

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050525 ((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 50: 2391-8 (2007) Article DOI: 10.1021/jm061321+ BindingDB Entry DOI: 10.7270/Q2NC6407 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 50: 2391-8 (2007) Article DOI: 10.1021/jm061321+ BindingDB Entry DOI: 10.7270/Q2NC6407 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050525 ((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050511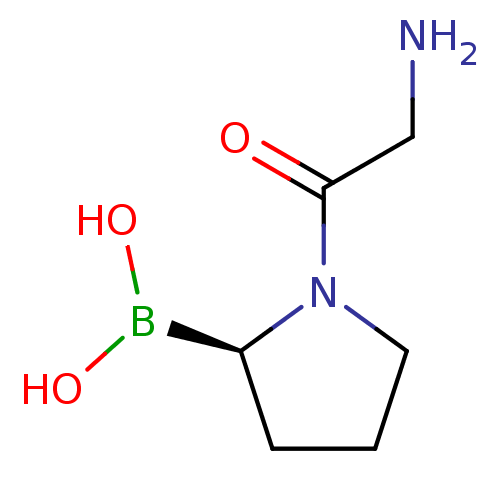 ((R)-1-(2-aminoacetyl)pyrrolidin-2-ylboronic acid |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50050511 ((R)-1-(2-aminoacetyl)pyrrolidin-2-ylboronic acid |...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP9 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50050525 ((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP9 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50431227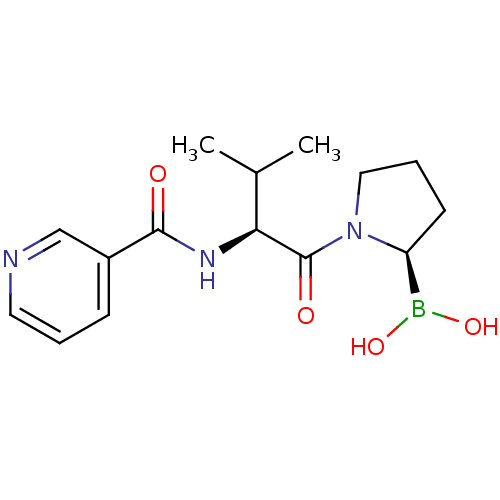 (CHEMBL2333024) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Sackler School of Biomedical Sciences Curated by ChEMBL | Assay Description Competitive inhibition of human PREP using Suc-GP-AMC as substrate by Lineweaver-Burk plot analysis | J Med Chem 56: 3467-77 (2013) Article DOI: 10.1021/jm400351a BindingDB Entry DOI: 10.7270/Q2C53N7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP9 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP8 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50253621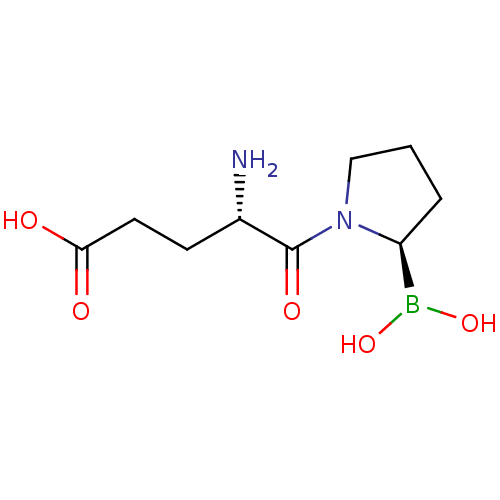 ((S)-4-amino-5-((R)-2-boronopyrrolidin-1-yl)-5-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM50050525 ((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP8 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50476305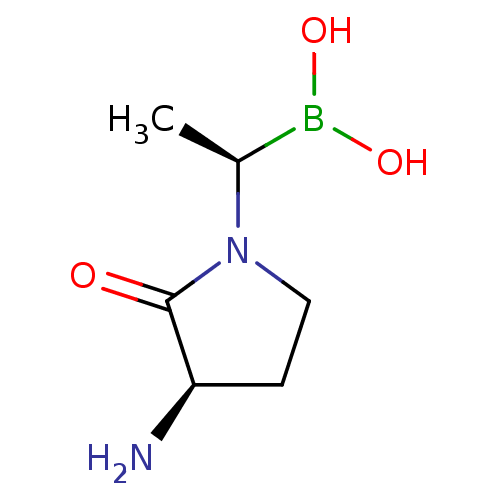 (CHEMBL2068511) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 50: 2391-8 (2007) Article DOI: 10.1021/jm061321+ BindingDB Entry DOI: 10.7270/Q2NC6407 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50253621 ((S)-4-amino-5-((R)-2-boronopyrrolidin-1-yl)-5-oxop...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP9 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM50050511 ((R)-1-(2-aminoacetyl)pyrrolidin-2-ylboronic acid |...