

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50324676 ((R)-3-amino-4-(3-hexylphenylamino)-4-oxobutylphosp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human S1P1 receptor expressed in CHO cells assessed as inhibition of SEW2871-induced [35S]GTPgamma binding | Nat Chem Biol 2: 434-41 (2006) Article DOI: 10.1038/nchembio804 BindingDB Entry DOI: 10.7270/Q2KD1Z4S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50324676 ((R)-3-amino-4-(3-hexylphenylamino)-4-oxobutylphosp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human S1P1 receptor expressed in CHO cells assessed as inhibition of S1P-induced [35S]GTPgamma binding | Nat Chem Biol 2: 434-41 (2006) Article DOI: 10.1038/nchembio804 BindingDB Entry DOI: 10.7270/Q2KD1Z4S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50324677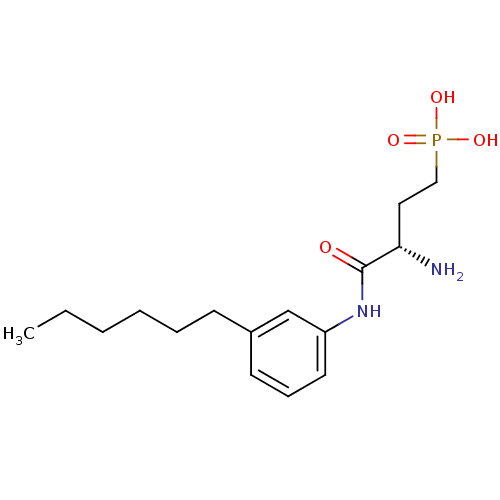 ((S)-3-amino-4-(3-hexylphenylamino)-4-oxobutylphosp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human S1P1 receptor expressed in CHO cells assessed as inhibition of SEW2871-induced [35S]GTPgamma binding | Nat Chem Biol 2: 434-41 (2006) Article DOI: 10.1038/nchembio804 BindingDB Entry DOI: 10.7270/Q2KD1Z4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50324677 ((S)-3-amino-4-(3-hexylphenylamino)-4-oxobutylphosp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human S1P1 receptor expressed in CHO cells assessed as inhibition of S1P-induced [35S]GTPgamma binding | Nat Chem Biol 2: 434-41 (2006) Article DOI: 10.1038/nchembio804 BindingDB Entry DOI: 10.7270/Q2KD1Z4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 reverse transcriptase | Eur J Med Chem 44: 2190-201 (2009) Article DOI: 10.1016/j.ejmech.2008.10.032 BindingDB Entry DOI: 10.7270/Q2X069TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 wild type reverse transcriptase (IIIB) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (Y181C) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibition of HIV1 virion-associated reverse transcriptase | Bioorg Med Chem 16: 6353-63 (2008) Article DOI: 10.1016/j.bmc.2008.05.010 BindingDB Entry DOI: 10.7270/Q2B56NJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168052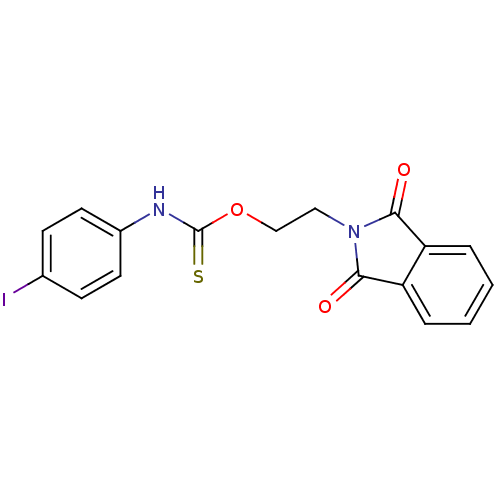 ((4-Iodo-phenyl)-thiocarbamic acid 2-(1,3-dioxo-1,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 wild type reverse transcriptase (IIIB) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50480227 (CHEMBL520032) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 reverse transcriptase | Eur J Med Chem 44: 2190-201 (2009) Article