 Found 237 hits with Last Name = 'sanderson' and Initial = 'v'
Found 237 hits with Last Name = 'sanderson' and Initial = 'v' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50301772
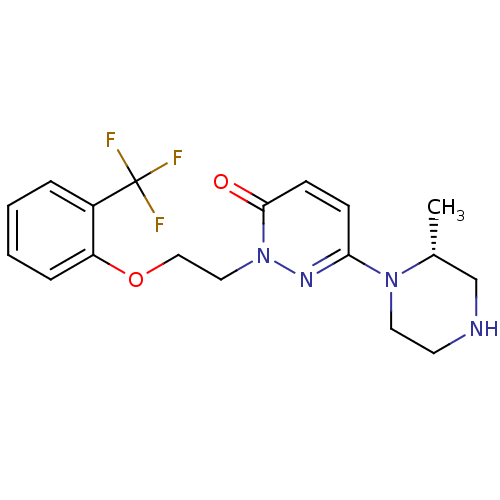
((R)-6-(2-methylpiperazin-1-yl)-2-(2-(2-(trifluorom...)Show SMILES C[C@@H]1CNCCN1c1ccc(=O)n(CCOc2ccccc2C(F)(F)F)n1 |r| Show InChI InChI=1S/C18H21F3N4O2/c1-13-12-22-8-9-24(13)16-6-7-17(26)25(23-16)10-11-27-15-5-3-2-4-14(15)18(19,20)21/h2-7,13,22H,8-12H2,1H3/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]meselurgine from human recombinant 5HT2C receptor expressed in Swiss mouse 3T3 cells by SPA |
Bioorg Med Chem Lett 19: 5791-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.136
BindingDB Entry DOI: 10.7270/Q2X0674Z |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50302145
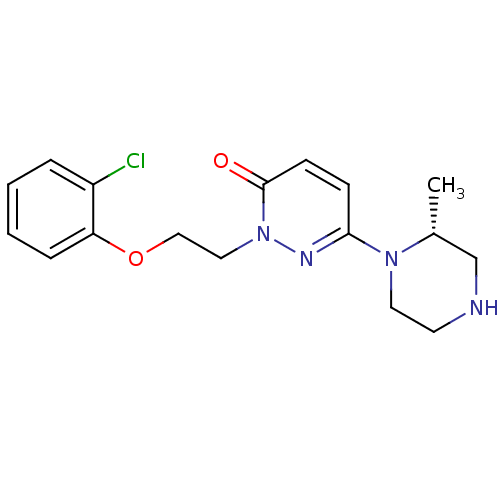
((R)-2-(2-(2-chlorophenoxy)ethyl)-6-(2-methylpipera...)Show SMILES C[C@@H]1CNCCN1c1ccc(=O)n(CCOc2ccccc2Cl)n1 |r| Show InChI InChI=1S/C17H21ClN4O2/c1-13-12-19-8-9-21(13)16-6-7-17(23)22(20-16)10-11-24-15-5-3-2-4-14(15)18/h2-7,13,19H,8-12H2,1H3/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 325 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]meselurgine from human recombinant 5HT2C receptor expressed in Swiss mouse 3T3 cells by SPA |
Bioorg Med Chem Lett 19: 5791-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.136
BindingDB Entry DOI: 10.7270/Q2X0674Z |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50302160
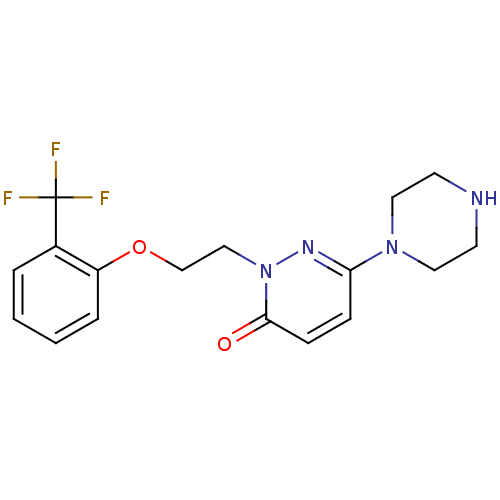
(6-(piperazin-1-yl)-2-(2-(2-(trifluoromethyl)phenox...)Show InChI InChI=1S/C17H19F3N4O2/c18-17(19,20)13-3-1-2-4-14(13)26-12-11-24-16(25)6-5-15(22-24)23-9-7-21-8-10-23/h1-6,21H,7-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 424 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]meselurgine from human recombinant 5HT2C receptor expressed in Swiss mouse 3T3 cells by SPA |
Bioorg Med Chem Lett 19: 5791-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.136
BindingDB Entry DOI: 10.7270/Q2X0674Z |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50302151
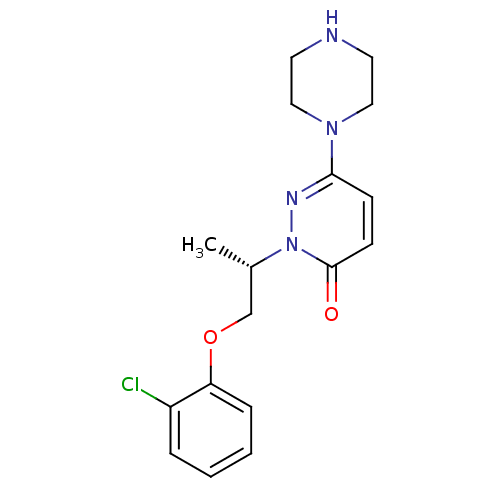
((S)-2-(1-(2-chlorophenoxy)propan-2-yl)-6-(piperazi...)Show SMILES C[C@@H](COc1ccccc1Cl)n1nc(ccc1=O)N1CCNCC1 |r| Show InChI InChI=1S/C17H21ClN4O2/c1-13(12-24-15-5-3-2-4-14(15)18)22-17(23)7-6-16(20-22)21-10-8-19-9-11-21/h2-7,13,19H,8-12H2,1H3/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 728 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]meselurgine from human recombinant 5HT2C receptor expressed in Swiss mouse 3T3 cells by SPA |
Bioorg Med Chem Lett 19: 5791-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.136
BindingDB Entry DOI: 10.7270/Q2X0674Z |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50226008
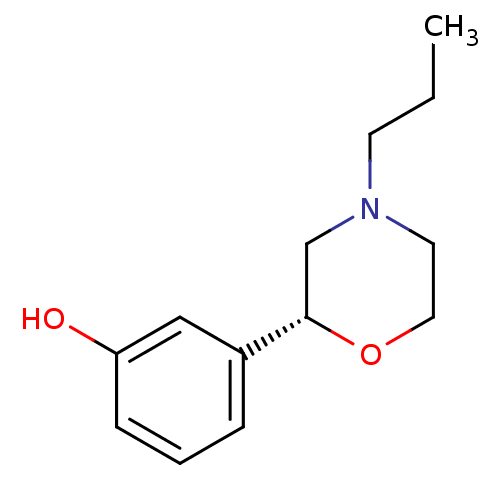
((R)-3-(4-propylmorpholin-2-yl)phenol | CHEMBL25040...)Show InChI InChI=1S/C13H19NO2/c1-2-6-14-7-8-16-13(10-14)11-4-3-5-12(15)9-11/h3-5,9,13,15H,2,6-8,10H2,1H3/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to rat 5HT2A receptor |
Bioorg Med Chem Lett 17: 6691-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.10.059
BindingDB Entry DOI: 10.7270/Q23J3CQ5 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50226008

