

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496270 (CHEMBL3127679) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496289 (CHEMBL3124968) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496273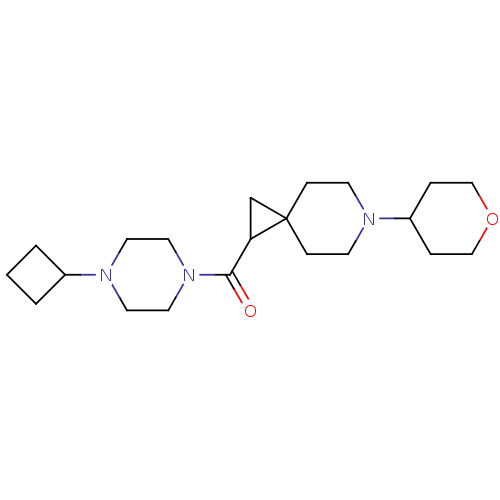 (CHEMBL3127700) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496272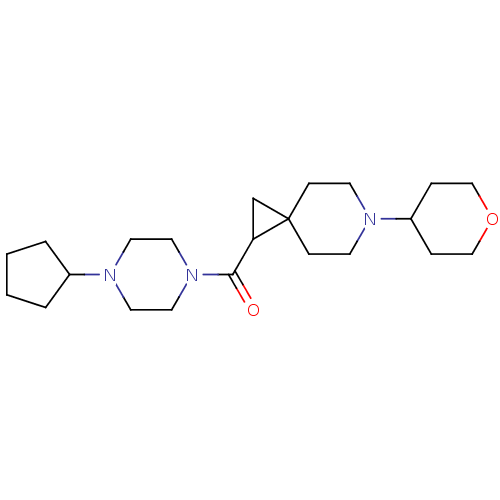 (CHEMBL3127701) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496296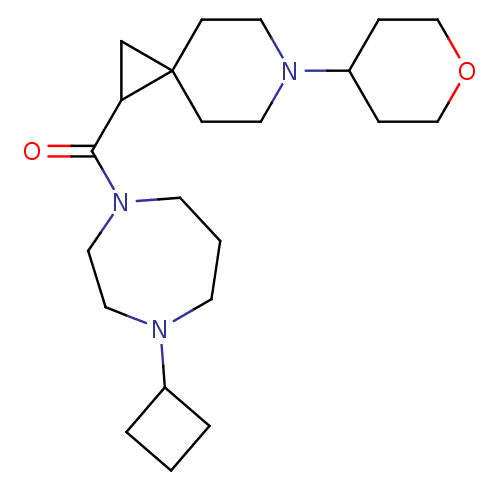 (CHEMBL3127704) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496281 (CHEMBL3127698) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50180955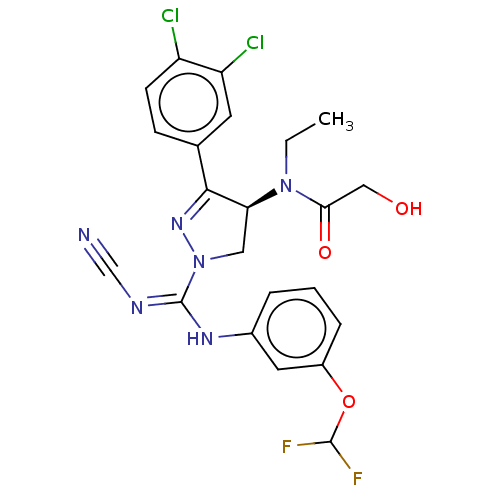 (CHEMBL3818617) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG Curated by ChEMBL | Assay Description Competitive inhibition of full length 6xHis-tagged SMYD2 (unknown origin) expressed in Escherichia coli using varying levels of Btn-Ahx-GSRAHSSHLKSKK... | J Med Chem 59: 4578-600 (2016) Article DOI: 10.1021/acs.jmedchem.5b01890 BindingDB Entry DOI: 10.7270/Q2RN39S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496290 (CHEMBL3127705) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496297 (CHEMBL3127699) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496259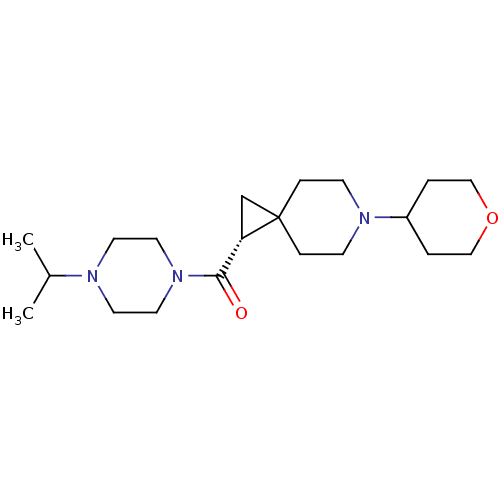 (CHEMBL3127672) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496295 (CHEMBL3127708) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496276 (CHEMBL3127670) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496280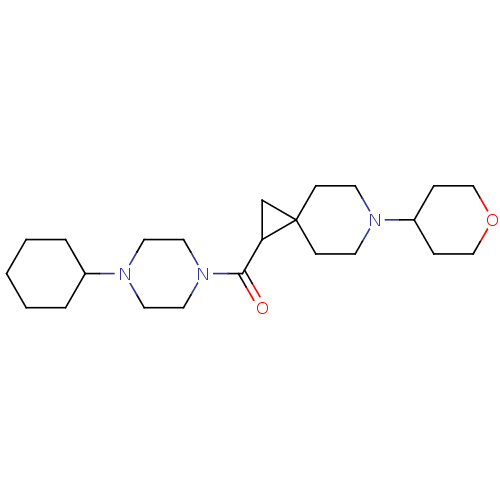 (CHEMBL3127702) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase SMYD2 (Homo sapiens (Human)) | BDBM50180955 (CHEMBL3818617) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG Curated by ChEMBL | Assay Description Uncompetitive inhibition of full length 6xHis-tagged SMYD2 (unknown origin) expressed in Escherichia coli using fixed levels of Btn-Ahx-GSRAHSSHLKSKK... | J Med Chem 59: 4578-600 (2016) Article DOI: 10.1021/acs.jmedchem.5b01890 BindingDB Entry DOI: 10.7270/Q2RN39S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496263 (CHEMBL3127709) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496275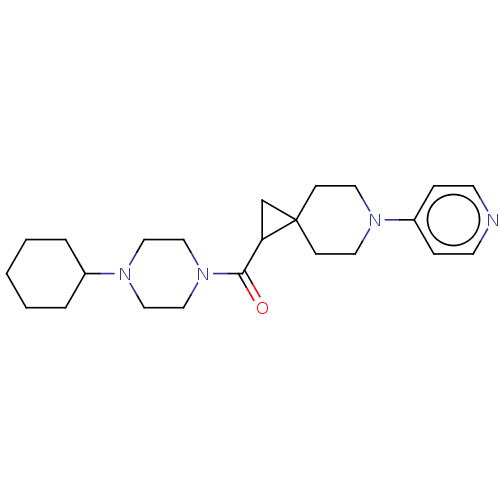 (CHEMBL3127671) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496279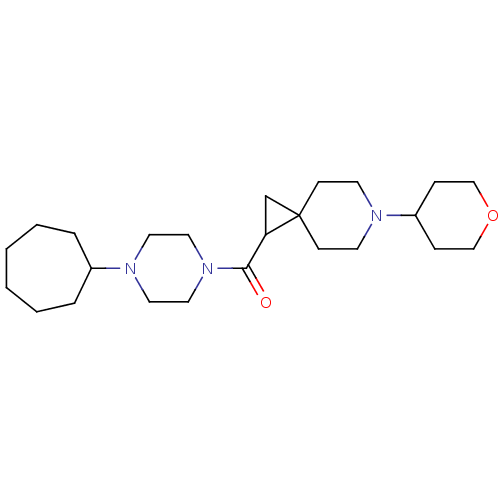 (CHEMBL3127703) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496278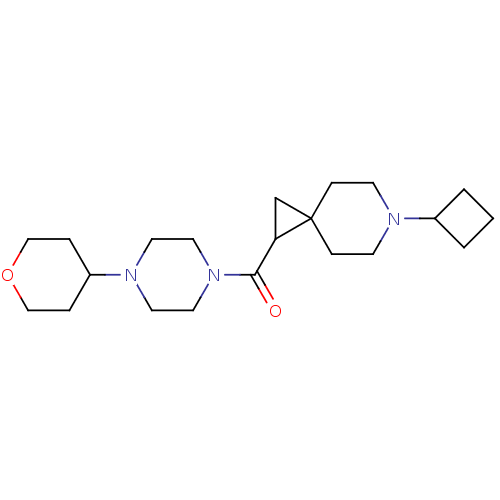 (CHEMBL3127706) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496261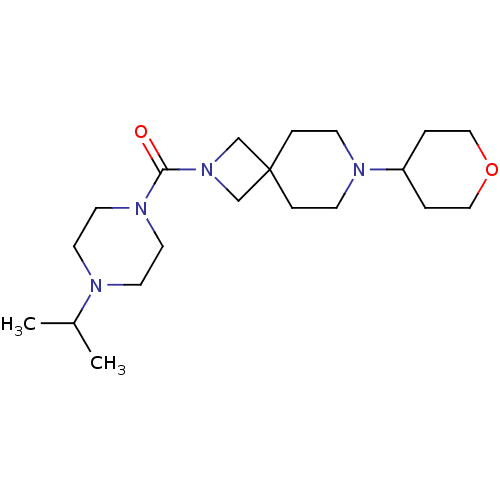 (CHEMBL3127680) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496287 (CHEMBL3127669) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496277 (CHEMBL3127707) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 439 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496291 (CHEMBL3127697) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496301 (CHEMBL3127668) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496274 (CHEMBL3127673) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human histamine H3 receptor expressed in CHOK1 cells after 1.5 hrs by scintillation proximity assay | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496294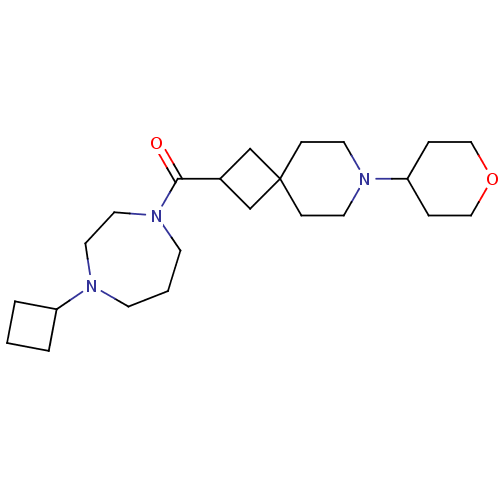 (CHEMBL3127674) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor long form expressed in CHOK1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]... | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496293 (CHEMBL3127676) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor long form expressed in CHOK1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]... | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496270 (CHEMBL3127679) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor long form expressed in CHOK1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]... | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496299 (CHEMBL3127675) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor long form expressed in CHOK1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]... | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 21 (Homo sapiens (Human)) | CHEMBL5279102 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496284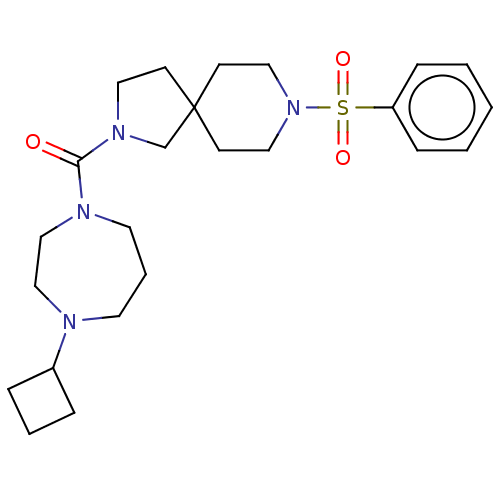 (CHEMBL3127691) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor long form expressed in CHOK1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]... | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496264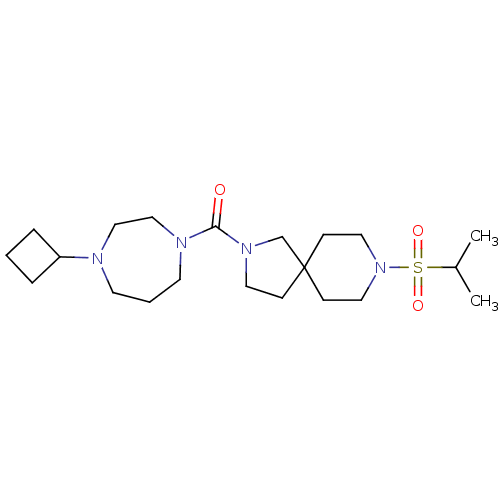 (CHEMBL3127692) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor long form expressed in CHOK1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]... | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496281 (CHEMBL3127698) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor long form expressed in CHOK1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]... | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496273 (CHEMBL3127700) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor long form expressed in CHOK1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]... | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496265 (CHEMBL3127690) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor long form expressed in CHOK1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]... | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496285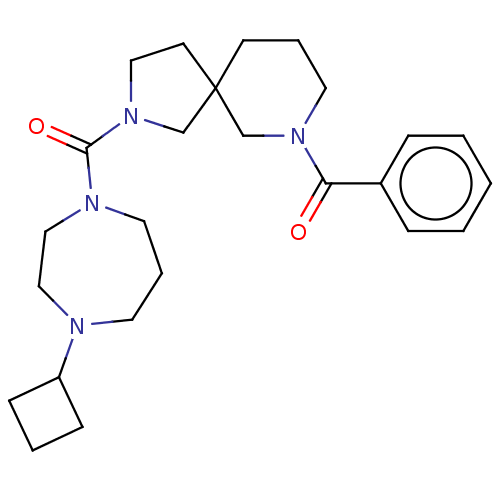 (CHEMBL3127688) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor long form expressed in CHOK1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]... | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496260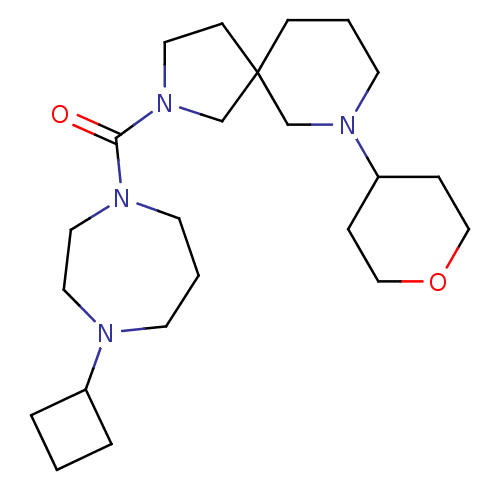 (CHEMBL3127681) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor long form expressed in CHOK1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]... | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 21 (Homo sapiens (Human)) | CHEMBL5279102 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496271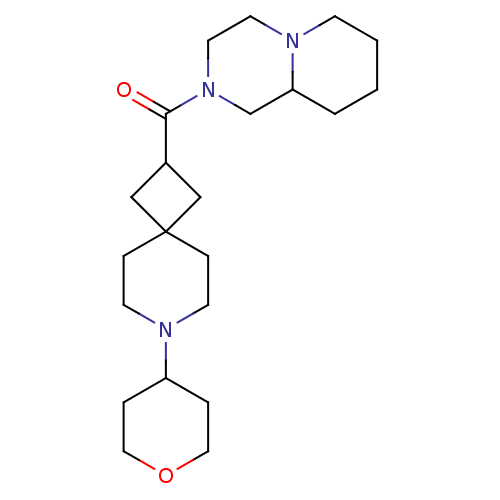 (CHEMBL3127677) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor long form expressed in CHOK1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]... | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496266 (CHEMBL3127689) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor long form expressed in CHOK1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]... | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 21 (Homo sapiens (Human)) | CHEMBL5268828 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496289 (CHEMBL3124968) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor long form expressed in