

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin B (Rattus norvegicus) | BDBM36331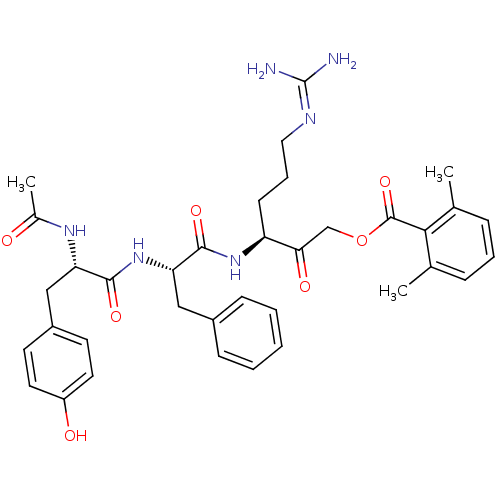 (Ac-YFR-AMOK 10b) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Stanford University | Assay Description Protease enzyme inhibition assay targeting diverse member of the cysteine protease families:cathepsin B, Z, and H | Nat Chem Biol 1: 33-8 (2005) Article DOI: 10.1038/nchembio707 BindingDB Entry DOI: 10.7270/Q22V2DGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Rattus norvegicus) | BDBM36328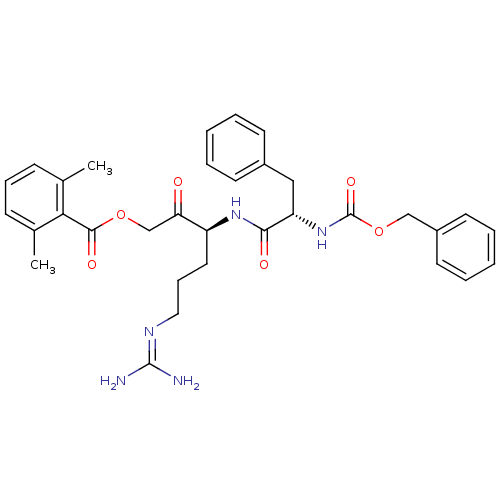 (Z-FR-AMOK 9b) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Stanford University | Assay Description Protease enzyme inhibition assay targeting diverse member of the cysteine protease families:cathepsin B, Z, and H | Nat Chem Biol 1: 33-8 (2005) Article DOI: 10.1038/nchembio707 BindingDB Entry DOI: 10.7270/Q22V2DGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Homo sapiens (Human)) | BDBM50143613 ((E)-3-{(S)-N'-[(S)-2-((S)-2-Benzyloxycarbonylamino...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry and the Parker H. Petit Institute for Bioengineering and Bioscience Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated for Irreversible Inhibition of S. mansoni Legumain | J Med Chem 47: 1889-92 (2004) Article DOI: 10.1021/jm049938j BindingDB Entry DOI: 10.7270/Q2BZ65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Homo sapiens (Human)) | BDBM50143614 ((E)-3-{(S)-N'-[(S)-2-((S)-2-Benzyloxycarbonylamino...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry and the Parker H. Petit Institute for Bioengineering and Bioscience Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated for Irreversible Inhibition of S. mansoni Legumain | J Med Chem 47: 1889-92 (2004) Article DOI: 10.1021/jm049938j BindingDB Entry DOI: 10.7270/Q2BZ65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Homo sapiens (Human)) | BDBM50143612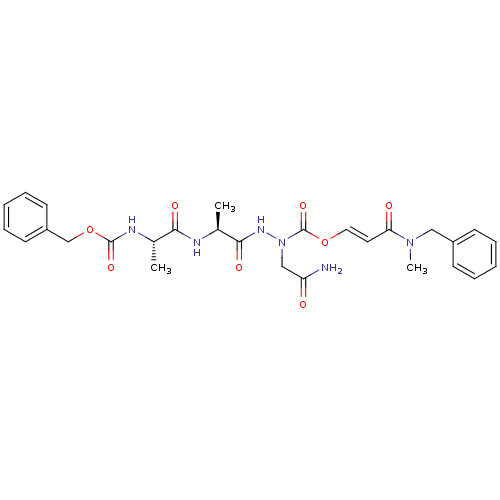 ((S)-N'-[(S)-2-((S)-2-Benzyloxycarbonylamino-propio...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry and the Parker H. Petit Institute for Bioengineering and Bioscience Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated for Irreversible Inhibition of S. mansoni Legumain | J Med Chem 47: 1889-92 (2004) Article DOI: 10.1021/jm049938j BindingDB Entry DOI: 10.7270/Q2BZ65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Rattus norvegicus) | BDBM36327 (Z-FG-AOMK 9a) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Stanford University | Assay Description Protease enzyme inhibition assay targeting diverse member of the cysteine protease families:cathepsin B, Z, and H | Nat Chem Biol 1: 33-8 (2005) Article DOI: 10.1038/nchembio707 BindingDB Entry DOI: 