((R)-3-(4-propylmorpholin-2-yl)phenol | CHEMBL25040...)Show InChI InChI=1S/C13H19NO2/c1-2-6-14-7-8-16-13(10-14)11-4-3-5-12(15)9-11/h3-5,9,13,15H,2,6-8,10H2,1H3/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to rat alpha1A adrenergic receptor |
Bioorg Med Chem Lett 17: 6691-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.10.059
BindingDB Entry DOI: 10.7270/Q23J3CQ5 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50226008

((R)-3-(4-propylmorpholin-2-yl)phenol | CHEMBL25040...)Show InChI InChI=1S/C13H19NO2/c1-2-6-14-7-8-16-13(10-14)11-4-3-5-12(15)9-11/h3-5,9,13,15H,2,6-8,10H2,1H3/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to human alpha2A adrenergic receptor |
Bioorg Med Chem Lett 17: 6691-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.10.059
BindingDB Entry DOI: 10.7270/Q23J3CQ5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50226008

((R)-3-(4-propylmorpholin-2-yl)phenol | CHEMBL25040...)Show InChI InChI=1S/C13H19NO2/c1-2-6-14-7-8-16-13(10-14)11-4-3-5-12(15)9-11/h3-5,9,13,15H,2,6-8,10H2,1H3/t13-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to human muscarinic M1 receptor |
Bioorg Med Chem Lett 17: 6691-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.10.059
BindingDB Entry DOI: 10.7270/Q23J3CQ5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50302141
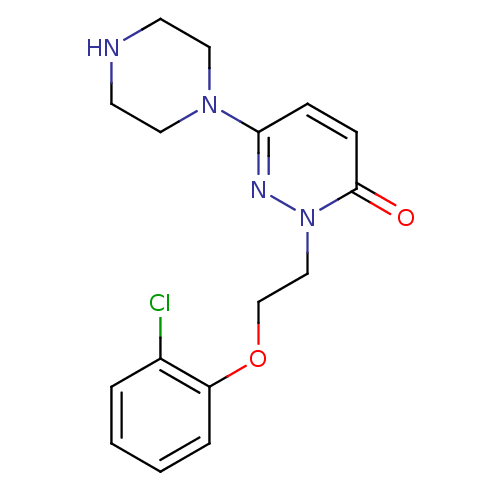
(2-(2-(2-chlorophenoxy)ethyl)-6-(piperazin-1-yl)pyr...)Show InChI InChI=1S/C16H19ClN4O2/c17-13-3-1-2-4-14(13)23-12-11-21-16(22)6-5-15(19-21)20-9-7-18-8-10-20/h1-6,18H,7-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]meselurgine from human recombinant 5HT2C receptor expressed in Swiss mouse 3T3 cells by SPA |
Bioorg Med Chem Lett 19: 5791-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.136
BindingDB Entry DOI: 10.7270/Q2X0674Z |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50226008

((R)-3-(4-propylmorpholin-2-yl)phenol | CHEMBL25040...)Show InChI InChI=1S/C13H19NO2/c1-2-6-14-7-8-16-13(10-14)11-4-3-5-12(15)9-11/h3-5,9,13,15H,2,6-8,10H2,1H3/t13-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H1 receptor |
Bioorg Med Chem Lett 17: 6691-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.10.059
BindingDB Entry DOI: 10.7270/Q23J3CQ5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50226008

((R)-3-(4-propylmorpholin-2-yl)phenol | CHEMBL25040...)Show InChI InChI=1S/C13H19NO2/c1-2-6-14-7-8-16-13(10-14)11-4-3-5-12(15)9-11/h3-5,9,13,15H,2,6-8,10H2,1H3/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Displacement of dofetilide from hERG |
Bioorg Med Chem Lett 17: 6691-6 (2007)
Article DOI: 10.1016/j.bmcl.2007.10.059
BindingDB Entry DOI: 10.7270/Q23J3CQ5 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50197517
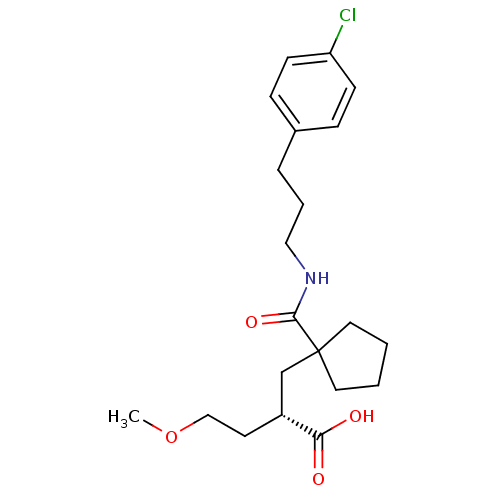
((S)-2-{1-[3-(4-chloro-phenyl)-propylcarbamoyl]-cyc...)Show SMILES COCC[C@H](CC1(CCCC1)C(=O)NCCCc1ccc(Cl)cc1)C(O)=O Show InChI InChI=1S/C21H30ClNO4/c1-27-14-10-17(19(24)25)15-21(11-2-3-12-21)20(26)23-13-4-5-16-6-8-18(22)9-7-16/h6-9,17H,2-5,10-15H2,1H3,(H,23,26)(H,24,25)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of rat NEP |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50197517

