

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50512416 (CHEMBL4447162) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Inhibition of human P2Y14R | J Med Chem 63: 9563-9589 (2020) Article DOI: 10.1021/acs.jmedchem.0c00745 BindingDB Entry DOI: 10.7270/Q20R9SZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50266666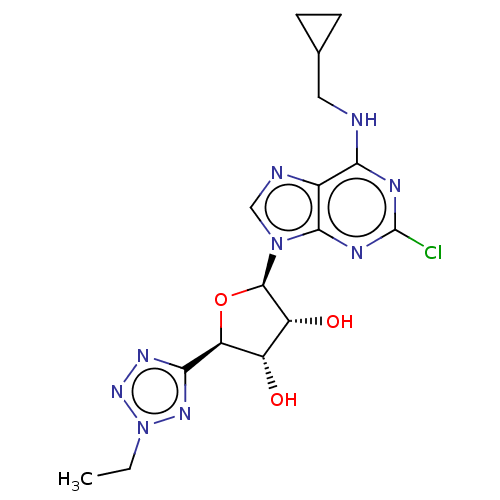 (CHEMBL4105164) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.313 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Displacement of [3H]HEMADO from recombinant human adenosine A3A receptor expressed in CHO cell membranes after 3 hrs by microbeta scintillation count... | J Med Chem 60: 4327-4341 (2017) Article DOI: 10.1021/acs.jmedchem.7b00291 BindingDB Entry DOI: 10.7270/Q2CJ8GZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50266664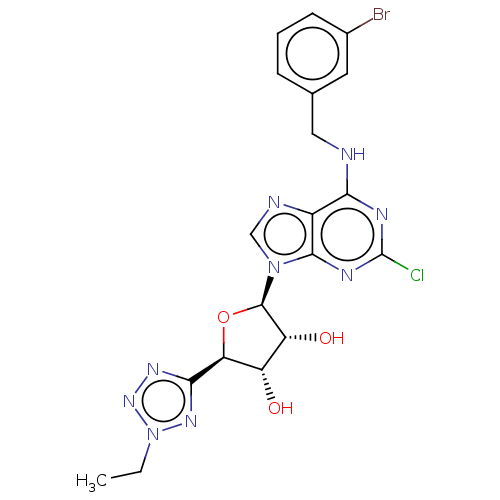 (CHEMBL4059961) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 0.345 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Displacement of [3H]HEMADO from recombinant human adenosine A3A receptor expressed in CHO cell membranes after 3 hrs by microbeta scintillation count... | J Med Chem 60: 4327-4341 (2017) Article DOI: 10.1021/acs.jmedchem.7b00291 BindingDB Entry DOI: 10.7270/Q2CJ8GZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50266649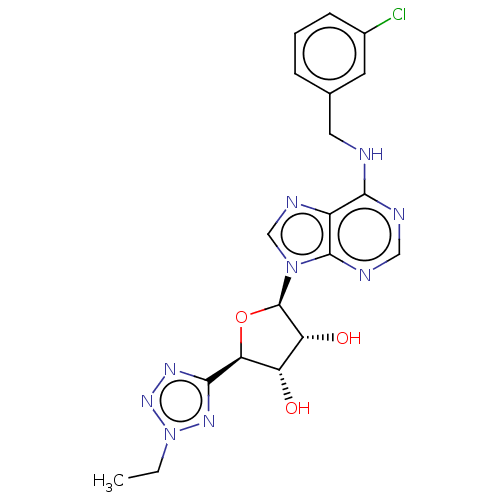 (CHEMBL4071338) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.386 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Displacement of [3H]HEMADO from recombinant human adenosine A3A receptor expressed in CHO cell membranes after 3 hrs by microbeta scintillation count... | J Med Chem 60: 4327-4341 (2017) Article DOI: 10.1021/acs.jmedchem.7b00291 BindingDB Entry DOI: 10.7270/Q2CJ8GZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50266650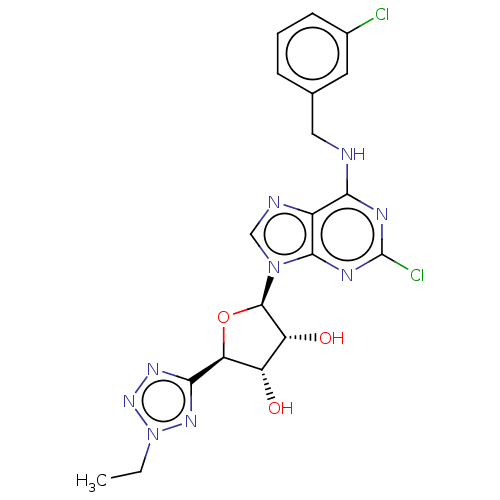 (CHEMBL4098377) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.387 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Displacement of [3H]HEMADO from recombinant human adenosine A3A receptor expressed in CHO cell membranes after 3 hrs by microbeta scintillation count... | J Med Chem 60: 4327-4341 (2017) Article DOI: 10.1021/acs.jmedchem.7b00291 BindingDB Entry DOI: 10.7270/Q2CJ8GZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50266651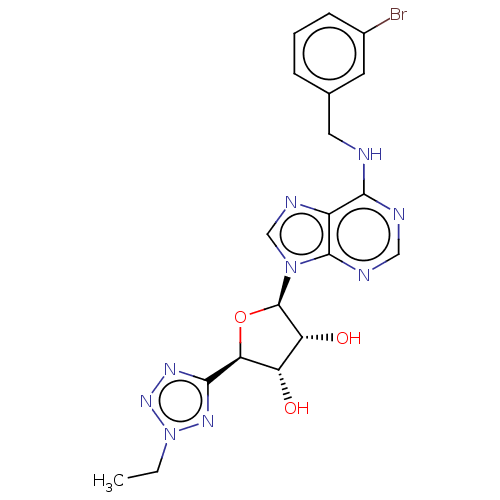 (CHEMBL4081876) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.393 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Displacement of [3H]HEMADO from recombinant human adenosine A3A receptor expressed in CHO cell membranes after 3 hrs by microbeta scintillation count... | J Med Chem 60: 4327-4341 (2017) Article DOI: 10.1021/acs.jmedchem.7b00291 BindingDB Entry DOI: 10.7270/Q2CJ8GZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 14 (Homo sapiens (Human)) | BDBM50456168 (CHEMBL1800685) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Antagonist activity at human P2Y14 expressed in CHO cells assessed as inhibition of forskolin-induced increase of cAMP accumulation incubated for 15 ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01964 BindingDB Entry DOI: 10.7270/Q2611470 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50266662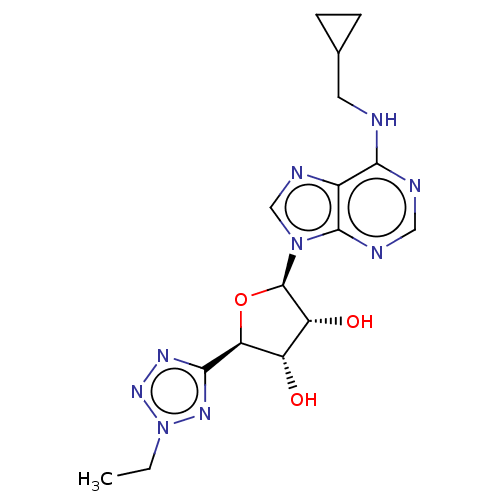 (CHEMBL4087306) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.438 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Displacement of [3H]CCPA from recombinant human adenosine A1 receptor expressed in CHO cell membranes after 3 hrs by microbeta scintillation counting | J Med Chem 60: 4327-4341 (2017) Article DOI: 10.1021/acs.jmedchem.7b00291 BindingDB Entry DOI: 10.7270/Q2CJ8GZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50069813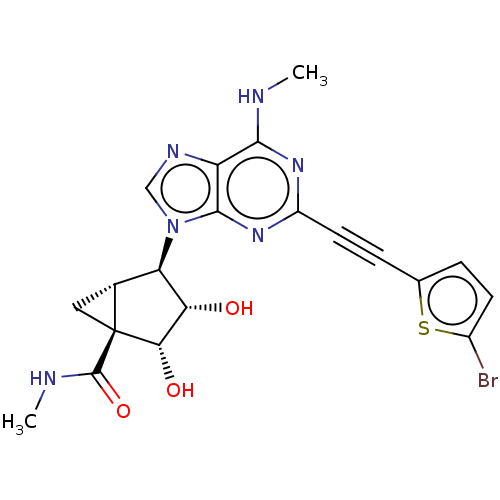 (CHEMBL3407785) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human recombinant A3 adenosine receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 57: 9901-14 (2014) Article DOI: 10.1021/jm501021n BindingDB Entry DOI: 10.7270/Q2S1846G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM434802 (US10577368, Compound 100) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by the Secretary, Department of Health and Human Services; Saint Louis University US Patent | Assay Description [3H]RóN6-Phenylisopropyladenosine (40, [3H]R-PIA, 63 Ci/mmol), [3H](2-[p-(2-carboxyethyl)phenyl-ethylamino]-5′-N-ethylcarboxamido-adenosine) (4... | US Patent US10577368 (2020) BindingDB Entry DOI: 10.7270/Q2HH6NGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50266666 (CHEMBL4105164) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.454 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Displacement of [3H]CCPA from recombinant human adenosine A1 receptor expressed in CHO cell membranes after 3 hrs by microbeta scintillation counting | J Med Chem 60: 4327-4341 (2017) Article DOI: 10.1021/acs.jmedchem.7b00291 BindingDB Entry DOI: 10.7270/Q2CJ8GZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50078427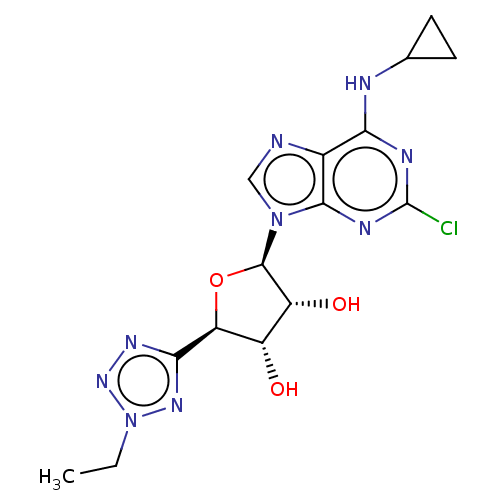 (CHEMBL3414941) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.461 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Displacement of [3H]HEMADO from recombinant human adenosine A3A receptor expressed in CHO cell membranes after 3 hrs by microbeta scintillation count... | J Med Chem 60: 4327-4341 (2017) Article DOI: 10.1021/acs.jmedchem.7b00291 BindingDB Entry DOI: 10.7270/Q2CJ8GZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM434800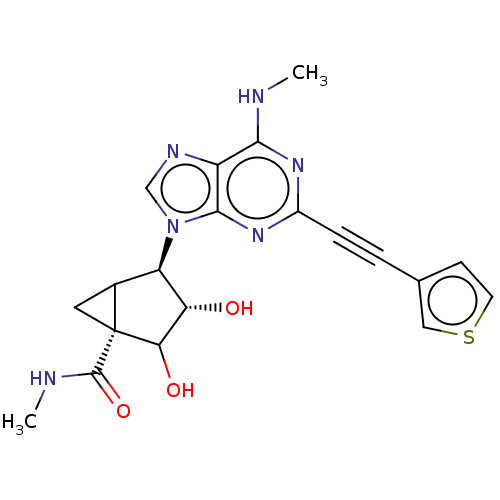 (US10577368, Compound 16) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by the Secretary, Department of Health and Human Services; Saint Louis University US Patent | Assay Description [3H]RóN6-Phenylisopropyladenosine (40, [3H]R-PIA, 63 Ci/mmol), [3H](2-[p-(2-carboxyethyl)phenyl-ethylamino]-5′-N-ethylcarboxamido-adenosine) (4... | US Patent US10577368 (2020) BindingDB Entry DOI: 10.7270/Q2HH6NGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50069988 (CHEMBL3407782) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human recombinant A3 adenosine receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 57: 9901-14 (2014) Article DOI: 10.1021/jm501021n BindingDB Entry DOI: 10.7270/Q2S1846G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50389134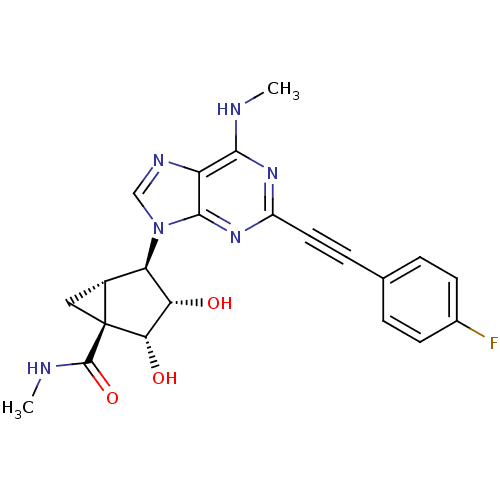 (CHEMBL2064639) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human recombinant A3 adenosine receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 57: 9901-14 (2014) Article DOI: 10.1021/jm501021n BindingDB Entry DOI: 10.7270/Q2S1846G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50266652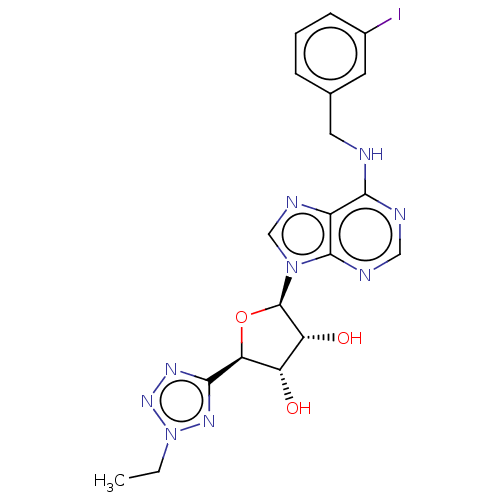 (CHEMBL4078479) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.532 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Displacement of [3H]HEMADO from recombinant human adenosine A3A receptor expressed in CHO cell membranes after 3 hrs by microbeta scintillation count... | J Med Chem 60: 4327-4341 (2017) Article DOI: 10.1021/acs.jmedchem.7b00291 BindingDB Entry DOI: 10.7270/Q2CJ8GZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50078427 (CHEMBL3414941) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.538 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Displacement of [3H]CCPA from recombinant human adenosine A1 receptor expressed in CHO cell membranes after 3 hrs by microbeta scintillation counting | J Med Chem 60: 4327-4341 (2017) Article DOI: 10.1021/acs.jmedchem.7b00291 BindingDB Entry DOI: 10.7270/Q2CJ8GZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM434831 (US10577368, Compound 113) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by the Secretary, Department of Health and Human Services; Saint Louis University US Patent | Assay Description [3H]RóN6-Phenylisopropyladenosine (40, [3H]R-PIA, 63 Ci/mmol), [3H](2-[p-(2-carboxyethyl)phenyl-ethylamino]-5′-N-ethylcarboxamido-adenosine) (4... | US Patent US10577368 (2020) BindingDB Entry DOI: 