

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldo-keto reductase family 1 member C2 (Homo sapiens (Human)) | BDBM50396691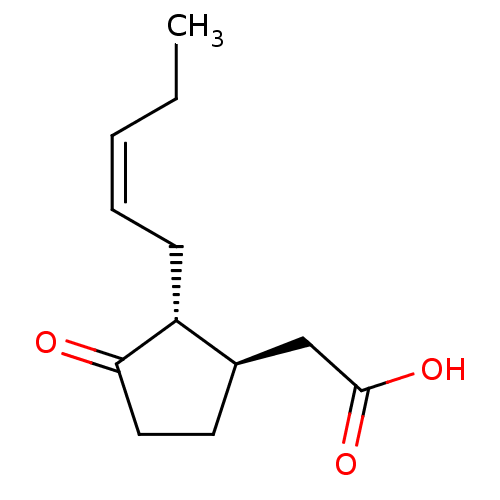 (CHEMBL449572 | US9271961, Jasmonic acid) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C2 expressed in Escherichia coli BL21(DE3) using phenanthrenequinone as substrate by spectrophotometric analysis | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C4 (Homo sapiens (Human)) | BDBM50396691 (CHEMBL449572 | US9271961, Jasmonic acid) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C4 expressed in Escherichia coli BL21(DE3) using phenanthrenequinone as substrate by spectrophotometric analysis | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50396691 (CHEMBL449572 | US9271961, Jasmonic acid) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | 1.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C1 expressed in Escherichia coli BL21(DE3) using phenanthrenequinone as substrate by spectrophotometric analysis | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396691 (CHEMBL449572 | US9271961, Jasmonic acid) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem | Article PubMed | 1.62E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant AKR1C3 expressed in Escherichia coli BL21(DE3) using phenanthrenequinone as substrate by spectrophotometric analysis | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50386447 (CHEMBL2047701) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-lys(Ac)-AMC as substrate preincubated for 20 mins with substrate measured after 60 mins by fluorescen... | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50386442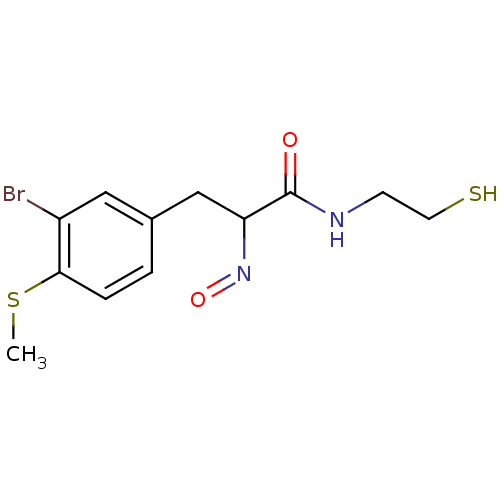 (CHEMBL2047694) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-lys(Ac)-AMC as substrate preincubated for 20 mins with substrate measured after 60 mins by fluorescen... | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50386435 (CHEMBL2047702) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-lys(Ac)-AMC as substrate preincubated for 20 mins with substrate measured after 60 mins by fluorescen... | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50386453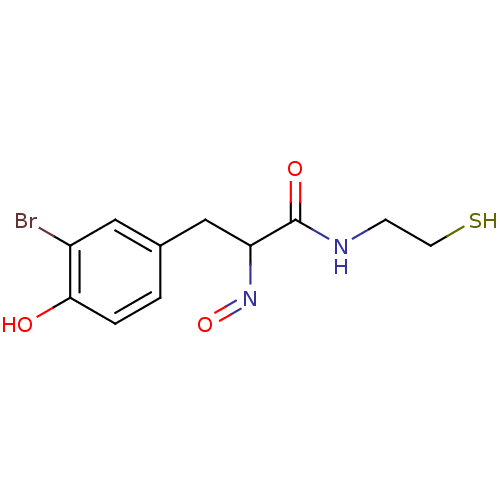 (CHEMBL2047540) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-lys(Ac)-AMC as substrate preincubated for 20 mins with substrate measured after 60 mins by fluorescen... | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50386441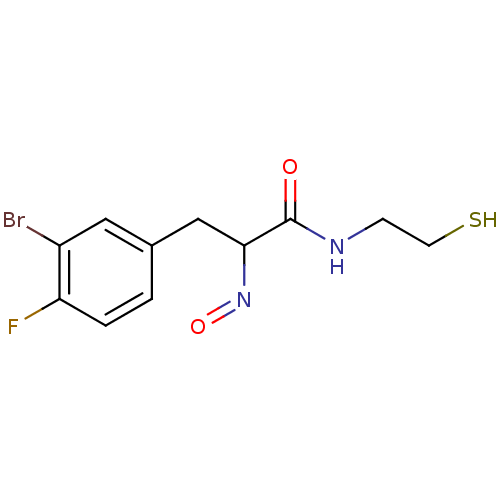 (CHEMBL2047692) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-lys(Ac)-AMC as substrate preincubated for 20 mins with substrate measured after 60 mins by fluorescen... | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50386434 (CHEMBL2047699) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-lys(Ac)-AMC as substrate preincubated for 20 mins with substrate measured