((S)-2-{1-[3-(4-chloro-phenyl)-propylcarbamoyl]-cyc...)Show SMILES COCC[C@H](CC1(CCCC1)C(=O)NCCCc1ccc(Cl)cc1)C(O)=O Show InChI InChI=1S/C21H30ClNO4/c1-27-14-10-17(19(24)25)15-21(11-2-3-12-21)20(26)23-13-4-5-16-6-8-18(22)9-7-16/h6-9,17H,2-5,10-15H2,1H3,(H,23,26)(H,24,25)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of rabbit NEP |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50197517

((S)-2-{1-[3-(4-chloro-phenyl)-propylcarbamoyl]-cyc...)Show SMILES COCC[C@H](CC1(CCCC1)C(=O)NCCCc1ccc(Cl)cc1)C(O)=O Show InChI InChI=1S/C21H30ClNO4/c1-27-14-10-17(19(24)25)15-21(11-2-3-12-21)20(26)23-13-4-5-16-6-8-18(22)9-7-16/h6-9,17H,2-5,10-15H2,1H3,(H,23,26)(H,24,25)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human kidney NEP at pH 7 |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50197513
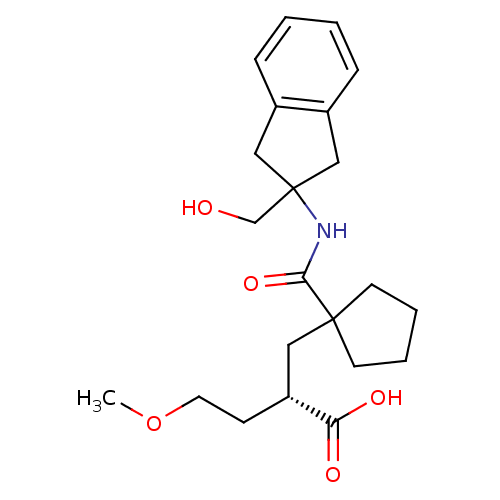
((S)-2-((1-((2-(hydroxymethyl)-2,3-dihydro-1H-inden...)Show SMILES COCC[C@H](CC1(CCCC1)C(=O)NC1(CO)Cc2ccccc2C1)C(O)=O Show InChI InChI=1S/C22H31NO5/c1-28-11-8-18(19(25)26)12-21(9-4-5-10-21)20(27)23-22(15-24)13-16-6-2-3-7-17(16)14-22/h2-3,6-7,18,24H,4-5,8-15H2,1H3,(H,23,27)(H,25,26)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human kidney NEP at pH 7 |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50197519

((S)-2-{1-[3-(4-fluoro-phenyl)-propylcarbamoyl]-cyc...)Show SMILES COCC[C@H](CC1(CCCC1)C(=O)NCCCc1ccc(F)cc1)C(O)=O Show InChI InChI=1S/C21H30FNO4/c1-27-14-10-17(19(24)25)15-21(11-2-3-12-21)20(26)23-13-4-5-16-6-8-18(22)9-7-16/h6-9,17H,2-5,10-15H2,1H3,(H,23,26)(H,24,25)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human kidney NEP at pH 7 |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50197517

((S)-2-{1-[3-(4-chloro-phenyl)-propylcarbamoyl]-cyc...)Show SMILES COCC[C@H](CC1(CCCC1)C(=O)NCCCc1ccc(Cl)cc1)C(O)=O Show InChI InChI=1S/C21H30ClNO4/c1-27-14-10-17(19(24)25)15-21(11-2-3-12-21)20(26)23-13-4-5-16-6-8-18(22)9-7-16/h6-9,17H,2-5,10-15H2,1H3,(H,23,26)(H,24,25)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NEP |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50197507

((S)-4-methoxy-2-{1-[(1R,2S)-2-(4-methoxy-phenyl)-c...)Show SMILES COCC[C@H](CC1(CCCC1)C(=O)N[C@@H]1C[C@H]1c1ccc(OC)cc1)C(O)=O Show InChI InChI=1S/C22H31NO5/c1-27-12-9-16(20(24)25)14-22(10-3-4-11-22)21(26)23-19-13-18(19)15-5-7-17(28-2)8-6-15/h5-8,16,18-19H,3-4,9-14H2,1-2H3,(H,23,26)(H,24,25)/t16-,18+,19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human kidney NEP at pH 7 |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50197513

((S)-2-((1-((2-(hydroxymethyl)-2,3-dihydro-1H-inden...)Show SMILES COCC[C@H](CC1(CCCC1)C(=O)NC1(CO)Cc2ccccc2C1)C(O)=O Show InChI InChI=1S/C22H31NO5/c1-28-11-8-18(19(25)26)12-21(9-4-5-10-21)20(27)23-22(15-24)13-16-6-2-3-7-17(16)14-22/h2-3,6-7,18,24H,4-5,8-15H2,1H3,(H,23,27)(H,25,26)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human kidney NEP at pH 7.4 |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50190746
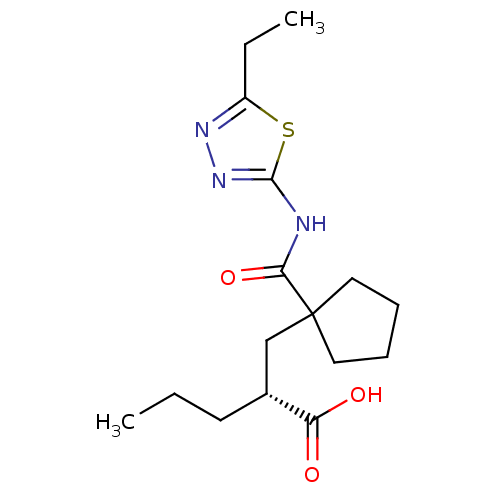
((R)-2-((1-((5-ethyl-1,3,4-thiadiazol-2-yl)carbamoy...)Show SMILES CCC[C@H](CC1(CCCC1)C(=O)Nc1nnc(CC)s1)C(O)=O Show InChI InChI=1S/C16H25N3O3S/c1-3-7-11(13(20)21)10-16(8-5-6-9-16)14(22)17-15-19-18-12(4-2)23-15/h11H,3-10H2,1-2H3,(H,20,21)(H,17,19,22)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of rabbit NEP |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50190746