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP8 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50171556 ((R)-1-((S)-2-amino-3-methylbutanamido)ethylboronic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50253639 ((S)-4-amino-5-((R)-1-boronoethylamino)-5-oxopentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM50431242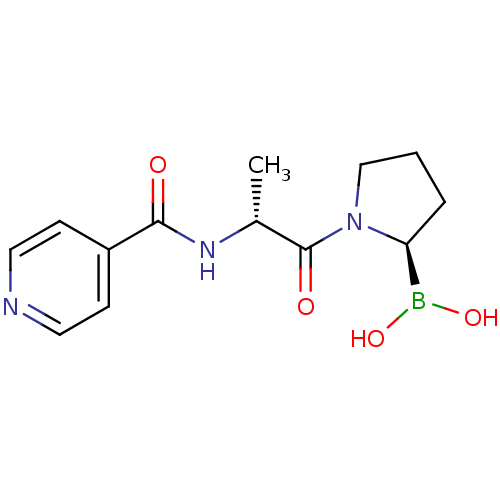 (CHEMBL2333026) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Sackler School of Biomedical Sciences Curated by ChEMBL | Assay Description Competitive inhibition of human FAP using Suc-GP-AMC as substrate by Lineweaver-Burk plot analysis | J Med Chem 56: 3467-77 (2013) Article DOI: 10.1021/jm400351a BindingDB Entry DOI: 10.7270/Q2C53N7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM50253621 ((S)-4-amino-5-((R)-2-boronopyrrolidin-1-yl)-5-oxop...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP8 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50253640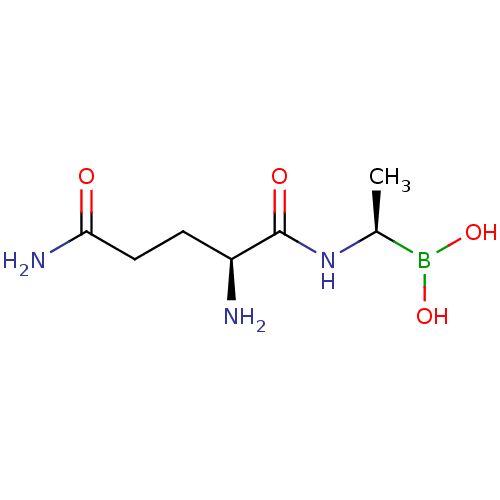 ((R)-1-((S)-2,5-diamino-5-oxopentanamido)ethylboron...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50171556 ((R)-1-((S)-2-amino-3-methylbutanamido)ethylboronic...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP9 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM50171556 ((R)-1-((S)-2-amino-3-methylbutanamido)ethylboronic...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP8 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM50253640 ((R)-1-((S)-2,5-diamino-5-oxopentanamido)ethylboron...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP8 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50253640 ((R)-1-((S)-2,5-diamino-5-oxopentanamido)ethylboron...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP9 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM50253639 ((S)-4-amino-5-((R)-1-boronoethylamino)-5-oxopentan...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP8 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50476304 (CHEMBL2068512) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 50: 2391-8 (2007) Article DOI: 10.1021/jm061321+ BindingDB Entry DOI: 10.7270/Q2NC6407 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50253639 ((S)-4-amino-5-((R)-1-boronoethylamino)-5-oxopentan...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP9 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50431242 (CHEMBL2333026) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Sackler School of Biomedical Sciences Curated by ChEMBL | Assay Description Competitive inhibition of human PREP using Suc-GP-AMC as substrate by Lineweaver-Burk plot analysis | J Med Chem 56: 3467-77 (2013) Article DOI: 10.1021/jm400351a BindingDB Entry DOI: 10.7270/Q2C53N7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50253641 ((R)-1-((S)-2-acetamido-3-methylbutanoyl)pyrrolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50253641 ((R)-1-((S)-2-acetamido-3-methylbutanoyl)pyrrolidin...