after 60 mins by fluorescen... | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50386444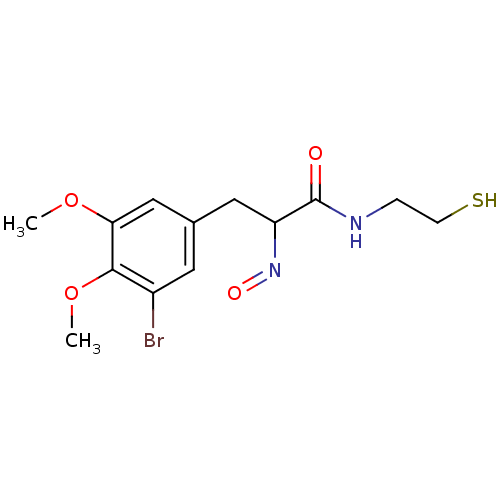 (CHEMBL2047696) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-lys(Ac)-AMC as substrate preincubated for 20 mins with substrate measured after 60 mins by fluorescen... | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50386443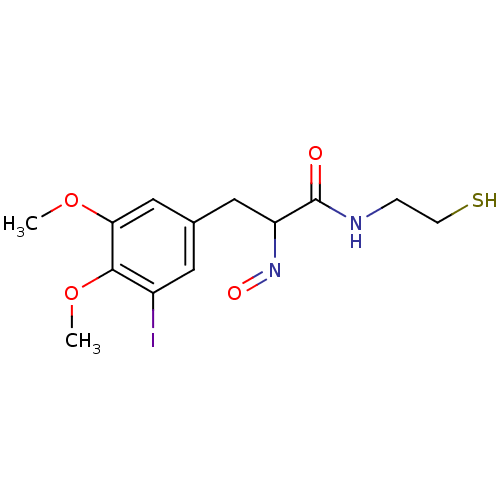 (CHEMBL2047695) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-lys(Ac)-AMC as substrate preincubated for 20 mins with substrate measured after 60 mins by fluorescen... | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50386448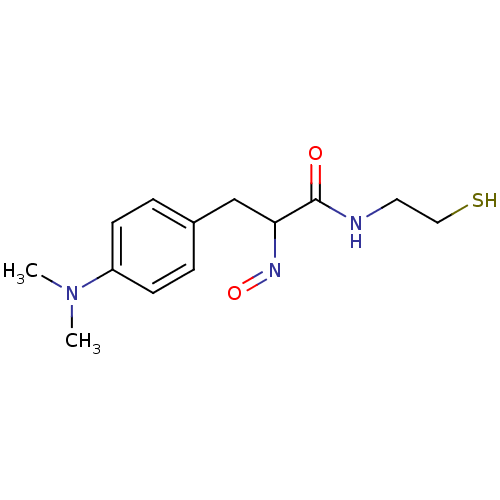 (CHEMBL2047703) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-lys(Ac)-AMC as substrate preincubated for 20 mins with substrate measured after 60 mins by fluorescen... | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50386440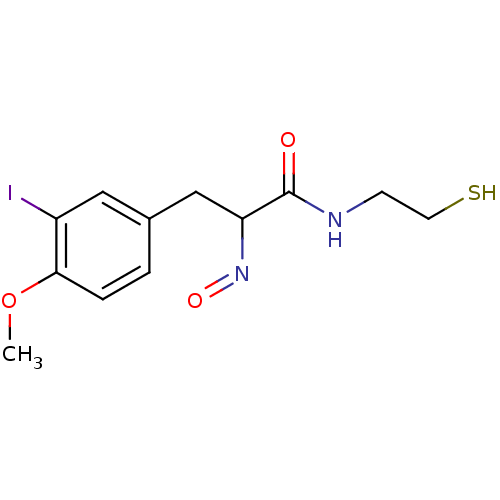 (CHEMBL2047691) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-lys(Ac)-AMC as substrate preincubated for 20 mins with substrate measured after 60 mins by fluorescen... | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50386457 (CHEMBL2047541) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-lys(Ac)-AMC as substrate preincubated for 20 mins with substrate measured after 60 mins by fluorescen... | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50386439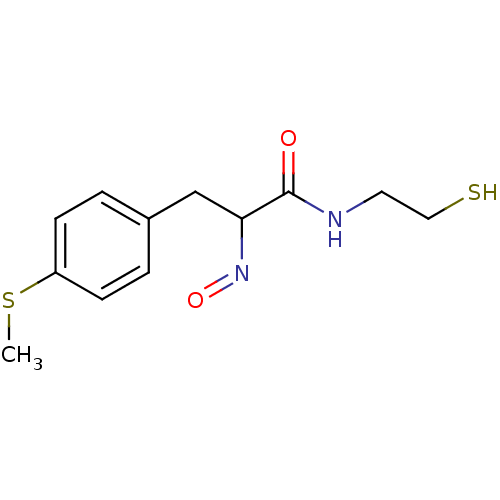 (CHEMBL2047690) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-lys(Ac)-AMC as substrate preincubated for 20 mins with substrate measured after 60 mins by fluorescen... | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50386450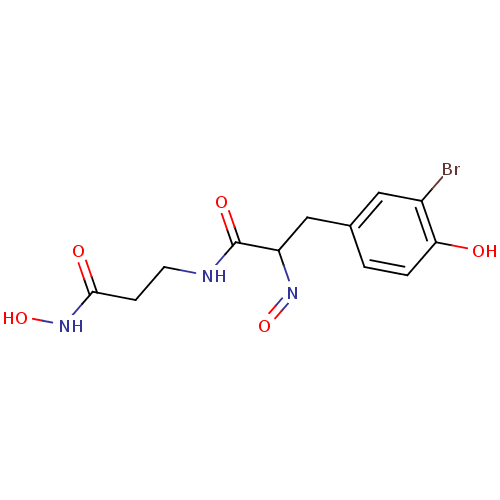 (CHEMBL2047684) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-lys(Ac)-AMC as substrate preincubated for 20 mins with substrate measured after 60 mins by fluorescen... | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50386445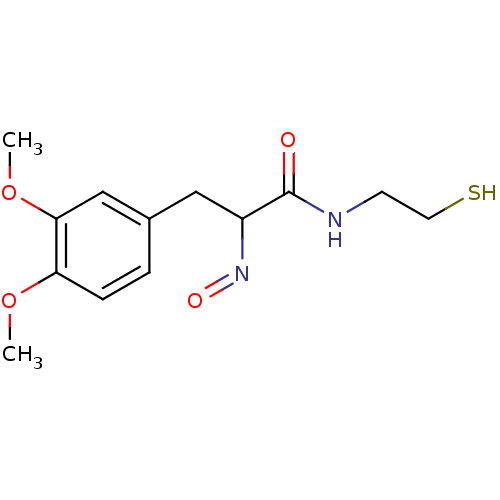 (CHEMBL2047698) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-lys(Ac)-AMC as substrate preincubated for 20 mins with substrate measured after 60 mins by fluorescen... | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50386437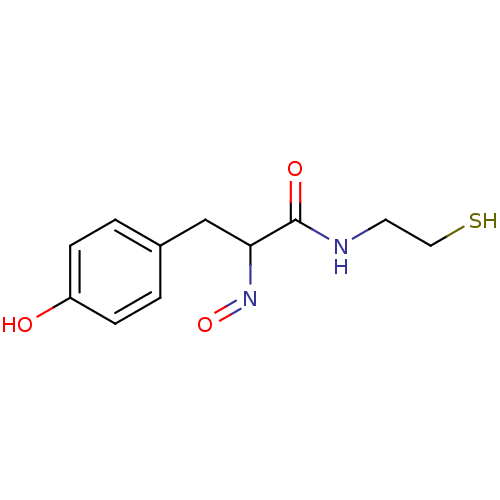 (CHEMBL2047688) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-lys(Ac)-AMC as substrate preincubated for 20 mins with substrate measured after 60 mins by fluorescen... | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50386449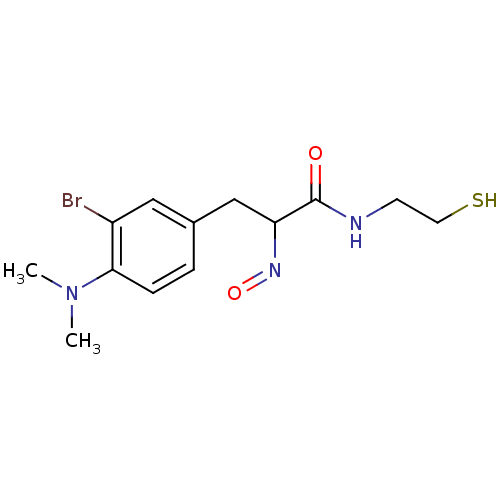 (CHEMBL2047704) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-lys(Ac)-AMC as substrate preincubated for 20 mins with substrate measured after 60 mins by fluorescen... | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50386446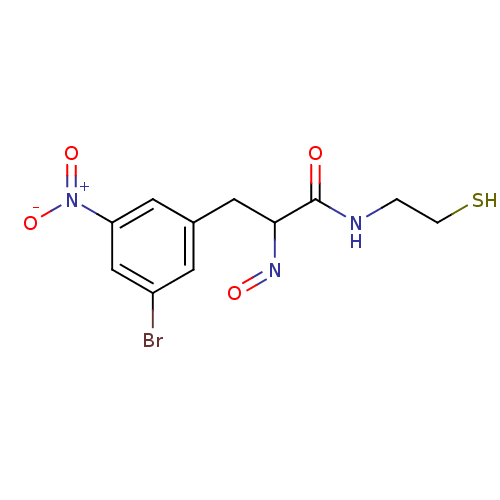 (CHEMBL2047700) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-lys(Ac)-AMC as substrate preincubated for 20 mins with substrate measured after 60 mins by fluorescen... | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50386452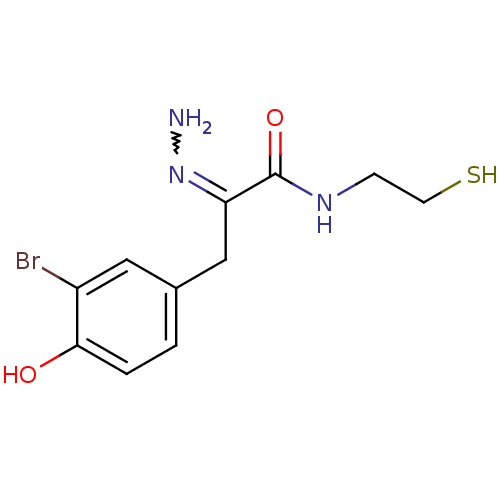 (CHEMBL2047680) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-lys(Ac)-AMC as substrate preincubated for 20 mins with substrate measured after 60 mins by fluorescen... | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50386472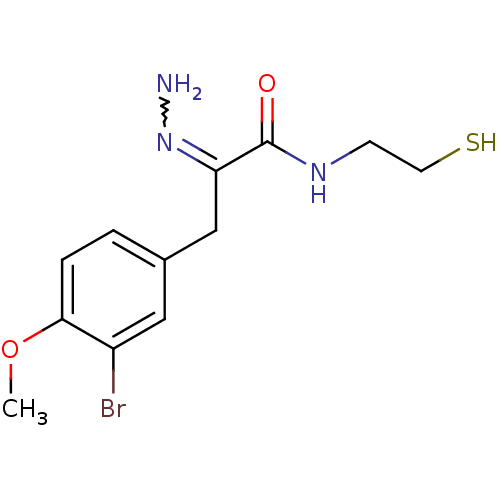 (CHEMBL2047681) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-lys(Ac)-AMC as substrate preincubated for 20 mins with substrate measured after 60 mins by fluorescen... | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396653 (CHEMBL2172122) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50386438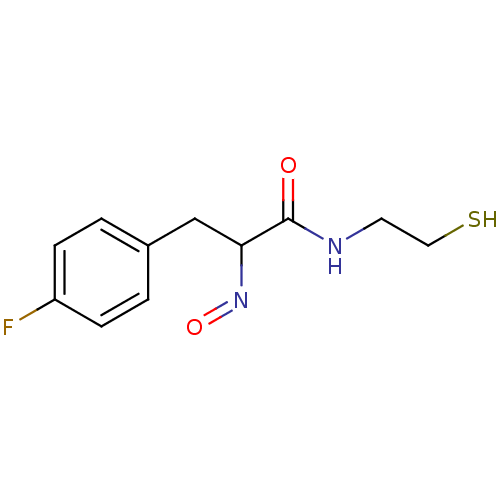 (CHEMBL2047689) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-lys(Ac)-AMC as substrate preincubated for 20 mins with substrate measured after 60 mins by fluorescen... | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396661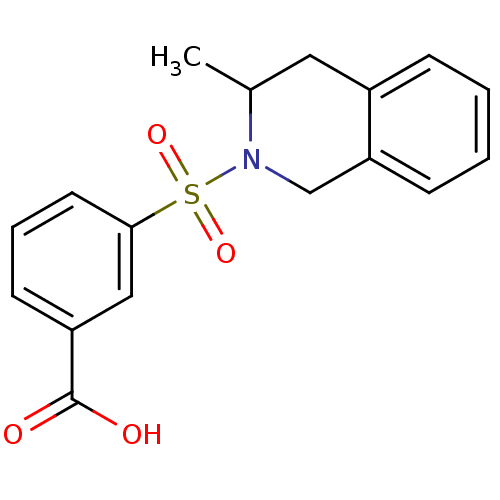 (CHEMBL2172110) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396695 (CHEMBL2172121) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396694 (CHEMBL2172112) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396693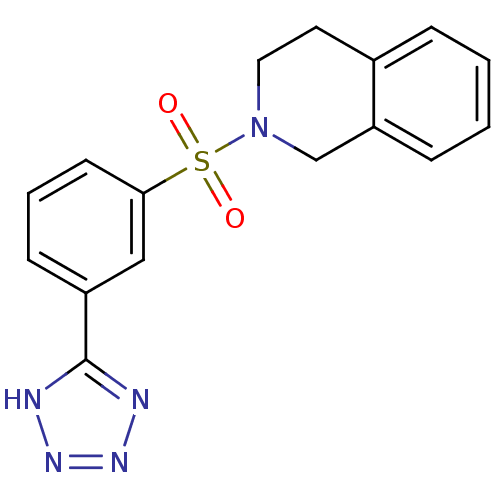 (CHEMBL2172067) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396659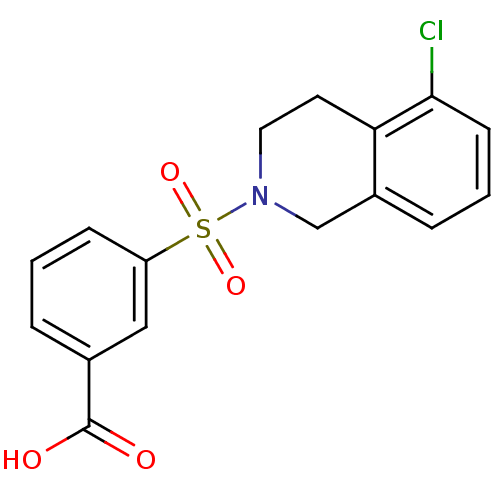 (CHEMBL2172113) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396658 (CHEMBL2172114) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50386436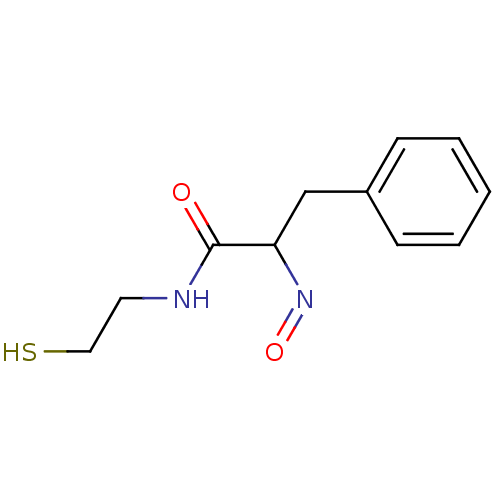 (CHEMBL2047687) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-lys(Ac)-AMC as substrate preincubated for 20 mins with substrate measured after 60 mins by fluorescen... | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396692 (CHEMBL2172087) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396653 (CHEMBL2172122) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of AKR1C3 overexpressed in human HCT116 cells assessed as inhibition of PR-104A conversion to hydroxylamine after 2 hrs by spectrophotomet... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396704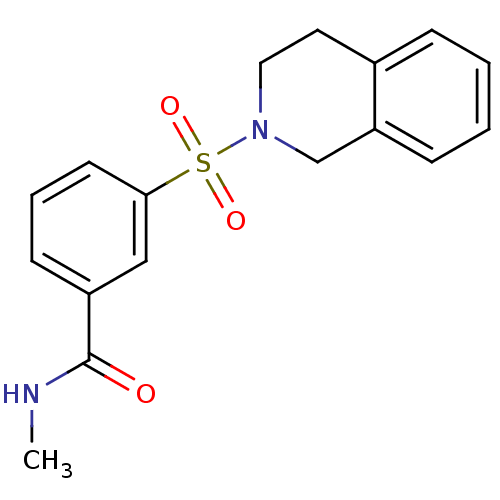 (CHEMBL2172072) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of AKR1C3 overexpressed in human HCT116 cells assessed as inhibition of PR-104A conversion to hydroxylamine after 2 hrs by spectrophotomet... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396676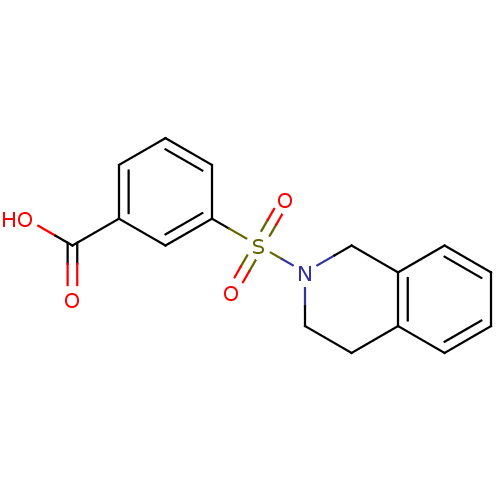 (CHEMBL1566492) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396650 (CHEMBL2172220) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396707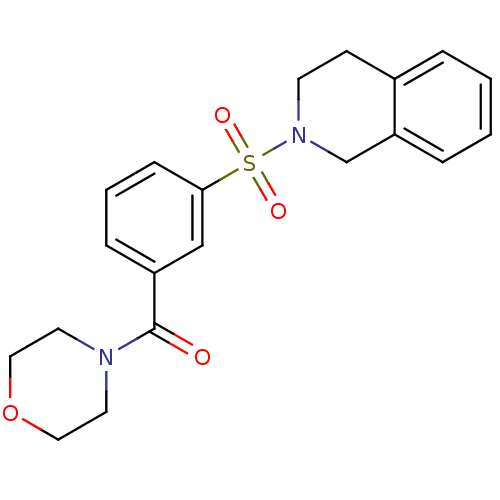 (CHEMBL2172069) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of AKR1C3 overexpressed in human HCT116 cells assessed as inhibition of PR-104A conversion to hydroxylamine after 2 hrs by spectrophotomet... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396703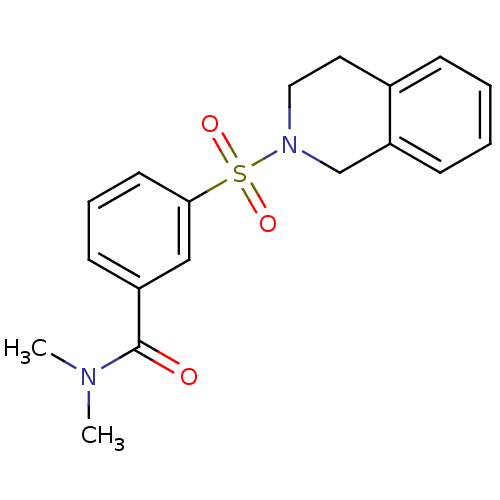 (CHEMBL2172073) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of AKR1C3 overexpressed in human HCT116 cells assessed as inhibition of PR-104A conversion to hydroxylamine after 2 hrs by spectrophotomet... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396657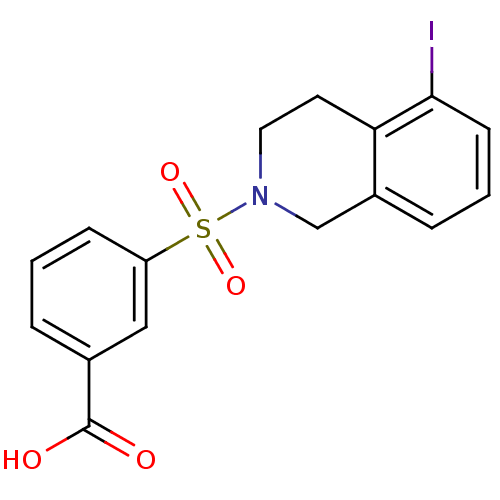 (CHEMBL2172115) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396719 (CHEMBL2172088) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396658 (CHEMBL2172114) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of AKR1C3 overexpressed in human HCT116 cells assessed as inhibition of PR-104A conversion to hydroxylamine after 2 hrs by spectrophotomet... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50386462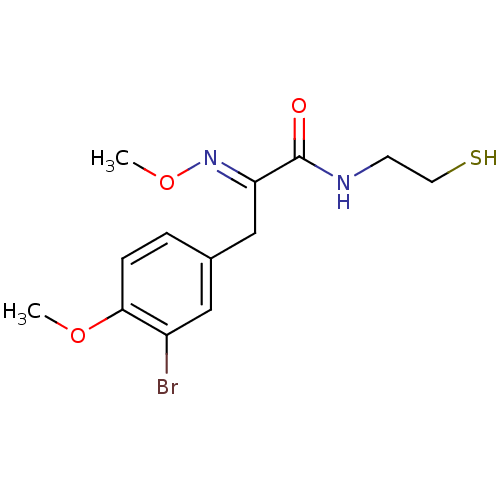 (CHEMBL2047670) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 using Boc-lys(Ac)-AMC as substrate preincubated for 20 mins with substrate measured after 60 mins by fluorescen... | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396659 (CHEMBL2172113) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of AKR1C3 overexpressed in human HCT116 cells assessed as inhibition of PR-104A conversion to hydroxylamine after 2 hrs by spectrophotomet... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396722 (CHEMBL2172116) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396656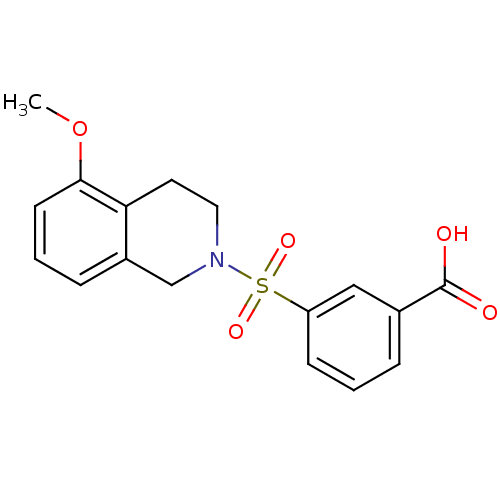 (CHEMBL2172117) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396655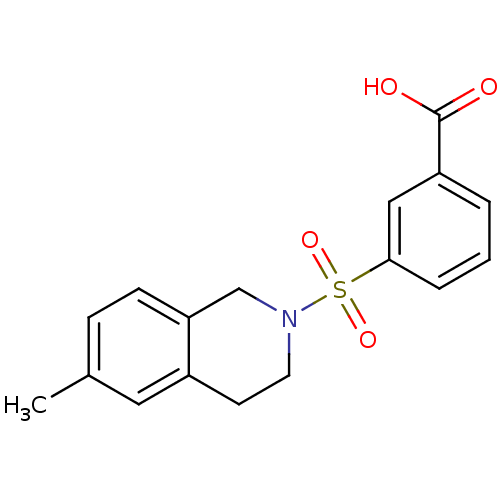 (CHEMBL2172118) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396646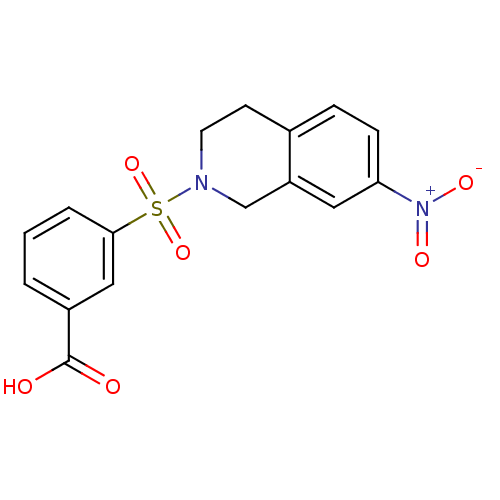 (CHEMBL2172084) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA (cytosine-5)-methyltransferase 1 (Homo sapiens (Human)) | BDBM50117090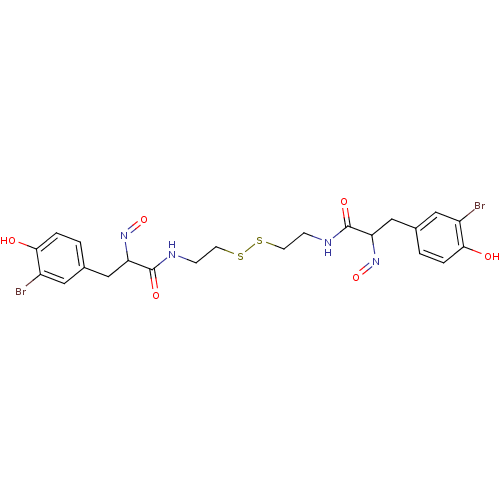 ((E,E)-Psammaplin A | 3-(3-Bromo-4-hydroxy-phenyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Imperial College London Curated by ChEMBL | Assay Description Inhibition of semi-purified DNMT1 | J Med Chem 55: 1731-50 (2012) Article DOI: 10.1021/jm2016182 BindingDB Entry DOI: 10.7270/Q22F7PHC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50396716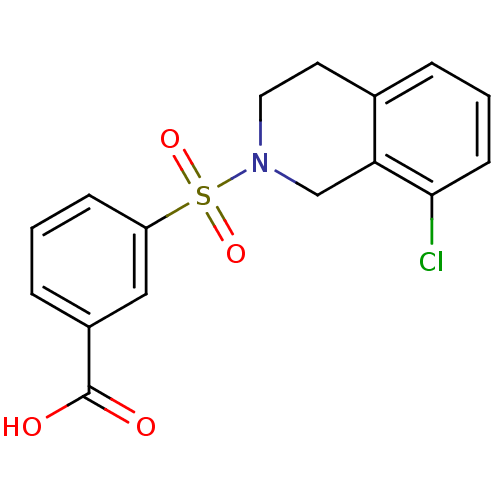 (CHEMBL2172091) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminal His6-tagged AKR1C3 expressed in Escherichia coli BL21(DE3) cells using 8-Acetyl-2,3,5,6-tetrahydro-1H,4H-1... | J Med Chem 55: 7746-58 (2012) Article DOI: 10.1021/jm3007867 BindingDB Entry DOI: 10.7270/Q28K7B6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 460 total ) | Next | Last >> |