

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini-CNR Curated by ChEMBL | Assay Description Inhibition of S-glutathionylated form of human recombinant carbonic anhydrase 7 using carbon-dioxide as substrate preincubated for 10 mins prior to s... | Bioorg Med Chem Lett 22: 1560-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.134 BindingDB Entry DOI: 10.7270/Q2NK3FHM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini-CNR Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 7 using carbon-dioxide as substrate preincubated for 10 mins prior to substrate addition by stoppe... | Bioorg Med Chem Lett 22: 1560-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.134 BindingDB Entry DOI: 10.7270/Q2NK3FHM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acylamino-acid-releasing enzyme (Sus scrofa) | BDBM50382729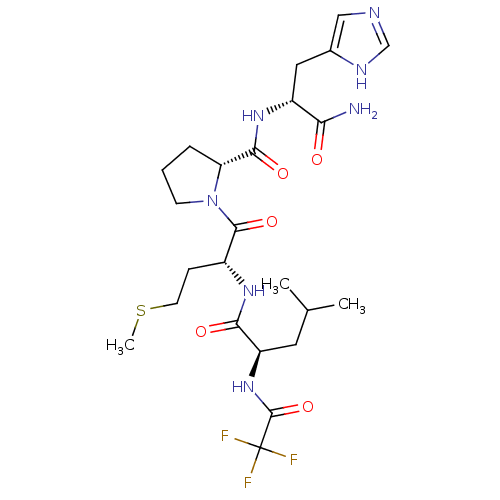 (CHEMBL2023560) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini Curated by ChEMBL | Assay Description Inhibition of pig APEH using acetyl-Ala-pNA as substrate incubated for 2 mins prior to substrate addition by spectrophotometry | J Med Chem 55: 2102-11 (2012) Article DOI: 10.1021/jm2013375 BindingDB Entry DOI: 10.7270/Q2BZ672F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 3 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 2.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini-CNR Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 3 using carbon-dioxide as substrate preincubated for 10 mins prior to substrate addition by stoppe... | Bioorg Med Chem Lett 22: 1560-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.134 BindingDB Entry DOI: 10.7270/Q2NK3FHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 265 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini-CNR Curated by ChEMBL | Assay Description Inhibition of S-glutathionylated form of human recombinant carbonic anhydrase 7 hydrolysis activity using 4-nitrophenyl acetate as substrate preincub... | Bioorg Med Chem Lett 22: 1560-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.134 BindingDB Entry DOI: 10.7270/Q2NK3FHM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 357 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini-CNR Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 7 hydrolysis activity using 4-nitrophenyl acetate as substrate preincubated for 10 mins prior to s... | Bioorg Med Chem Lett 22: 1560-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.134 BindingDB Entry DOI: 10.7270/Q2NK3FHM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 3 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini-CNR Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 3 hydrolysis activity using 4-nitrophenyl acetate as substrate preincubated for 10 mins prior to s... | Bioorg Med Chem Lett 22: 1560-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.134 BindingDB Entry DOI: 10.7270/Q2NK3FHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 2.