DOI: 10.1016/j.ejmech.2008.10.032 BindingDB Entry DOI: 10.7270/Q2X069TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (K103N+Y181C) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168128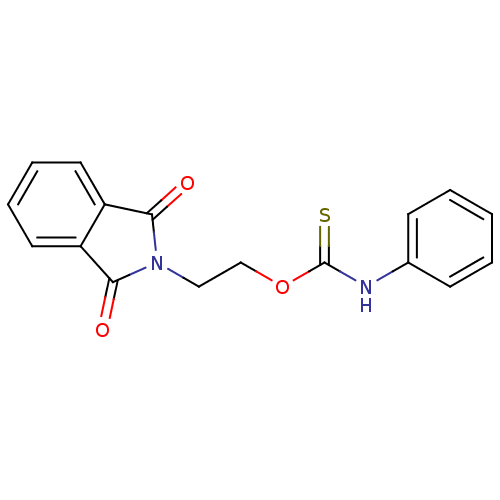 (CHEMBL191655 | O-2-(1,3-dioxoisoindolin-2-yl)ethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 virion reverse transcriptase | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50480228 (CHEMBL484982) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibition of wild-type HIV1 reverse transcriptase | Eur J Med Chem 44: 2190-201 (2009) Article DOI: 10.1016/j.ejmech.2008.10.032 BindingDB Entry DOI: 10.7270/Q2X069TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478732 (CHEMBL443403) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibition of HIV1 virion-associated reverse transcriptase | Bioorg Med Chem 16: 6353-63 (2008) Article DOI: 10.1016/j.bmc.2008.05.010 BindingDB Entry DOI: 10.7270/Q2B56NJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1866 (3-(5-bromopyridin-2-yl)-1-[2-(pyridin-2-yl)ethyl]t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 wild type reverse transcriptase (IIIB) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM1866 (3-(5-bromopyridin-2-yl)-1-[2-(pyridin-2-yl)ethyl]t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibition of HIV1 virion-associated reverse transcriptase | Bioorg Med Chem 16: 6353-63 (2008) Article DOI: 10.1016/j.bmc.2008.05.010 BindingDB Entry DOI: 10.7270/Q2B56NJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168060 (CHEMBL195108 | N-[2-(4-Iodo-phenylthiocarbamoyloxy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 wild type reverse transcriptase (IIIB) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (K103R) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168052 ((4-Iodo-phenyl)-thiocarbamic acid 2-(1,3-dioxo-1,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (K103R) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168060 (CHEMBL195108 | N-[2-(4-Iodo-phenylthiocarbamoyloxy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (Y181C) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1866 (3-(5-bromopyridin-2-yl)-1-[2-(pyridin-2-yl)ethyl]t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (Y181C) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168052 ((4-Iodo-phenyl)-thiocarbamic acid 2-(1,3-dioxo-1,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (Y181C) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168060 (CHEMBL195108 | N-[2-(4-Iodo-phenylthiocarbamoyloxy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (K103N+Y181C) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1866 (3-(5-bromopyridin-2-yl)-1-[2-(pyridin-2-yl)ethyl]t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (K103N+Y181C) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1866 (3-(5-bromopyridin-2-yl)-1-[2-(pyridin-2-yl)ethyl]t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (K103R) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168060 (CHEMBL195108 | N-[2-(4-Iodo-phenylthiocarbamoyloxy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (K103R) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50168052 ((4-Iodo-phenyl)-thiocarbamic acid 2-(1,3-dioxo-1,3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Genova Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 mutant reverse transcriptase (K103N+Y181C) | J Med Chem 48: 3858-73 (2005) Article DOI: 10.1021/jm049252r BindingDB Entry DOI: 10.7270/Q2GH9JQK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM430984 (US10550102, Example 40-1 (-)) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Chinese hamster ovary (CHO) cells overexpressing soluble guanylate cyclase were generated to test the effect of sGC activators in a cellular context.... | US Patent US10550102 (2020) BindingDB Entry DOI: 10.7270/Q2PC34SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM430985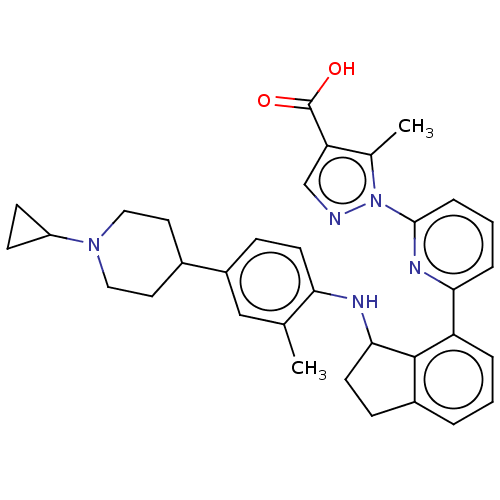 (Example 60-A. (+-)-Ethyl 1-(6-(3-((4-(1-cyclopropy...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Chinese hamster ovary (CHO) cells overexpressing soluble guanylate cyclase were generated to test the effect of sGC activators in a cellular context.... | US Patent US10550102 (2020) BindingDB Entry DOI: 10.7270/Q2PC34SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM430986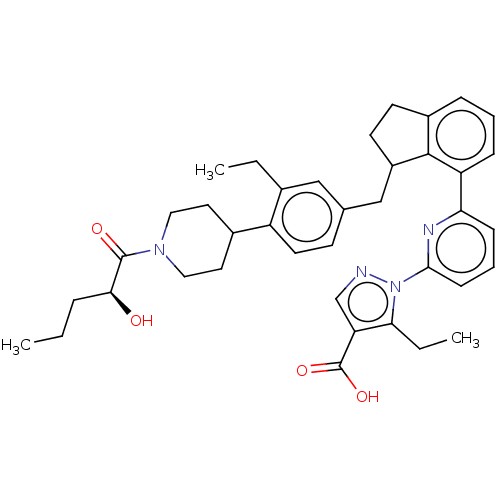 (US10550102, Example 40-1 (+)) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Chinese hamster ovary (CHO) cells overexpressing soluble guanylate cyclase were generated to test the effect of sGC activators in a cellular context.... | US Patent US10550102 (2020) BindingDB Entry DOI: 10.7270/Q2PC34SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM430987 (US10550102, Example 61. b). (enantiomer-1) | US105...