((R)-2-((1-((5-ethyl-1,3,4-thiadiazol-2-yl)carbamoy...)Show SMILES CCC[C@H](CC1(CCCC1)C(=O)Nc1nnc(CC)s1)C(O)=O Show InChI InChI=1S/C16H25N3O3S/c1-3-7-11(13(20)21)10-16(8-5-6-9-16)14(22)17-15-19-18-12(4-2)23-15/h11H,3-10H2,1-2H3,(H,20,21)(H,17,19,22)/t11-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of rabbit NEP |
J Med Chem 49: 4409-24 (2006)
Article DOI: 10.1021/jm060133g
BindingDB Entry DOI: 10.7270/Q2930SS8 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50197517

((S)-2-{1-[3-(4-chloro-phenyl)-propylcarbamoyl]-cyc...)Show SMILES COCC[C@H](CC1(CCCC1)C(=O)NCCCc1ccc(Cl)cc1)C(O)=O Show InChI InChI=1S/C21H30ClNO4/c1-27-14-10-17(19(24)25)15-21(11-2-3-12-21)20(26)23-13-4-5-16-6-8-18(22)9-7-16/h6-9,17H,2-5,10-15H2,1H3,(H,23,26)(H,24,25)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10.5 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human kidney NEP at pH 7.4 |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50190771
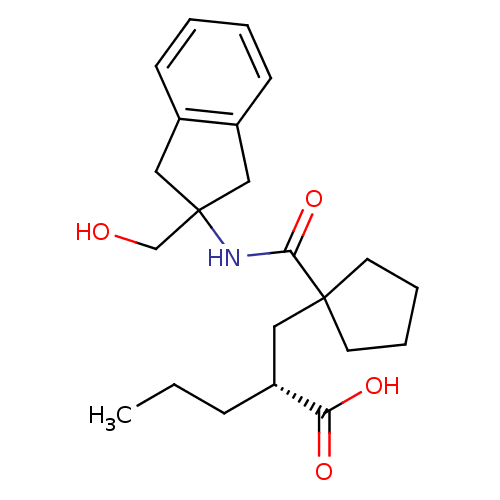
((R)-2-((1-((2-(hydroxymethyl)-2,3-dihydro-1H-inden...)Show SMILES CCC[C@H](CC1(CCCC1)C(=O)NC1(CO)Cc2ccccc2C1)C(O)=O Show InChI InChI=1S/C22H31NO4/c1-2-7-18(19(25)26)12-21(10-5-6-11-21)20(27)23-22(15-24)13-16-8-3-4-9-17(16)14-22/h3-4,8-9,18,24H,2,5-7,10-15H2,1H3,(H,23,27)(H,25,26)/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of dog NEP |
J Med Chem 49: 4409-24 (2006)
Article DOI: 10.1021/jm060133g
BindingDB Entry DOI: 10.7270/Q2930SS8 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50197519

((S)-2-{1-[3-(4-fluoro-phenyl)-propylcarbamoyl]-cyc...)Show SMILES COCC[C@H](CC1(CCCC1)C(=O)NCCCc1ccc(F)cc1)C(O)=O Show InChI InChI=1S/C21H30FNO4/c1-27-14-10-17(19(24)25)15-21(11-2-3-12-21)20(26)23-13-4-5-16-6-8-18(22)9-7-16/h6-9,17H,2-5,10-15H2,1H3,(H,23,26)(H,24,25)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human kidney NEP at pH 7.4 |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50197507

((S)-4-methoxy-2-{1-[(1R,2S)-2-(4-methoxy-phenyl)-c...)Show SMILES COCC[C@H](CC1(CCCC1)C(=O)N[C@@H]1C[C@H]1c1ccc(OC)cc1)C(O)=O Show InChI InChI=1S/C22H31NO5/c1-27-12-9-16(20(24)25)14-22(10-3-4-11-22)21(26)23-19-13-18(19)15-5-7-17(28-2)8-6-15/h5-8,16,18-19H,3-4,9-14H2,1-2H3,(H,23,26)(H,24,25)/t16-,18+,19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13.8 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human kidney NEP at pH 7.4 |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50190746

((R)-2-((1-((5-ethyl-1,3,4-thiadiazol-2-yl)carbamoy...)Show SMILES CCC[C@H](CC1(CCCC1)C(=O)Nc1nnc(CC)s1)C(O)=O Show InChI InChI=1S/C16H25N3O3S/c1-3-7-11(13(20)21)10-16(8-5-6-9-16)14(22)17-15-19-18-12(4-2)23-15/h11H,3-10H2,1-2H3,(H,20,21)(H,17,19,22)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of rat NEP |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50190746

((R)-2-((1-((5-ethyl-1,3,4-thiadiazol-2-yl)carbamoy...)Show SMILES CCC[C@H](CC1(CCCC1)C(=O)Nc1nnc(CC)s1)C(O)=O Show InChI InChI=1S/C16H25N3O3S/c1-3-7-11(13(20)21)10-16(8-5-6-9-16)14(22)17-15-19-18-12(4-2)23-15/h11H,3-10H2,1-2H3,(H,20,21)(H,17,19,22)/t11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of rat NEP |
J Med Chem 49: 4409-24 (2006)
Article DOI: 10.1021/jm060133g
BindingDB Entry DOI: 10.7270/Q2930SS8 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50190746