10.7270/Q22V2DGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Rattus norvegicus) | BDBM36330 (AC-YFG-AMOK 10a) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Stanford University | Assay Description Protease enzyme inhibition assay targeting diverse member of the cysteine protease families:cathepsin B, Z, and H | Nat Chem Biol 1: 33-8 (2005) Article DOI: 10.1038/nchembio707 BindingDB Entry DOI: 10.7270/Q22V2DGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM81515 (VEA-499) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM81516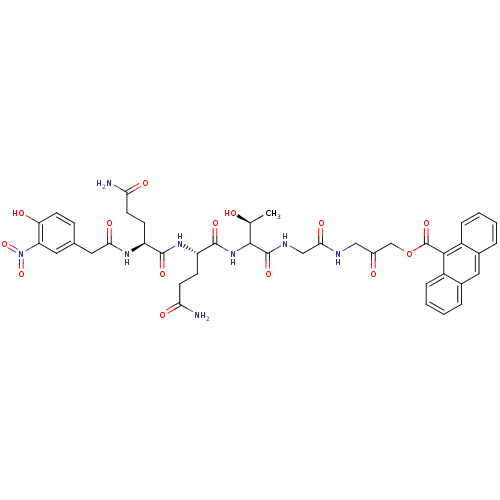 (VEA-500) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Rattus norvegicus) | BDBM36329 (Z-LG-AOMK 9c) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Stanford University | Assay Description Protease enzyme inhibition assay targeting diverse member of the cysteine protease families:cathepsin B, Z, and H | Nat Chem Biol 1: 33-8 (2005) Article DOI: 10.1038/nchembio707 BindingDB Entry DOI: 10.7270/Q22V2DGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Homo sapiens (Human)) | BDBM50292159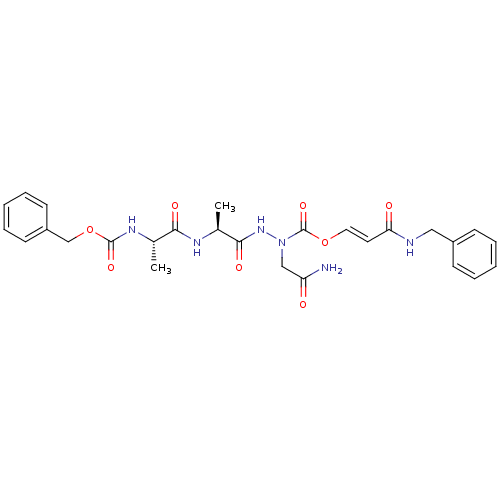 ((S)-N'-[(S)-2-((S)-2-Benzyloxycarbonylamino-propio...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry and the Parker H. Petit Institute for Bioengineering and Bioscience Curated by ChEMBL | Assay Description Inhibitory concentration of the compound was evaluated for irreversible inhibition of S. mansoni Legumain | J Med Chem 47: 1889-92 (2004) Article DOI: 10.1021/jm049938j BindingDB Entry DOI: 10.7270/Q2BZ65G0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Rattus norvegicus) | BDBM36325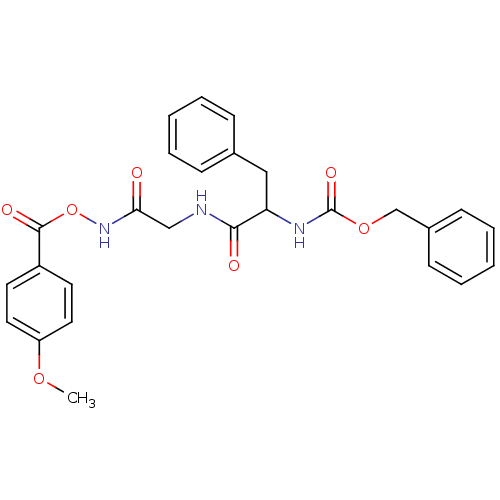 (Cathepsin Inhibitor III) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Stanford University | Assay Description Protease enzyme inhibition assay targeting diverse member of the cysteine protease families:cathepsin B, Z, and H | Nat Chem Biol 1: 33-8 (2005) Article DOI: 10.1038/nchembio707 BindingDB Entry DOI: 10.7270/Q22V2DGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin Z (Rattus norvegicus) | BDBM36325 (Cathepsin Inhibitor III) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Stanford University | Assay Description Protease enzyme inhibition assay targeting diverse member of the cysteine protease families:cathepsin B, Z, and H | Nat Chem Biol 1: 33-8 (2005) Article DOI: 10.1038/nchembio707 BindingDB Entry DOI: 10.7270/Q22V2DGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 1 (Homo sapiens (Human)) | BDBM81515 (VEA-499) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM81511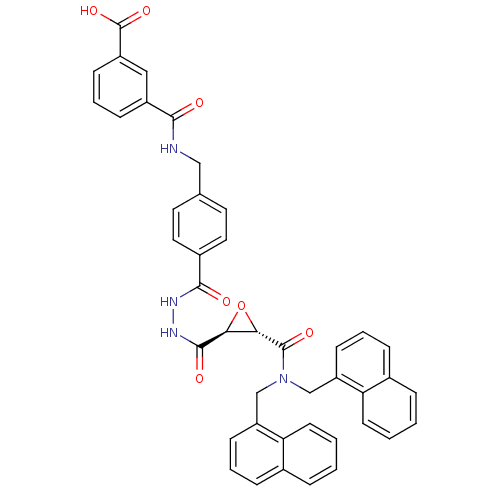 (VEA-260) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 6 (Homo sapiens (Human)) | BDBM81517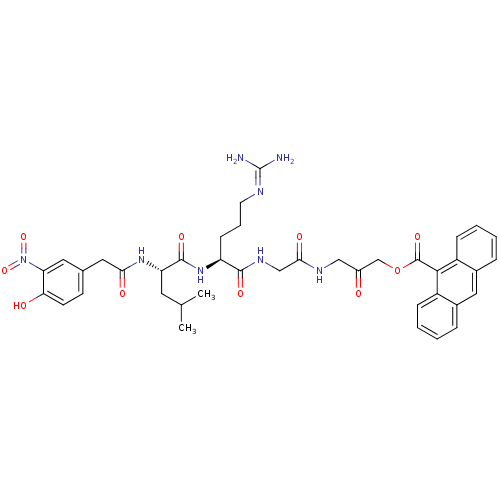 (VEA-561) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 