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Chinese hamster ovary (CHO) cells overexpressing soluble guanylate cyclase were generated to test the effect of sGC activators in a cellular context.... | US Patent US10550102 (2020) BindingDB Entry DOI: 10.7270/Q2PC34SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM430988 (US10550102, Example 40-2 (-)) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Chinese hamster ovary (CHO) cells overexpressing soluble guanylate cyclase were generated to test the effect of sGC activators in a cellular context.... | US Patent US10550102 (2020) BindingDB Entry DOI: 10.7270/Q2PC34SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM430987 (US10550102, Example 61. b). (enantiomer-1) | US105...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Chinese hamster ovary (CHO) cells overexpressing soluble guanylate cyclase were generated to test the effect of sGC activators in a cellular context.... | US Patent US10550102 (2020) BindingDB Entry DOI: 10.7270/Q2PC34SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM430990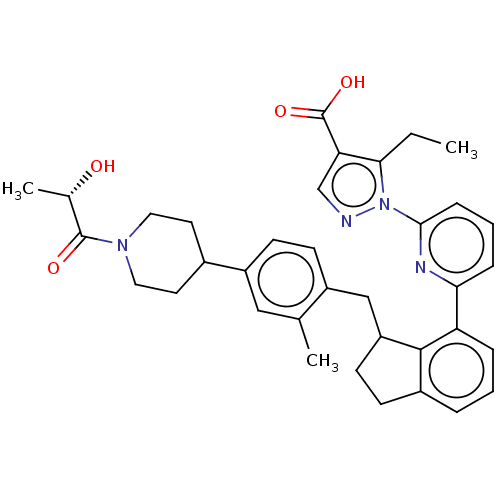 (US10550102, Example 40-2 (+)) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Chinese hamster ovary (CHO) cells overexpressing soluble guanylate cyclase were generated to test the effect of sGC activators in a cellular context.... | US Patent US10550102 (2020) BindingDB Entry DOI: 10.7270/Q2PC34SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM430991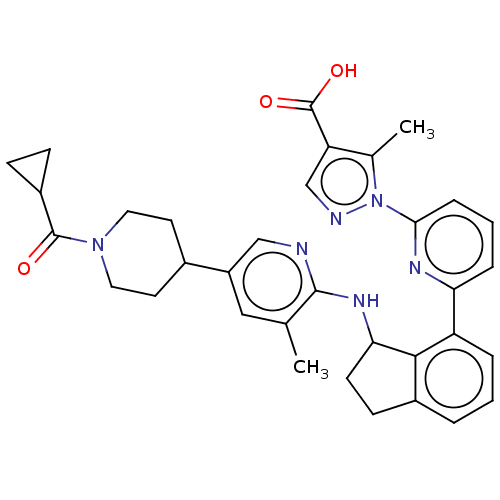 (Example 62-A. (+-)-Ethyl 1-(6-(3-((tert-butoxycarb...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Chinese hamster ovary (CHO) cells overexpressing soluble guanylate cyclase were generated to test the effect of sGC activators in a cellular context.... | US Patent US10550102 (2020) BindingDB Entry DOI: 10.7270/Q2PC34SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM430992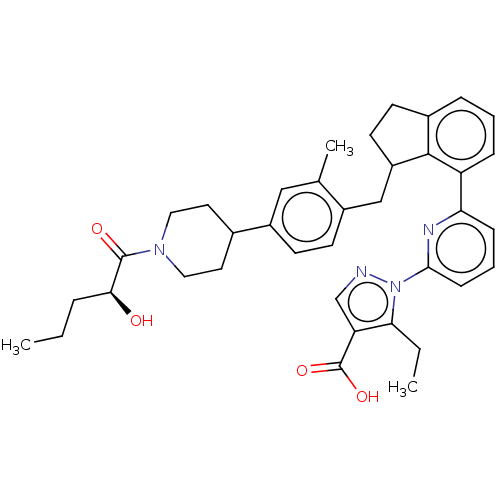 (US10550102, Example 40-3 (-)) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 49 | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Chinese hamster ovary (CHO) cells overexpressing soluble guanylate cyclase were generated to test the effect of sGC activators in a cellular context.... | US Patent US10550102 (2020) BindingDB Entry DOI: 10.7270/Q2PC34SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM430993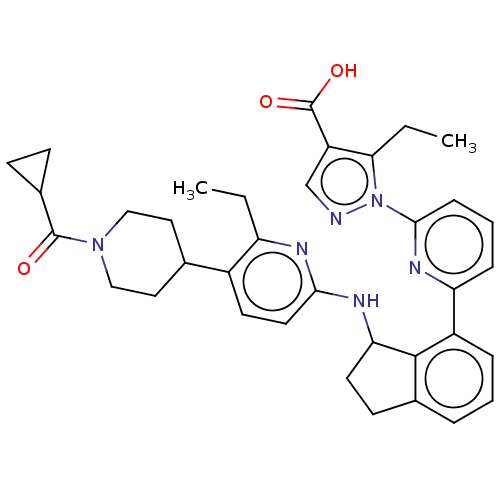 (US10550102, Example 63a) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Chinese hamster ovary (CHO) cells overexpressing soluble guanylate cyclase were generated to test the effect of sGC activators in a cellular context.... | US Patent US10550102 (2020) BindingDB Entry DOI: 10.7270/Q2PC34SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM430994 (US10550102, Example 40-3 (+)) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Chinese hamster ovary (CHO) cells overexpressing soluble guanylate cyclase were generated to test the effect of sGC activators in a cellular context.... | US Patent US10550102 (2020) BindingDB Entry DOI: 10.7270/Q2PC34SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM430995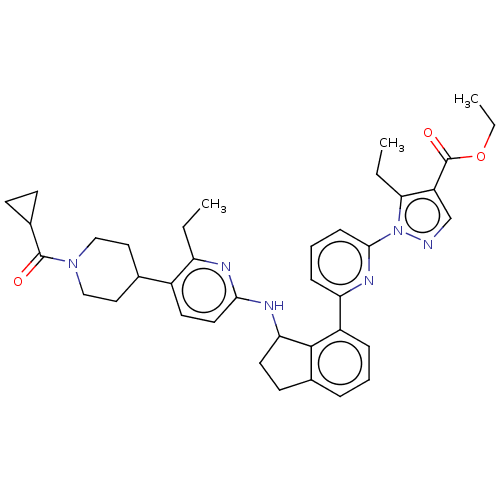 (US10550102, Example 63b) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 60 | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Chinese hamster ovary (CHO) cells overexpressing soluble guanylate cyclase were generated to test the effect of sGC activators in a cellular context.... | US Patent US10550102 (2020) BindingDB Entry DOI: 10.7270/Q2PC34SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM430996 (US10550102, Example 41. b). (-)) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Chinese hamster ovary (CHO) cells overexpressing soluble guanylate cyclase were generated to test the effect of sGC activators in a cellular context.... | US Patent US10550102 (2020) BindingDB Entry DOI: 10.7270/Q2PC34SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM430997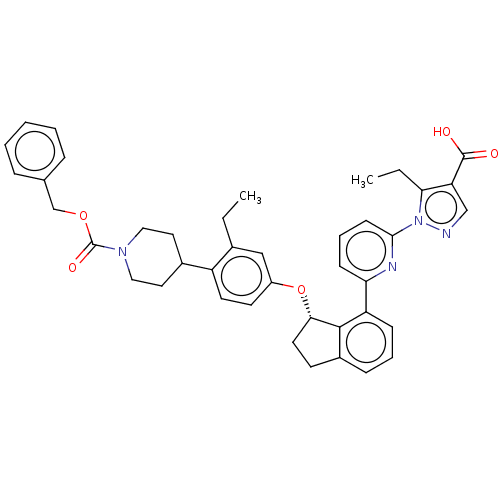 (US10550102, Example 64-1) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Chinese hamster ovary (CHO) cells overexpressing soluble guanylate cyclase were generated to test the effect of sGC activators in a cellular context.... | US Patent US10550102 (2020) BindingDB Entry DOI: 10.7270/Q2PC34SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM430998 (US10550102, Example 41. b). (+)) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Chinese hamster ovary (CHO) cells overexpressing soluble guanylate cyclase were generated to test the effect of sGC activators in a cellular context.... | US Patent US10550102 (2020) BindingDB Entry DOI: 10.7270/Q2PC34SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM430999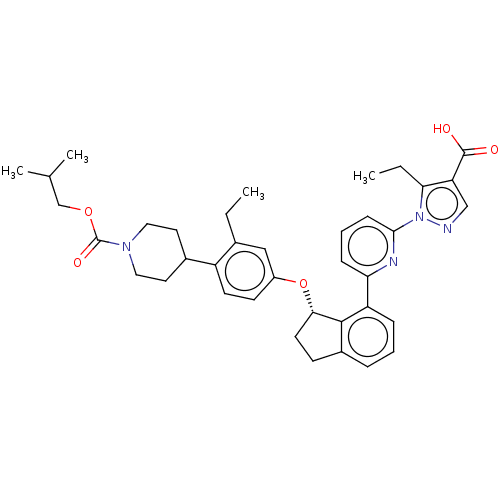 (US10550102, Example 64-2) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Chinese hamster ovary (CHO) cells overexpressing soluble guanylate cyclase were generated to test the effect of sGC activators in a cellular context.... | US Patent US10550102 (2020) BindingDB Entry DOI: 10.7270/Q2PC34SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM431000 (US10550102, Example 42. b). (-)) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 93 | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Chinese hamster ovary (CHO) cells overexpressing soluble guanylate cyclase were generated