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP9 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50005111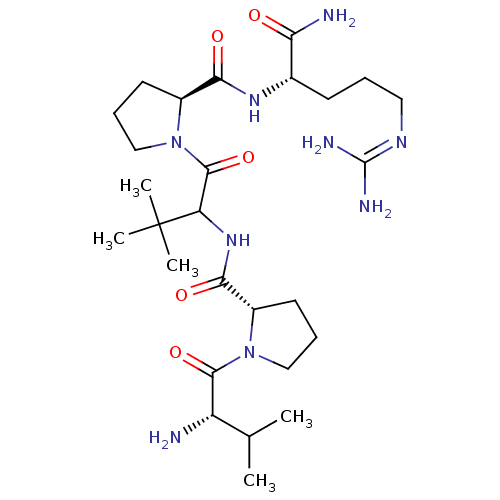 (CHEMBL3086656) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant DPP4 using AP-pNA as substrate preincubated for 30 mins followed by substrate addition measured after 30 ... | J Med Chem 56: 8339-51 (2013) Article DOI: 10.1021/jm400423p BindingDB Entry DOI: 10.7270/Q2PK0HMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50005109 (CHEMBL3086660) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant DPP4 using AP-pNA as substrate preincubated for 30 mins followed by substrate addition measured after 30 ... | J Med Chem 56: 8339-51 (2013) Article DOI: 10.1021/jm400423p BindingDB Entry DOI: 10.7270/Q2PK0HMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50005107 (CHEMBL3086661) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant DPP4 using AP-pNA as substrate preincubated for 30 mins followed by substrate addition measured after 30 ... | J Med Chem 56: 8339-51 (2013) Article DOI: 10.1021/jm400423p BindingDB Entry DOI: 10.7270/Q2PK0HMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM50253641 ((R)-1-((S)-2-acetamido-3-methylbutanoyl)pyrrolidin...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP8 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50005112 (CHEMBL3086658) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant DPP4 using AP-pNA as substrate preincubated for 30 mins followed by substrate addition measured after 30 ... | J Med Chem 56: 8339-51 (2013) Article DOI: 10.1021/jm400423p BindingDB Entry DOI: 10.7270/Q2PK0HMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50005110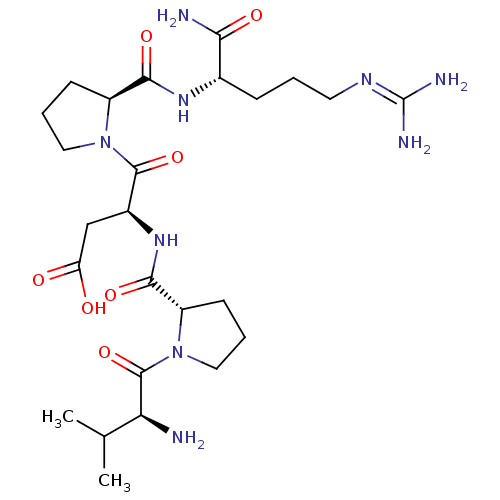 (CHEMBL3086657) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant DPP4 using AP-pNA as substrate preincubated for 30 mins followed by substrate addition measured after 30 ... | J Med Chem 56: 8339-51 (2013) Article DOI: 10.1021/jm400423p BindingDB Entry DOI: 10.7270/Q2PK0HMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50389480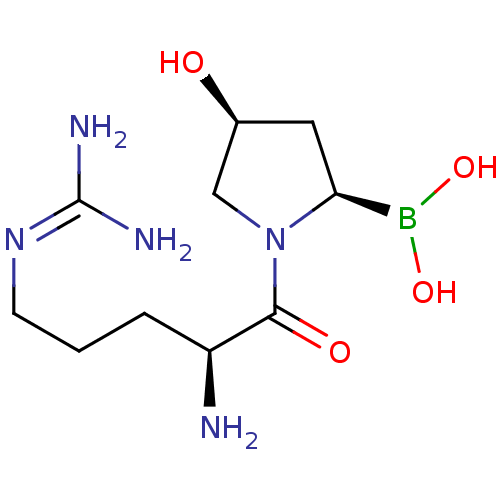 (CHEMBL2063040) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human DPP4 expressed in HEK293 cells using Ala-Pro-p-nitroanilide hydrochloride salt as substrate preincubated for 10 mins prior to add... | Bioorg Med Chem Lett 22: 5536-40 (2012) Article DOI: 10.1016/j.bmcl.2012.07.033 BindingDB Entry DOI: 