10.7270/Q2HH6NGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane domain-containing protein TMIGD3 (Homo sapiens (Human)) | BDBM50500133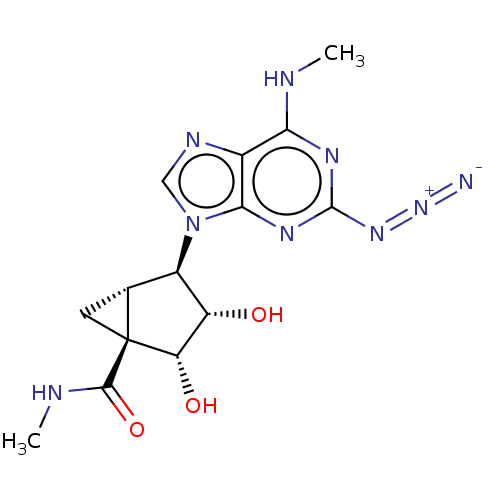 (CHEMBL3747303) | UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyl-uronamide from human adenosine A3 receptor stably expressed in CHO cell membrane... | Medchemcomm 6: 555-563 Article DOI: 10.1039/c4md00571f BindingDB Entry DOI: 10.7270/Q2DB84W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50069857 (CHEMBL3407783) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human recombinant A3 adenosine receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 57: 9901-14 (2014) Article DOI: 10.1021/jm501021n BindingDB Entry DOI: 10.7270/Q2S1846G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM434799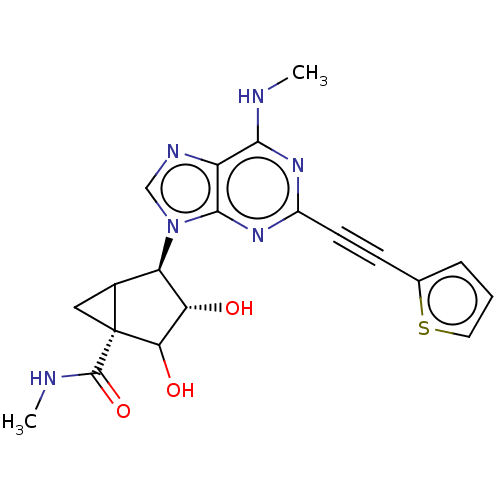 (US10577368, Compound 8) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by the Secretary, Department of Health and Human Services; Saint Louis University US Patent | Assay Description [3H]RóN6-Phenylisopropyladenosine (40, [3H]R-PIA, 63 Ci/mmol), [3H](2-[p-(2-carboxyethyl)phenyl-ethylamino]-5′-N-ethylcarboxamido-adenosine) (4... | US Patent US10577368 (2020) BindingDB Entry DOI: 10.7270/Q2HH6NGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50266662 (CHEMBL4087306) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.575 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Displacement of [3H]HEMADO from recombinant human adenosine A3A receptor expressed in CHO cell membranes after 3 hrs by microbeta scintillation count... | J Med Chem 60: 4327-4341 (2017) Article DOI: 10.1021/acs.jmedchem.7b00291 BindingDB Entry DOI: 10.7270/Q2CJ8GZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM434826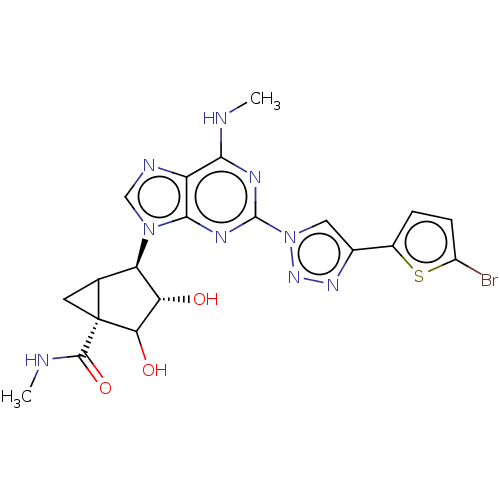 (US10577368, Compound 111) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by the Secretary, Department of Health and Human Services; Saint Louis University US Patent | Assay Description [3H]RóN6-Phenylisopropyladenosine (40, [3H]R-PIA, 63 Ci/mmol), [3H](2-[p-(2-carboxyethyl)phenyl-ethylamino]-5′-N-ethylcarboxamido-adenosine) (4... | US Patent US10577368 (2020) BindingDB Entry DOI: 10.7270/Q2HH6NGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane domain-containing protein TMIGD3 (Homo sapiens (Human)) | BDBM50500127 (CHEMBL3746778) | UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyl-uronamide from human adenosine A3 receptor stably expressed in CHO cell membrane... | Medchemcomm 6: 555-563 Article DOI: 10.1039/c4md00571f BindingDB Entry DOI: 10.7270/Q2DB84W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50389135 (CHEMBL2064640) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human recombinant A3 adenosine receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 57: 9901-14 (2014) Article DOI: 10.1021/jm501021n BindingDB Entry DOI: 10.7270/Q2S1846G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM434839 (US10577368, Compound 123) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by the Secretary, Department of Health and Human Services; Saint Louis University US Patent | Assay Description [3H]RóN6-Phenylisopropyladenosine (40, [3H]R-PIA, 63 Ci/mmol), [3H](2-[p-(2-carboxyethyl)phenyl-ethylamino]-5′-N-ethylcarboxamido-adenosine) (4... | US Patent US10577368 (2020) BindingDB Entry DOI: 10.7270/Q2HH6NGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM434795 (US10577368, Compound 17) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by the Secretary, Department of Health and Human Services; Saint Louis University US Patent | Assay Description [3H]RóN6-Phenylisopropyladenosine (40, [3H]R-PIA, 63 Ci/mmol), [3H](2-[p-(2-carboxyethyl)phenyl-ethylamino]-5′-N-ethylcarboxamido-adenosine) (4... | US Patent US10577368 (2020) BindingDB Entry DOI: 10.7270/Q2HH6NGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50069982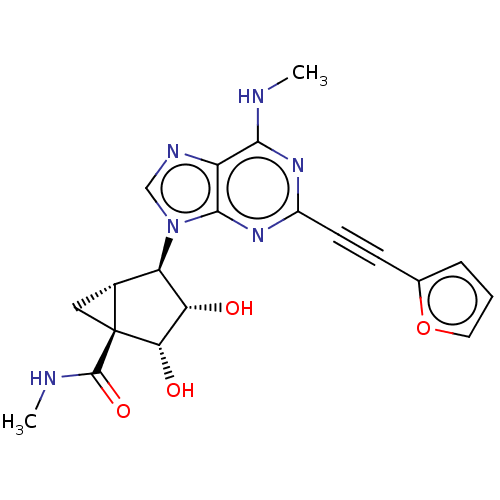 (CHEMBL3407778) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human recombinant A3 adenosine receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 57: 9901-14 (2014) Article DOI: 10.1021/jm501021n BindingDB Entry DOI: 10.7270/Q2S1846G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM434783 (US10577368, Compound 11) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by the Secretary, Department of Health and Human Services; Saint Louis University US Patent | Assay Description [3H]RóN6-Phenylisopropyladenosine (40, [3H]R-PIA, 63 Ci/mmol), [3H](2-[p-(2-carboxyethyl)phenyl-ethylamino]-5′-N-ethylcarboxamido-adenosine) (4... | US Patent US10577368 (2020) BindingDB Entry DOI: 10.7270/Q2HH6NGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50069991 (CHEMBL3407768) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human recombinant A3 adenosine receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 57: 9901-14 (2014) Article DOI: 10.1021/jm501021n BindingDB Entry DOI: 10.7270/Q2S1846G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane domain-containing protein TMIGD3 (Homo sapiens (Human)) | BDBM50500126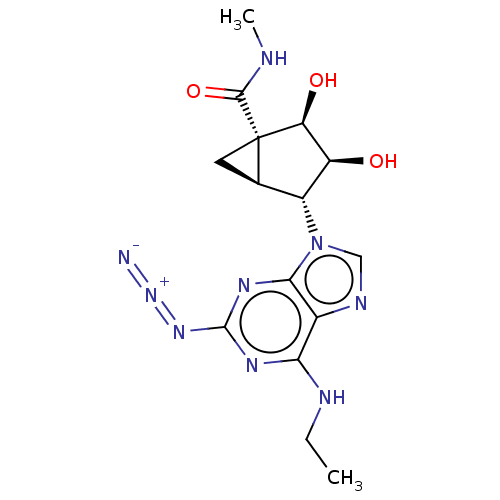 (CHEMBL3746260) | UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyl-uronamide from human adenosine A3 receptor stably expressed in CHO cell membrane... | Medchemcomm 6: 555-563 Article DOI: 10.1039/c4md00571f BindingDB