((R)-2-((1-((5-ethyl-1,3,4-thiadiazol-2-yl)carbamoy...)Show SMILES CCC[C@H](CC1(CCCC1)C(=O)Nc1nnc(CC)s1)C(O)=O Show InChI InChI=1S/C16H25N3O3S/c1-3-7-11(13(20)21)10-16(8-5-6-9-16)14(22)17-15-19-18-12(4-2)23-15/h11H,3-10H2,1-2H3,(H,20,21)(H,17,19,22)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human NEP |
J Med Chem 49: 4409-24 (2006)
Article DOI: 10.1021/jm060133g
BindingDB Entry DOI: 10.7270/Q2930SS8 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50190746

((R)-2-((1-((5-ethyl-1,3,4-thiadiazol-2-yl)carbamoy...)Show SMILES CCC[C@H](CC1(CCCC1)C(=O)Nc1nnc(CC)s1)C(O)=O Show InChI InChI=1S/C16H25N3O3S/c1-3-7-11(13(20)21)10-16(8-5-6-9-16)14(22)17-15-19-18-12(4-2)23-15/h11H,3-10H2,1-2H3,(H,20,21)(H,17,19,22)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18.9 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human kidney NEP at pH 7 |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50190746

((R)-2-((1-((5-ethyl-1,3,4-thiadiazol-2-yl)carbamoy...)Show SMILES CCC[C@H](CC1(CCCC1)C(=O)Nc1nnc(CC)s1)C(O)=O Show InChI InChI=1S/C16H25N3O3S/c1-3-7-11(13(20)21)10-16(8-5-6-9-16)14(22)17-15-19-18-12(4-2)23-15/h11H,3-10H2,1-2H3,(H,20,21)(H,17,19,22)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NEP |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50190746

((R)-2-((1-((5-ethyl-1,3,4-thiadiazol-2-yl)carbamoy...)Show SMILES CCC[C@H](CC1(CCCC1)C(=O)Nc1nnc(CC)s1)C(O)=O Show InChI InChI=1S/C16H25N3O3S/c1-3-7-11(13(20)21)10-16(8-5-6-9-16)14(22)17-15-19-18-12(4-2)23-15/h11H,3-10H2,1-2H3,(H,20,21)(H,17,19,22)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant NEP |
J Med Chem 49: 4409-24 (2006)
Article DOI: 10.1021/jm060133g
BindingDB Entry DOI: 10.7270/Q2930SS8 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50190746

((R)-2-((1-((5-ethyl-1,3,4-thiadiazol-2-yl)carbamoy...)Show SMILES CCC[C@H](CC1(CCCC1)C(=O)Nc1nnc(CC)s1)C(O)=O Show InChI InChI=1S/C16H25N3O3S/c1-3-7-11(13(20)21)10-16(8-5-6-9-16)14(22)17-15-19-18-12(4-2)23-15/h11H,3-10H2,1-2H3,(H,20,21)(H,17,19,22)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of dog NEP |
J Med Chem 49: 4409-24 (2006)
Article DOI: 10.1021/jm060133g
BindingDB Entry DOI: 10.7270/Q2930SS8 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50190753
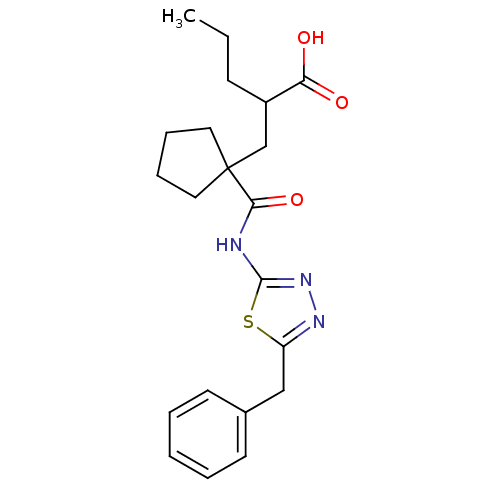
(2-((1-((5-benzyl-1,3,4-thiadiazol-2-yl)carbamoyl)c...)Show SMILES CCCC(CC1(CCCC1)C(=O)Nc1nnc(Cc2ccccc2)s1)C(O)=O Show InChI InChI=1S/C21H27N3O3S/c1-2-8-16(18(25)26)14-21(11-6-7-12-21)19(27)22-20-24-23-17(28-20)13-15-9-4-3-5-10-15/h3-5,9-10,16H,2,6-8,11-14H2,1H3,(H,25,26)(H,22,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of dog NEP |
J Med Chem 49: 4409-24 (2006)
Article DOI: 10.1021/jm060133g
BindingDB Entry DOI: 10.7270/Q2930SS8 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50190746

((R)-2-((1-((5-ethyl-1,3,4-thiadiazol-2-yl)carbamoy...)Show SMILES CCC[C@H](CC1(CCCC1)C(=O)Nc1nnc(CC)s1)C(O)=O Show InChI InChI=1S/C16H25N3O3S/c1-3-7-11(13(20)21)10-16(8-5-6-9-16)14(22)17-15-19-18-12(4-2)23-15/h11H,3-10H2,1-2H3,(H,20,21)(H,17,19,22)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31.8 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human kidney NEP at pH 7.4 |
Bioorg Med Chem 15: 142-59 (2006)
Article DOI: 10.1016/j.bmc.2006.10.002
BindingDB Entry DOI: 10.7270/Q2ZS2W4J |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50190762
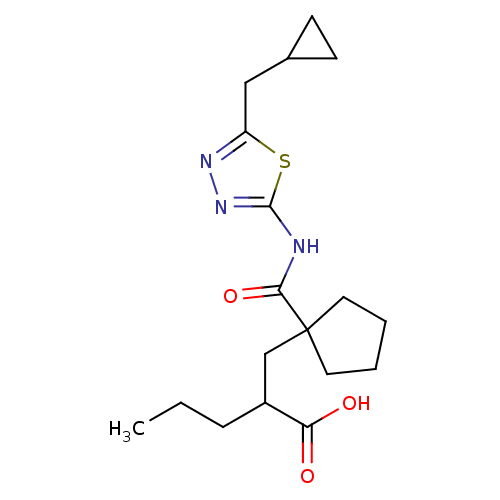
(2-((1-((5-(cyclopropylmethyl)-1,3,4-thiadiazol-2-y...)Show SMILES CCCC(CC1(CCCC1)C(=O)Nc1nnc(CC2CC2)s1)C(O)=O Show InChI InChI=1S/C18H27N3O3S/c1-2-5-13(15(22)23)11-18(8-3-4-9-18)16(24)19-17-21-20-14(25-17)10-12-6-7-12/h12-13H,2-11H2,1H3,(H,22,23)(H,19,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of dog NEP |
J Med Chem 49: 4409-24 (2006)
Article DOI: 10.1021/jm060133g
BindingDB Entry DOI: 10.7270/Q2930SS8 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50190737