10.7270/Q2M909QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50389480 (CHEMBL2063040) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human DPP9 expressed in HEK293 cells using Ala-Pro-p-nitroanilide hydrochloride salt as substrate preincubated for 10 mins prior to add... | Bioorg Med Chem Lett 22: 5536-40 (2012) Article DOI: 10.1016/j.bmcl.2012.07.033 BindingDB Entry DOI: 10.7270/Q2M909QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050525 ((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human DPP4 expressed in HEK293 cells using Ala-Pro-p-nitroanilide hydrochloride salt as substrate preincubated for 10 mins prior to add... | Bioorg Med Chem Lett 22: 5536-40 (2012) Article DOI: 10.1016/j.bmcl.2012.07.033 BindingDB Entry DOI: 10.7270/Q2M909QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM50431243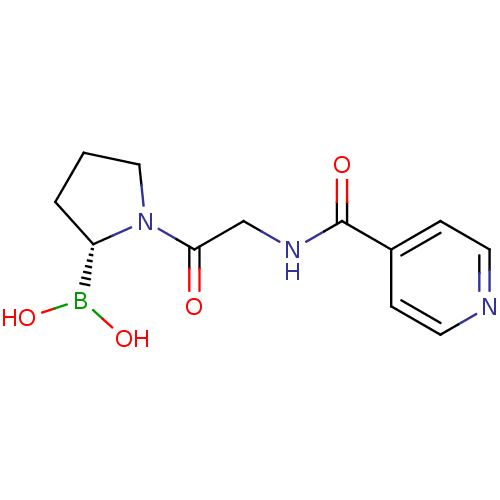 (CHEMBL2333025) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Sackler School of Biomedical Sciences Curated by ChEMBL | Assay Description Inhibition of human FAP using Z-Gly-Pro-AMC as substrate preincubated for 10 mins prior to substrate addition by fluorescence assay | J Med Chem 56: 3467-77 (2013) Article DOI: 10.1021/jm400351a BindingDB Entry DOI: 10.7270/Q2C53N7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050525 ((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 at pH 2 | J Med Chem 50: 2391-8 (2007) Article DOI: 10.1021/jm061321+ BindingDB Entry DOI: 10.7270/Q2NC6407 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50341487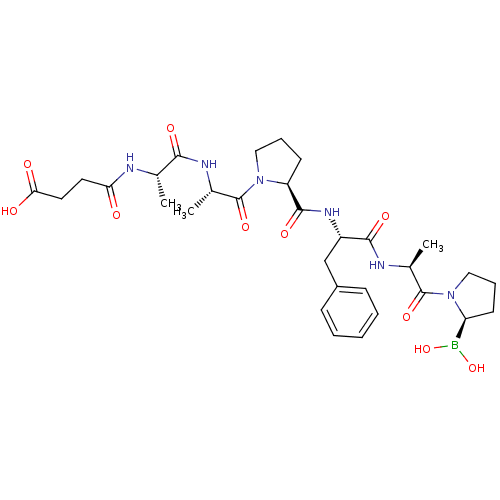 (CHEMBL1765236 | Succinyl-alaninyl-alaninyl-proliny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Tufts University Sackler School of Graduate Biomedical Sciences Curated by ChEMBL | Assay Description Inhibition of DDP4 at pH 7.4 preincubated with alpha-chymotrypsin up to 8 hrs | J Med Chem 54: 2022-8 (2011) Article DOI: 10.1021/jm100972f BindingDB Entry DOI: 10.7270/Q23F4PXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Sackler School of Biomedical Sciences Curated by ChEMBL | Assay Description Inhibition of human DPP4 using H-Gly-Pro-AMC as substrate preincubated for 10 mins prior to substrate addition by fluorescence assay | J Med Chem 56: 3467-77 (2013) Article DOI: 10.1021/jm400351a BindingDB Entry DOI: 10.7270/Q2C53N7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050525 ((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 2.0 | n/a |