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini-CNR Curated by ChEMBL | Assay Description Inhibition of S-glutathionylated form of human recombinant carbonic anhydrase 7 phosphatase activity using 4-nitrophenyl phosphate as substrate prein... | Bioorg Med Chem Lett 22: 1560-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.134 BindingDB Entry DOI: 10.7270/Q2NK3FHM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 3.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini-CNR Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 7 phosphatase activity using 4-nitrophenyl phosphate as substrate preincubated for 10 mins prior t... | Bioorg Med Chem Lett 22: 1560-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.134 BindingDB Entry DOI: 10.7270/Q2NK3FHM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 3 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini-CNR Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 3 phosphatase activity using 4-nitrophenyl phosphate as substrate preincubated for 10 mins prior t... | Bioorg Med Chem Lett 22: 1560-4 (2012) Article DOI: 10.1016/j.bmcl.2011.12.134 BindingDB Entry DOI: 10.7270/Q2NK3FHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acylamino-acid-releasing enzyme (Sus scrofa) | BDBM50382729 (CHEMBL2023560) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto di Biostrutture e Bioimmagini Curated by ChEMBL | Assay Description Inhibition of pig APEH using acetyl-Ala-pNA as substrate incubated for 2 mins prior to substrate addition by spectrophotometry | J Med Chem 55: 2102-11 (2012) Article DOI: 10.1021/jm2013375 BindingDB Entry DOI: 10.7270/Q2BZ672F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50428477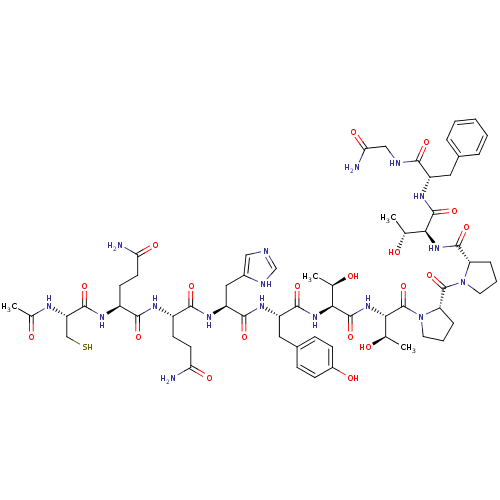 (CHEMBL2335173) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a |
Institute of Biostructures and Bioimages Curated by ChEMBL | Assay Description Binding affinity to synthetic peptide mimicking HER2 subdomain 4 (unknown origin) by fluorescence spectroscopic analysis | Eur J Med Chem 61: 116-21 (2013) Article DOI: 10.1016/j.ejmech.2012.09.024 BindingDB Entry DOI: 10.7270/Q2R78GK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50428475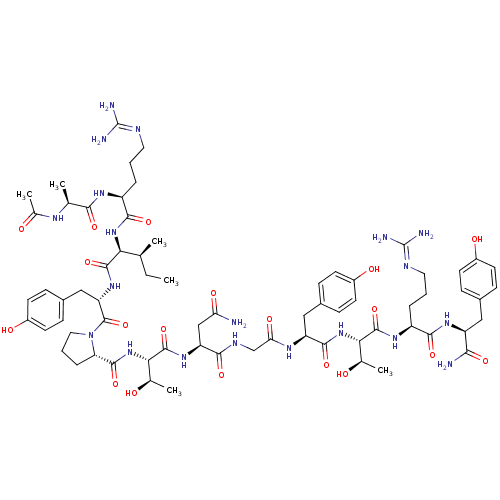 (CHEMBL2335174) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 446 | n/a | n/a | n/a | n/a | n/a |
Institute of Biostructures and Bioimages Curated by ChEMBL | Assay Description Binding affinity to synthetic peptide mimicking HER2 subdomain 4 (unknown origin) by fluorescence spectroscopic analysis | Eur J Med Chem 61: 116-21 (2013) Article DOI: 10.1016/j.ejmech.2012.09.024 BindingDB Entry DOI: 10.7270/Q2R78GK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50428476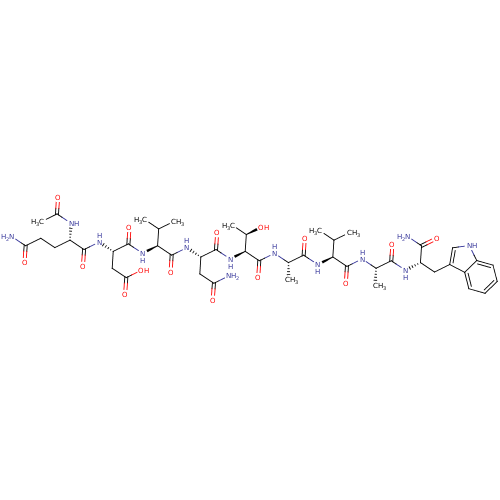 (CHEMBL2335172) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a |
Institute of Biostructures and Bioimages Curated by ChEMBL | Assay Description Binding affinity to synthetic peptide mimicking HER2 subdomain 4 (unknown origin) by fluorescence spectroscopic analysis | Eur J Med Chem 61: 116-21 (2013) Article DOI: 10.1016/j.ejmech.2012.09.024 BindingDB Entry DOI: 10.7270/Q2R78GK9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||