(2-((1-((5-ethyl-1,3,4-thiadiazol-2-yl)carbamoyl)cy...)Show SMILES CCc1nnc(NC(=O)C2(CC(CCc3ccccc3)C(O)=O)CCCC2)s1 Show InChI InChI=1S/C21H27N3O3S/c1-2-17-23-24-20(28-17)22-19(27)21(12-6-7-13-21)14-16(18(25)26)11-10-15-8-4-3-5-9-15/h3-5,8-9,16H,2,6-7,10-14H2,1H3,(H,25,26)(H,22,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of dog NEP |
J Med Chem 49: 4409-24 (2006)
Article DOI: 10.1021/jm060133g
BindingDB Entry DOI: 10.7270/Q2930SS8 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50190773
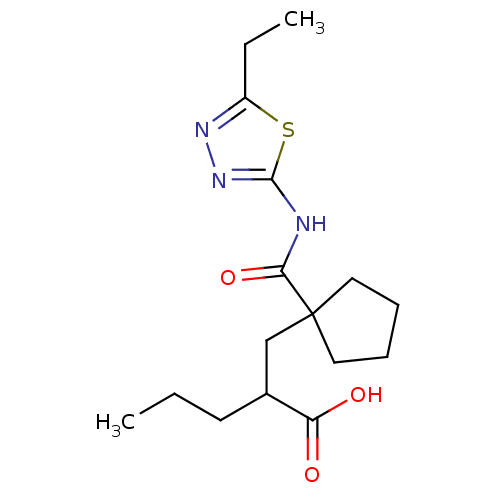
(2-[1-(5-ethyl-[1,3,4]thiadiazol-2-ylcarbamoyl)-cyc...)Show InChI InChI=1S/C16H25N3O3S/c1-3-7-11(13(20)21)10-16(8-5-6-9-16)14(22)17-15-19-18-12(4-2)23-15/h11H,3-10H2,1-2H3,(H,20,21)(H,17,19,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of dog NEP |
J Med Chem 49: 4409-24 (2006)
Article DOI: 10.1021/jm060133g
BindingDB Entry DOI: 10.7270/Q2930SS8 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50190752
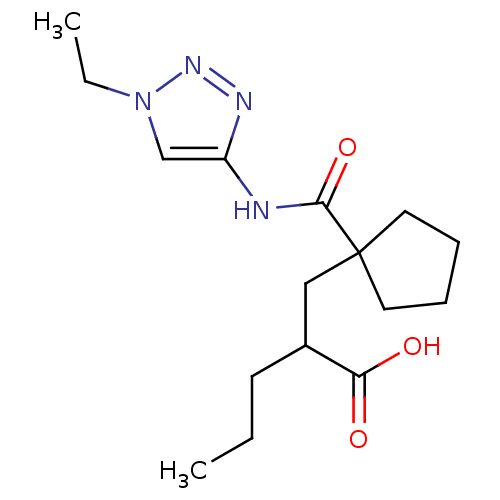
(2-((1-((1-ethyl-1H-1,2,3-triazol-4-yl)carbamoyl)cy...)Show InChI InChI=1S/C16H26N4O3/c1-3-7-12(14(21)22)10-16(8-5-6-9-16)15(23)17-13-11-20(4-2)19-18-13/h11-12H,3-10H2,1-2H3,(H,17,23)(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of dog NEP |
J Med Chem 49: 4409-24 (2006)
Article DOI: 10.1021/jm060133g
BindingDB Entry DOI: 10.7270/Q2930SS8 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50190764
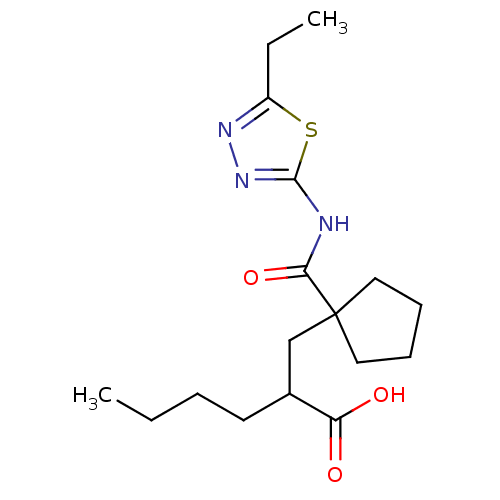
(2-((1-((5-ethyl-1,3,4-thiadiazol-2-yl)carbamoyl)cy...)Show InChI InChI=1S/C17H27N3O3S/c1-3-5-8-12(14(21)22)11-17(9-6-7-10-17)15(23)18-16-20-19-13(4-2)24-16/h12H,3-11H2,1-2H3,(H,21,22)(H,18,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of dog NEP |
J Med Chem 49: 4409-24 (2006)
Article DOI: 10.1021/jm060133g
BindingDB Entry DOI: 10.7270/Q2930SS8 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50190750