Entry DOI: 10.7270/Q2DB84W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM434801 (US10577368, Compound 32) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by the Secretary, Department of Health and Human Services; Saint Louis University US Patent | Assay Description [3H]RóN6-Phenylisopropyladenosine (40, [3H]R-PIA, 63 Ci/mmol), [3H](2-[p-(2-carboxyethyl)phenyl-ethylamino]-5′-N-ethylcarboxamido-adenosine) (4... | US Patent US10577368 (2020) BindingDB Entry DOI: 10.7270/Q2HH6NGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50069812 (CHEMBL3407784) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human recombinant A3 adenosine receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 57: 9901-14 (2014) Article DOI: 10.1021/jm501021n BindingDB Entry DOI: 10.7270/Q2S1846G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50069812 (CHEMBL3407784) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human adenosine A3 receptor expressed in CHO cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM434824 (US10577368, Compound 109) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by the Secretary, Department of Health and Human Services; Saint Louis University US Patent | Assay Description [3H]RóN6-Phenylisopropyladenosine (40, [3H]R-PIA, 63 Ci/mmol), [3H](2-[p-(2-carboxyethyl)phenyl-ethylamino]-5′-N-ethylcarboxamido-adenosine) (4... | US Patent US10577368 (2020) BindingDB Entry DOI: 10.7270/Q2HH6NGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50116875 (CHEMBL3612943) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human adenosine A3 receptor expressed in CHO cell membranes | ACS Med Chem Lett 6: 804-8 (2015) Article DOI: 10.1021/acsmedchemlett.5b00150 BindingDB Entry DOI: 10.7270/Q23R0VPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane domain-containing protein TMIGD3 (Homo sapiens (Human)) | BDBM50116875 (CHEMBL3612943) | UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyl-uronamide from human adenosine A3 receptor stably expressed in CHO cell membrane... | Medchemcomm 6: 555-563 Article DOI: 10.1039/c4md00571f BindingDB Entry DOI: 10.7270/Q2DB84W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50266652 (CHEMBL4078479) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.768 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Displacement of [3H]CCPA from recombinant human adenosine A1 receptor expressed in CHO cell membranes after 3 hrs by microbeta scintillation counting | J Med Chem 60: 4327-4341 (2017) Article DOI: 10.1021/acs.jmedchem.7b00291 BindingDB Entry DOI: 10.7270/Q2CJ8GZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM434785 (US10577368, Compound 13) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by the Secretary, Department of Health and Human Services; Saint Louis University US Patent | Assay Description [3H]RóN6-Phenylisopropyladenosine (40, [3H]R-PIA, 63 Ci/mmol), [3H](2-[p-(2-carboxyethyl)phenyl-ethylamino]-5′-N-ethylcarboxamido-adenosine) (4... | US Patent US10577368 (2020) BindingDB Entry DOI: 10.7270/Q2HH6NGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50069992 (CHEMBL3407766) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human recombinant A3 adenosine receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 57: 9901-14 (2014) Article DOI: 10.1021/jm501021n BindingDB Entry DOI: 10.7270/Q2S1846G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50266663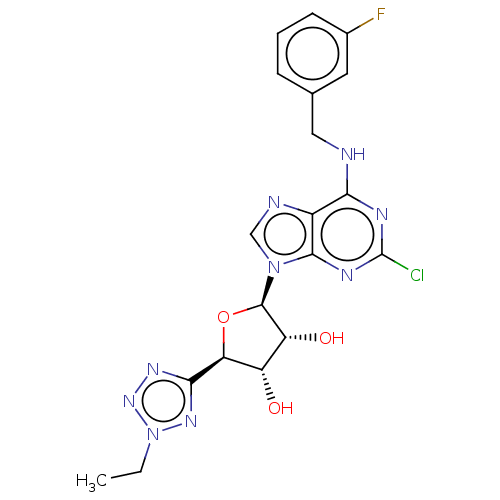 (CHEMBL4079433) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.806 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Displacement of [3H]HEMADO from recombinant human adenosine A3A receptor expressed in CHO cell membranes after 3 hrs by microbeta scintillation count... | J Med Chem 60: 4327-4341 (2017) Article DOI: 10.1021/acs.jmedchem.7b00291 BindingDB Entry DOI: 10.7270/Q2CJ8GZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane domain-containing protein TMIGD3 (Homo sapiens (Human)) | BDBM50500115 (CHEMBL3747209) | UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyl-uronamide from human adenosine A3 receptor stably expressed in CHO cell membrane... | Medchemcomm 6: 555-563 Article DOI: 10.1039/c4md00571f BindingDB Entry DOI: 10.7270/Q2DB84W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane domain-containing protein TMIGD3 (Homo sapiens (Human)) | BDBM50500124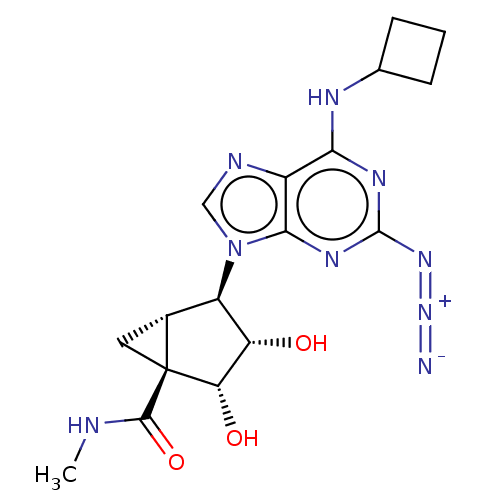 (CHEMBL3747005) | UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyl-uronamide from human adenosine A3 receptor stably expressed in CHO cell membrane... | Medchemcomm 6: 555-563 Article DOI: 10.1039/c4md00571f BindingDB Entry DOI: 10.7270/Q2DB84W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50389130 (CHEMBL2064635) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]I-AB-MECA from human recombinant A3 adenosine receptor expressed in CHO cells after 60 mins by liquid scintillation counting | J Med Chem 57: 9901-14 (2014) Article DOI: 10.1021/jm501021n BindingDB Entry DOI: 10.7270/Q2S1846G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM434780 (US10577368, Compound 400) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by the Secretary, Department of Health and Human Services; Saint Louis University US Patent | Assay Description [3H]RóN6-Phenylisopropyladenosine (40, [3H]R-PIA, 63 