Tufts University Sackler School of Graduate Biomedical Sciences Curated by ChEMBL | Assay Description Inhibition of DDP4 at pH 2 preincubated for 10 mins | J Med Chem 54: 2022-8 (2011) Article DOI: 10.1021/jm100972f BindingDB Entry DOI: 10.7270/Q23F4PXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM50431228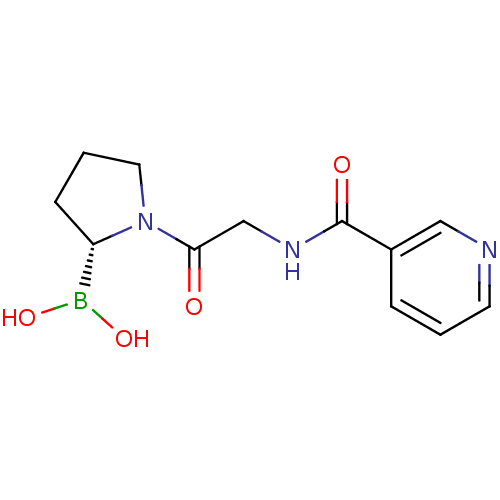 (CHEMBL2333023) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Sackler School of Biomedical Sciences Curated by ChEMBL | Assay Description Inhibition of human FAP using Z-Gly-Pro-AMC as substrate preincubated for 10 mins prior to substrate addition by fluorescence assay | J Med Chem 56: 3467-77 (2013) Article DOI: 10.1021/jm400351a BindingDB Entry DOI: 10.7270/Q2C53N7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM50389480 (CHEMBL2063040) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human DPP8 expressed in HEK293 cells using Ala-Pro-p-nitroanilide hydrochloride salt as substrate preincubated for 10 mins prior to add... | Bioorg Med Chem Lett 22: 5536-40 (2012) Article DOI: 10.1016/j.bmcl.2012.07.033 BindingDB Entry DOI: 10.7270/Q2M909QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50431227 (CHEMBL2333024) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Sackler School of Biomedical Sciences Curated by ChEMBL | Assay Description Inhibition of human PREP using Z-Gly-Pro-AMC as substrate preincubated for 10 mins prior to substrate addition by fluorescence assay | J Med Chem 56: 3467-77 (2013) Article DOI: 10.1021/jm400351a BindingDB Entry DOI: 10.7270/Q2C53N7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon-like peptide 1 receptor (Homo sapiens (Human)) | BDBM50152769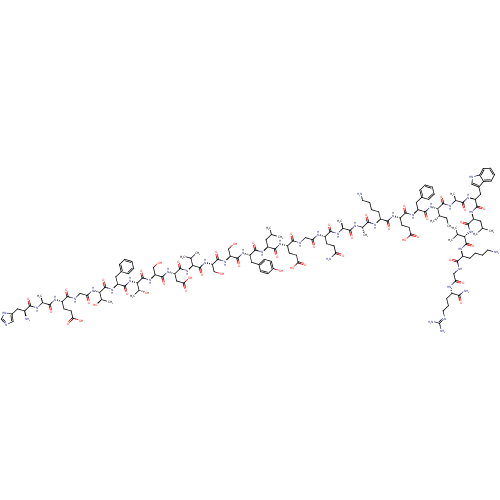 (CHEMBL410972 | GLP-1(7-36)-NH2 | GLP-17-(7-36) der...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Displacement of [125I]-exendin (9 to 39) from human GLP-1R expressed in African green monkey COS7 cells by liquid scintillation counting analysis | J Med Chem 56: 8339-51 (2013) Article DOI: 10.1021/jm400423p BindingDB Entry DOI: 10.7270/Q2PK0HMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 2.0 | n/a |
Tufts University Sackler School of Graduate Biomedical Sciences Curated by ChEMBL | Assay Description Inhibition of DDP4 at pH 2 preincubated for 10 mins | J Med Chem 54: 2022-8 (2011) Article DOI: 10.1021/jm100972f BindingDB Entry DOI: 10.7270/Q23F4PXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 at pH 2 | J Med Chem 50: 2391-8 (2007) Article DOI: 10.1021/jm061321+ BindingDB Entry DOI: 10.7270/Q2NC6407 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 188 total ) | Next | Last >> |