/C6H8N2O2S/c7-5-1-3-6(4-2-5)11(8,9)10/h1-4H,7H2,(H2,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 628 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gebze Technical University
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 2 esterase activity isolated from human serum using 4-nitrophenylacetate as substrate measured over 3 mins... |
Bioorg Med Chem 23: 7353-8 (2015)
Article DOI: 10.1016/j.bmc.2015.10.009
BindingDB Entry DOI: 10.7270/Q2ZS2ZB2 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM12414
(CHEMBL27601 | benzenesulfonamide | hCA inhibitor, ...)Show InChI InChI=1S/C6H7NO2S/c7-10(8,9)6-4-2-1-3-5-6/h1-5H,(H2,7,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gebze Technical University
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 2 esterase activity isolated from human serum using 4-nitrophenylacetate as substrate measured over 3 mins... |
Bioorg Med Chem 23: 7353-8 (2015)
Article DOI: 10.1016/j.bmc.2015.10.009
BindingDB Entry DOI: 10.7270/Q2ZS2ZB2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10859
(4-methylbenzene-1-sulfonamide | CHEMBL574 | aromat...)Show InChI InChI=1S/C7H9NO2S/c1-6-2-4-7(5-3-6)11(8,9)10/h2-5H,1H3,(H2,8,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gebze Technical University
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 2 esterase activity isolated from human serum using 4-nitrophenylacetate as substrate measured over 3 mins... |
Bioorg Med Chem 23: 7353-8 (2015)
Article DOI: 10.1016/j.bmc.2015.10.009
BindingDB Entry DOI: 10.7270/Q2ZS2ZB2 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50129949
(CHEMBL3627999)Show SMILES Cc1ccc(cc1)S(=O)(=O)N[C@@H](Cc1ccccc1)C(=O)OCc1ccccc1 |r| Show InChI InChI=1S/C23H23NO4S/c1-18-12-14-21(15-13-18)29(26,27)24-22(16-19-8-4-2-5-9-19)23(25)28-17-20-10-6-3-7-11-20/h2-15,22,24H,16-17H2,1H3/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gebze Technical University
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 1 esterase activity isolated from human erythrocytes using 4-nitrophenylacetate as substrate measured over... |
Bioorg Med Chem 23: 7353-8 (2015)
Article DOI: 10.1016/j.bmc.2015.10.009
BindingDB Entry DOI: 10.7270/Q2ZS2ZB2 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50130030

(CHEMBL1885780)Show InChI InChI=1S/C16H17NO4S/c1-13-7-9-15(10-8-13)22(19,20)17-11-16(18)21-12-14-5-3-2-4-6-14/h2-10,17H,11-12H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gebze Technical University
Curated by ChEMBL
| Assay Description
Inhibition of cytosolic carbonic anhydrase 2 esterase activity isolated from human erythrocytes using 4-nitrophenylacetate as substrate measured over... |
Bioorg Med Chem 23: 7353-8 (2015)
Article DOI: 10.1016/j.bmc.2015.10.009
BindingDB Entry DOI: 10.7270/Q2ZS2ZB2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data