(2-((1-((4-benzylpyridin-2-yl)carbamoyl)cyclopentyl...)Show SMILES CCCC(CC1(CCCC1)C(=O)Nc1cc(Cc2ccccc2)ccn1)C(O)=O Show InChI InChI=1S/C24H30N2O3/c1-2-8-20(22(27)28)17-24(12-6-7-13-24)23(29)26-21-16-19(11-14-25-21)15-18-9-4-3-5-10-18/h3-5,9-11,14,16,20H,2,6-8,12-13,15,17H2,1H3,(H,27,28)(H,25,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of dog NEP |
J Med Chem 49: 4409-24 (2006)
Article DOI: 10.1021/jm060133g
BindingDB Entry DOI: 10.7270/Q2930SS8 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50190769
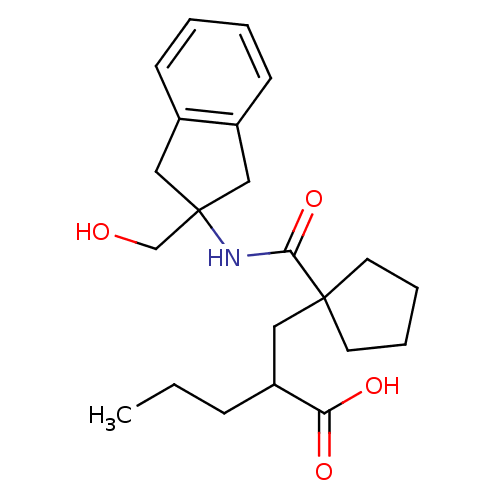
(CHEMBL377037 | rac-2-((1-((2-(hydroxymethyl)-2,3-d...)Show SMILES CCCC(CC1(CCCC1)C(=O)NC1(CO)Cc2ccccc2C1)C(O)=O Show InChI InChI=1S/C22H31NO4/c1-2-7-18(19(25)26)12-21(10-5-6-11-21)20(27)23-22(15-24)13-16-8-3-4-9-17(16)14-22/h3-4,8-9,18,24H,2,5-7,10-15H2,1H3,(H,23,27)(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of dog NEP |
J Med Chem 49: 4409-24 (2006)
Article DOI: 10.1021/jm060133g
BindingDB Entry DOI: 10.7270/Q2930SS8 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50190739

((R)-2-((1-((5-methyl-1,3,4-thiadiazol-2-yl)carbamo...)Show InChI InChI=1S/C15H23N3O3S/c1-3-6-11(12(19)20)9-15(7-4-5-8-15)13(21)16-14-18-17-10(2)22-14/h11H,3-9H2,1-2H3,(H,19,20)(H,16,18,21)/t11-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of dog NEP |
J Med Chem 49: 4409-24 (2006)
Article DOI: 10.1021/jm060133g
BindingDB Entry DOI: 10.7270/Q2930SS8 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50190738

(2-((1-((5-ethyl-1,3,4-thiadiazol-2-yl)carbamoyl)cy...)Show SMILES CCc1nnc(NC(=O)C2(CC(CCC(C)C)C(O)=O)CCCC2)s1 Show InChI InChI=1S/C18H29N3O3S/c1-4-14-20-21-17(25-14)19-16(24)18(9-5-6-10-18)11-13(15(22)23)8-7-12(2)3/h12-13H,4-11H2,1-3H3,(H,22,23)(H,19,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of dog NEP |
J Med Chem 49: 4409-24 (2006)
Article DOI: 10.1021/jm060133g
BindingDB Entry DOI: 10.7270/Q2930SS8 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50190758
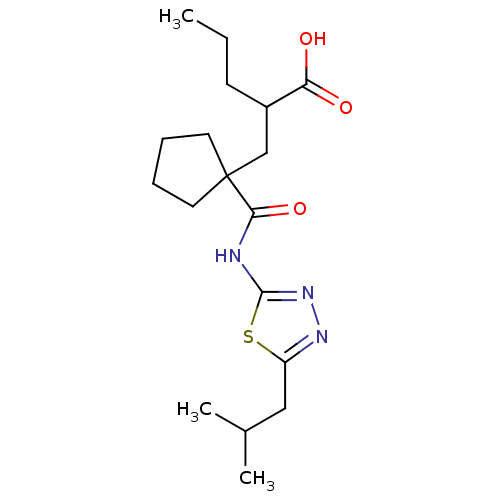
(2-((1-((5-isobutyl-1,3,4-thiadiazol-2-yl)carbamoyl...)Show SMILES CCCC(CC1(CCCC1)C(=O)Nc1nnc(CC(C)C)s1)C(O)=O Show InChI InChI=1S/C18H29N3O3S/c1-4-7-13(15(22)23)11-18(8-5-6-9-18)16(24)19-17-21-20-14(25-17)10-12(2)3/h12-13H,4-11H2,1-3H3,(H,22,23)(H,19,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of dog NEP |
J Med Chem 49: 4409-24 (2006)
Article DOI: 10.1021/jm060133g
BindingDB Entry DOI: 10.7270/Q2930SS8 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50190757
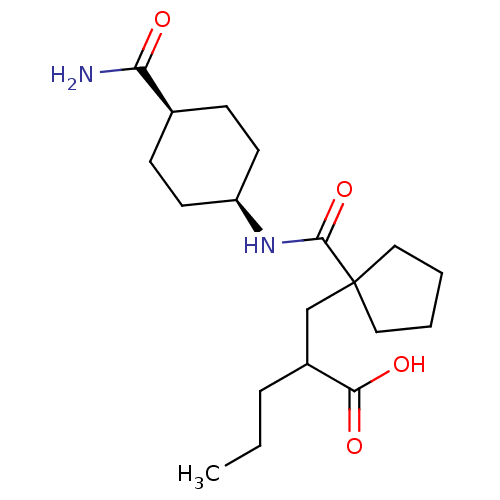
(CHEMBL439508 | cis-2-((1-((4-carbamoylcyclohexyl)c...)Show SMILES CCCC(CC1(CCCC1)C(=O)N[C@H]1CC[C@H](CC1)C(N)=O)C(O)=O |wU:13.13,16.20,(-5.69,-23.39,;-5.69,-24.93,;-4.36,-25.7,;-4.36,-27.24,;-3.03,-28.01,;-1.69,-27.24,;-2.94,-26.34,;-2.46,-24.87,;-.92,-24.87,;-.45,-26.34,;-.36,-28.01,;-.36,-29.55,;.97,-27.24,;2.31,-28.01,;3.63,-27.24,;4.97,-28.02,;4.96,-29.56,;3.62,-30.32,;2.3,-29.55,;6.29,-30.33,;7.63,-29.57,;6.29,-31.87,;-5.69,-28.01,;-7.03,-27.24,;-5.69,-29.55,)| Show InChI InChI=1S/C19H32N2O4/c1-2-5-14(17(23)24)12-19(10-3-4-11-19)18(25)21-15-8-6-13(7-9-15)16(20)22/h13-15H,2-12H2,1H3,(H2,20,22)(H,21,25)(H,23,24)/t13-,14?,15+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of dog NEP |
J Med Chem 49: 4409-24 (2006)
Article DOI: 10.1021/jm060133g
BindingDB Entry DOI: 10.7270/Q2930SS8 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50190761