to test the effect of sGC activators in a cellular context.... | US Patent US10550102 (2020) BindingDB Entry DOI: 10.7270/Q2PC34SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM431001 (US10550102, Example 64-3) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Chinese hamster ovary (CHO) cells overexpressing soluble guanylate cyclase were generated to test the effect of sGC activators in a cellular context.... | US Patent US10550102 (2020) BindingDB Entry DOI: 10.7270/Q2PC34SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM431002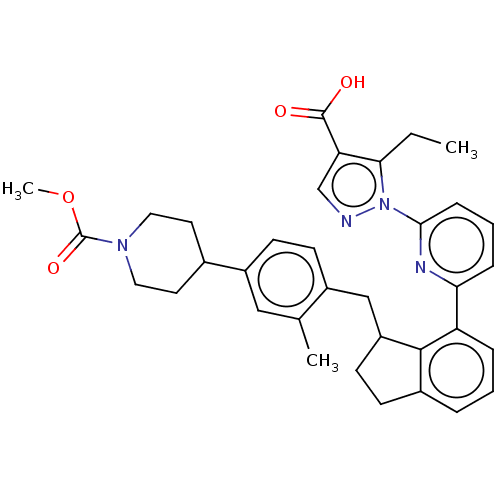 (US10550102, Example 42. b). (+)) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Chinese hamster ovary (CHO) cells overexpressing soluble guanylate cyclase were generated to test the effect of sGC activators in a cellular context.... | US Patent US10550102 (2020) BindingDB Entry DOI: 10.7270/Q2PC34SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM431003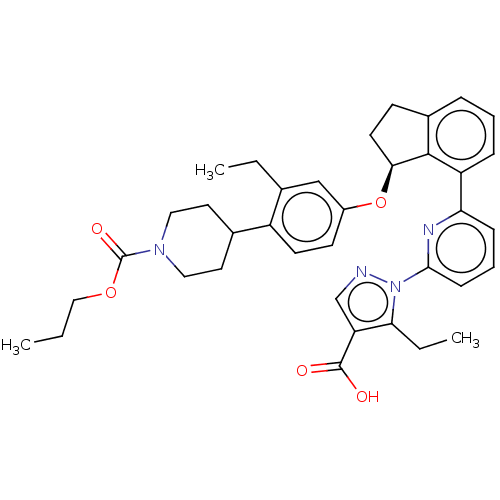 (US10550102, Example 64-4) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Chinese hamster ovary (CHO) cells overexpressing soluble guanylate cyclase were generated to test the effect of sGC activators in a cellular context.... | US Patent US10550102 (2020) BindingDB Entry DOI: 10.7270/Q2PC34SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM431004 (US10550102, Example 43 (+)) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Chinese hamster ovary (CHO) cells overexpressing soluble guanylate cyclase were generated to test the effect of sGC activators in a cellular context.... | US Patent US10550102 (2020) BindingDB Entry DOI: 10.7270/Q2PC34SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM431005 (US10550102, Example 64-5) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Chinese hamster ovary (CHO) cells overexpressing soluble guanylate cyclase were generated to test the effect of sGC activators in a cellular context.... | US Patent US10550102 (2020) BindingDB Entry DOI: 10.7270/Q2PC34SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Guanylate cyclase soluble subunit alpha-1/beta-1 (Homo sapiens (Human)) | BDBM431006 (US10550102, Example 43 (-)) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Chinese hamster ovary (CHO) cells overexpressing soluble guanylate cyclase were generated to test the effect of sGC activators in a cellular context.... | US Patent US10550102 (2020) BindingDB Entry DOI: 10.7270/Q2PC34SQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 188 total ) | Next | Last >> |