Ci/mmol), [3H](2-[p-(2-carboxyethyl)phenyl-ethylamino]-5′-N-ethylcarboxamido-adenosine) (4... | US Patent US10577368 (2020) BindingDB Entry DOI: 10.7270/Q2HH6NGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM434835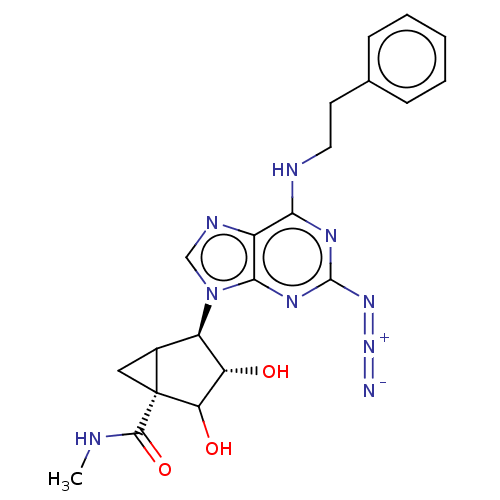 (US10577368, Compound 117) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by the Secretary, Department of Health and Human Services; Saint Louis University US Patent | Assay Description [3H]RóN6-Phenylisopropyladenosine (40, [3H]R-PIA, 63 Ci/mmol), [3H](2-[p-(2-carboxyethyl)phenyl-ethylamino]-5′-N-ethylcarboxamido-adenosine) (4... | US Patent US10577368 (2020) BindingDB Entry DOI: 10.7270/Q2HH6NGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM434833 (US10577368, Compound 115) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The United States of America, as represented by the Secretary, Department of Health and Human Services; Saint Louis University US Patent | Assay Description [3H]RóN6-Phenylisopropyladenosine (40, [3H]R-PIA, 63 Ci/mmol), [3H](2-[p-(2-carboxyethyl)phenyl-ethylamino]-5′-N-ethylcarboxamido-adenosine) (4... | US Patent US10577368 (2020) BindingDB Entry DOI: 10.7270/Q2HH6NGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50047917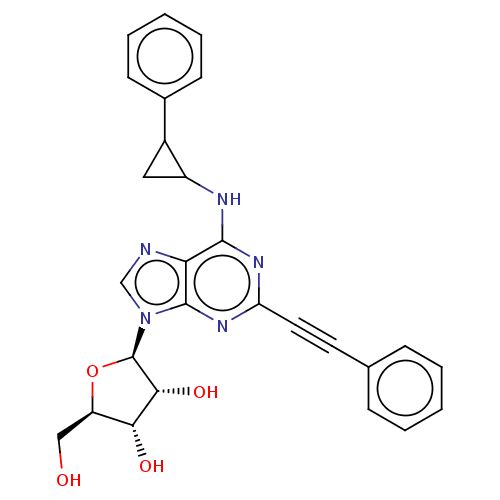 (CHEMBL3311282) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]N6-(4-amino-3-iodobenzyl)adenosine-5'-N-methyluronamide from human A3AR expressed in CHO cells after 60 mins by scintillation c... | Bioorg Med Chem Lett 24: 3302-6 (2014) Article DOI: 10.1016/j.bmcl.2014.06.006 BindingDB Entry DOI: 10.7270/Q2V40WVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50078426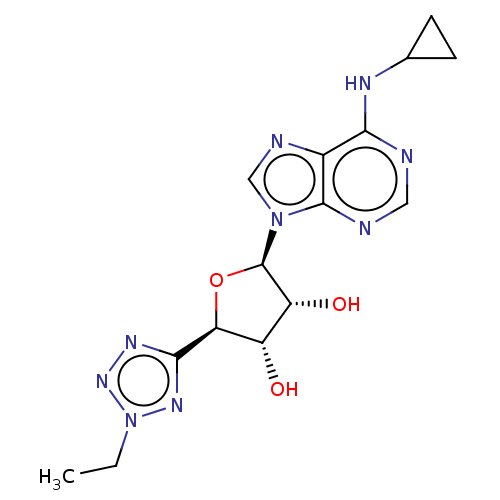 (CHEMBL3414940) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.864 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Displacement of [3H]CCPA from recombinant human adenosine A1 receptor expressed in CHO cell membranes after 3 hrs by microbeta scintillation counting | J Med Chem 60: 4327-4341 (2017) Article DOI: 10.1021/acs.jmedchem.7b00291 BindingDB Entry DOI: 10.7270/Q2CJ8GZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50078426 (CHEMBL3414940) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.875 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Camerino Curated by ChEMBL | Assay Description Displacement of [3H]HEMADO from recombinant human adenosine A3A receptor expressed in CHO cell membranes after 3 hrs by microbeta scintillation count... | J Med Chem 60: 4327-4341 (2017) Article DOI: 10.1021/acs.jmedchem.7b00291 BindingDB Entry DOI: 10.7270/Q2CJ8GZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 959 total ) | Next | Last >> |