(CHEMBL209493 | rac-2-((1-((5-methyl-1,3,4-thiadiaz...)Show InChI InChI=1S/C15H23N3O3S/c1-3-6-11(12(19)20)9-15(7-4-5-8-15)13(21)16-14-18-17-10(2)22-14/h11H,3-9H2,1-2H3,(H,19,20)(H,16,18,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 176 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of dog NEP |
J Med Chem 49: 4409-24 (2006)
Article DOI: 10.1021/jm060133g
BindingDB Entry DOI: 10.7270/Q2930SS8 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50190780

(2-((1-((4-butylpyridin-2-yl)carbamoyl)cyclopentyl)...)Show InChI InChI=1S/C21H32N2O3/c1-3-5-9-16-10-13-22-18(14-16)23-20(26)21(11-6-7-12-21)15-17(8-4-2)19(24)25/h10,13-14,17H,3-9,11-12,15H2,1-2H3,(H,24,25)(H,22,23,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 184 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of dog NEP |
J Med Chem 49: 4409-24 (2006)
Article DOI: 10.1021/jm060133g
BindingDB Entry DOI: 10.7270/Q2930SS8 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50190777
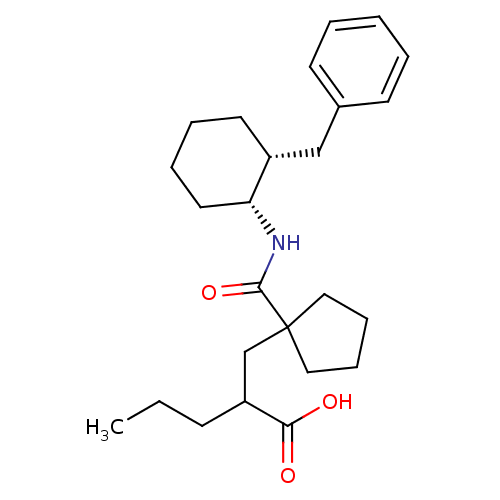
(2-((1-(((1R,2R)-2-benzylcyclohexyl)carbamoyl)cyclo...)Show SMILES CCCC(CC1(CCCC1)C(=O)N[C@@H]1CCCC[C@@H]1Cc1ccccc1)C(O)=O Show InChI InChI=1S/C25H37NO3/c1-2-10-21(23(27)28)18-25(15-8-9-16-25)24(29)26-22-14-7-6-13-20(22)17-19-11-4-3-5-12-19/h3-5,11-12,20-22H,2,6-10,13-18H2,1H3,(H,26,29)(H,27,28)/t20-,21?,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of dog NEP |
J Med Chem 49: 4409-24 (2006)
Article DOI: 10.1021/jm060133g
BindingDB Entry DOI: 10.7270/Q2930SS8 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50190743
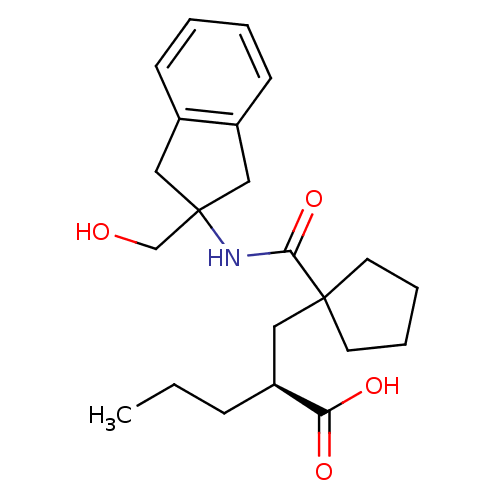
((S)-2-((1-((2-(hydroxymethyl)-2,3-dihydro-1H-inden...)Show SMILES CCC[C@@H](CC1(CCCC1)C(=O)NC1(CO)Cc2ccccc2C1)C(O)=O Show InChI InChI=1S/C22H31NO4/c1-2-7-18(19(25)26)12-21(10-5-6-11-21)20(27)23-22(15-24)13-16-8-3-4-9-17(16)14-22/h3-4,8-9,18,24H,2,5-7,10-15H2,1H3,(H,23,27)(H,25,26)/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 205 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of dog NEP |
J Med Chem 49: 4409-24 (2006)
Article DOI: 10.1021/jm060133g
BindingDB Entry DOI: 10.7270/Q2930SS8 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Homo sapiens (Human)) | BDBM50190782
(2-((1-(((1R,3S)-3-carbamoylcyclopentyl)carbamoyl)c...)Show SMILES CCCC(CC1(CCCC1)C(=O)N[C@@H]1CC[C@@H](C1)C(N)=O)C(O)=O Show InChI InChI=1S/C18H30N2O4/c1-2-5-13(16(22)23)11-18(8-3-4-9-18)17(24)20-14-7-6-12(10-14)15(19)21/h12-14H,2-11H2,1H3,(H2,19,21)(H,20,24)(H,22,23)/t12-,13?,14+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 213 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of dog NEP |
J Med Chem 49: 4409-24 (2006)
Article DOI: 10.1021/jm060133g
BindingDB Entry DOI: 10.7270/Q2930SS8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data