7 (Homo sapiens (Human)) | BDBM81517 (VEA-561) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin Z (Rattus norvegicus) | BDBM36331 (Ac-YFR-AMOK 10b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Stanford University | Assay Description Protease enzyme inhibition assay targeting diverse member of the cysteine protease families:cathepsin B, Z, and H | Nat Chem Biol 1: 33-8 (2005) Article DOI: 10.1038/nchembio707 BindingDB Entry DOI: 10.7270/Q22V2DGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM81517 (VEA-561) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Rattus norvegicus) | BDBM36325 (Cathepsin Inhibitor III) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Stanford University | Assay Description Protease enzyme inhibition assay targeting diverse member of the cysteine protease families:cathepsin B, Z, and H | Nat Chem Biol 1: 33-8 (2005) Article DOI: 10.1038/nchembio707 BindingDB Entry DOI: 10.7270/Q22V2DGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM81510 (JCP-666) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 1 (Homo sapiens (Human)) | BDBM81511 (VEA-260) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Rattus norvegicus) | BDBM36330 (AC-YFG-AMOK 10a) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Stanford University | Assay Description Protease enzyme inhibition assay targeting diverse member of the cysteine protease families:cathepsin B, Z, and H | Nat Chem Biol 1: 33-8 (2005) Article DOI: 10.1038/nchembio707 BindingDB Entry DOI: 10.7270/Q22V2DGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Rattus norvegicus) | BDBM36329 (Z-LG-AOMK 9c) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Stanford University | Assay Description Protease enzyme inhibition assay targeting diverse member of the cysteine protease families:cathepsin B, Z, and H | Nat Chem Biol 1: 33-8 (2005) Article DOI: 10.1038/nchembio707 BindingDB Entry DOI: 10.7270/Q22V2DGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM81513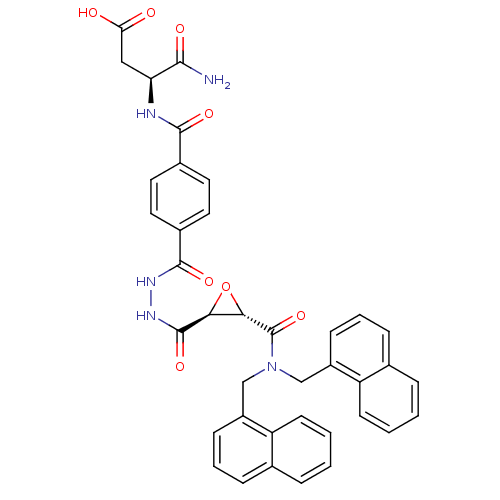 (VEA-422) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pro-cathepsin H (Rattus norvegicus) | BDBM36331 (Ac-YFR-AMOK 10b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Stanford University | Assay Description Protease enzyme inhibition assay targeting diverse member of the cysteine protease families:cathepsin B, Z, and H | Nat Chem Biol 1: 33-8 (2005) Article DOI: 10.1038/nchembio707 BindingDB Entry DOI: 10.7270/Q22V2DGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM81512 (VEA-417) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM81514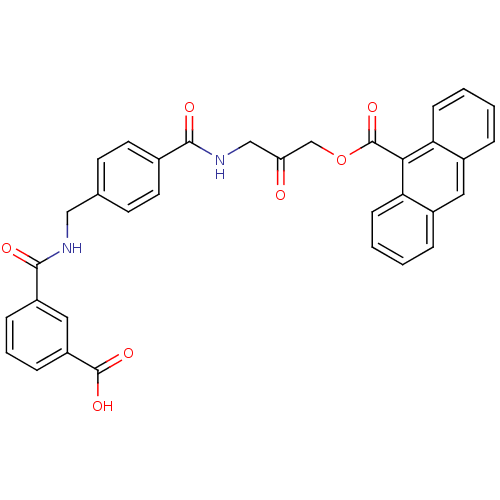 (VEA-401) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.34E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 1 (Homo sapiens (Human)) | BDBM81510 (JCP-666) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 1 (Homo sapiens (Human)) | BDBM81516 (VEA-500) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 7 (Homo sapiens (Human)) | BDBM81514 (VEA-401) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 1 (Homo sapiens (Human)) | BDBM81513 (VEA-422) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.24E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 1 (Homo sapiens (Human)) | BDBM81514 (VEA-401) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.31E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 1 (Homo sapiens (Human)) | BDBM81517 (VEA-561) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 1 (Homo sapiens (Human)) | BDBM81512 (VEA-417) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.25E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 7 (Homo sapiens (Human)) | BDBM81511 (VEA-260) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.65E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin Z (Rattus norvegicus) | BDBM36330 (AC-YFG-AMOK 10a) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Stanford University | Assay Description Protease enzyme inhibition assay targeting diverse member of the cysteine protease families:cathepsin B, Z, and H | Nat Chem Biol 1: 33-8 (2005) Article DOI: 10.1038/nchembio707 BindingDB Entry DOI: 10.7270/Q22V2DGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 6 (Homo sapiens (Human)) | BDBM81514 (VEA-401) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 6 (Homo sapiens (Human)) | BDBM81516 (VEA-500) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 6 (Homo sapiens (Human)) | BDBM81515 (VEA-499) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 7 (Homo sapiens (Human)) | BDBM81510 (JCP-666) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >7.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 7 (Homo sapiens (Human)) | BDBM81513 (VEA-422) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >7.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 7 (Homo sapiens (Human)) | BDBM81512 (VEA-417) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >7.00E+4 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 6 (Homo sapiens (Human)) | BDBM81511 (VEA-260) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 6 (Homo sapiens (Human)) | BDBM81510 (JCP-666) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 6 (Homo sapiens (Human)) | BDBM81512 (VEA-417) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 7 (Homo sapiens (Human)) | BDBM81515 (VEA-499) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 7 (Homo sapiens (Human)) | BDBM81516 (VEA-500) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 6 (Homo sapiens (Human)) | BDBM81513 (VEA-422) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||