CHOK1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]... | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496272 (CHEMBL3127701) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor long form expressed in CHOK1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]... | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50181506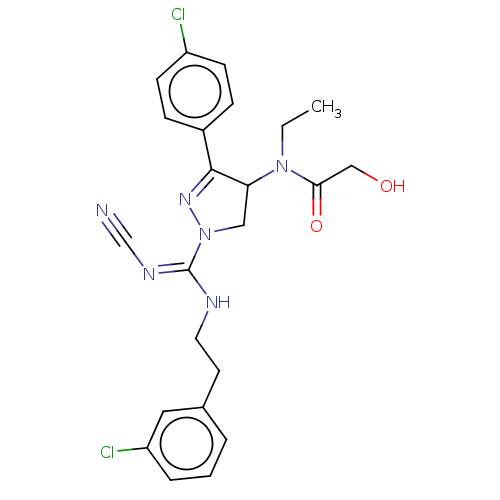 (CHEMBL3819038) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER Pharma AG Curated by ChEMBL | Assay Description Antagonist activity at PAR1 (unknown origin) | J Med Chem 59: 4578-600 (2016) Article DOI: 10.1021/acs.jmedchem.5b01890 BindingDB Entry DOI: 10.7270/Q2RN39S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496295 (CHEMBL3127708) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor long form expressed in CHOK1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]... | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50151363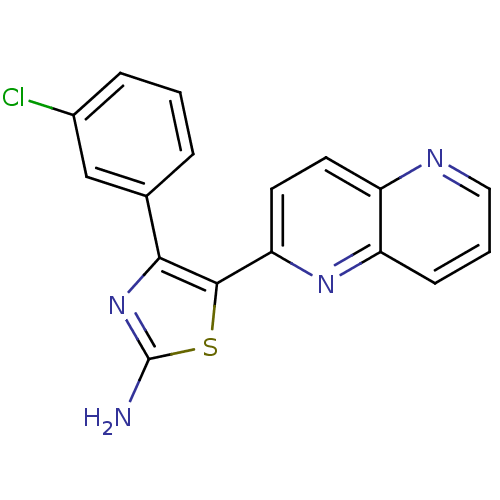 (4-(3-Chloro-phenyl)-5-[1,5]naphthyridin-2-yl-thiaz...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of TGFbeta-induced ALK5 in human HepG2 cells pretreated for 30 mins followed by TGFbeta stimulation measured after overnight incubation by... | Bioorg Med Chem 25: 1672-1680 (2017) Article DOI: 10.1016/j.bmc.2017.01.036 BindingDB Entry DOI: 10.7270/Q2PV6NXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496286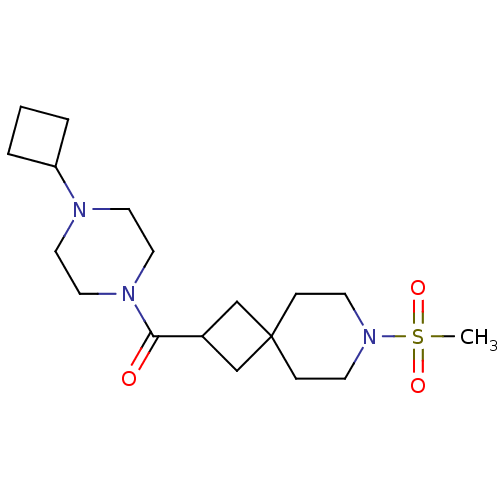 (CHEMBL3127684) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor long form expressed in CHOK1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]... | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 21 (Homo sapiens (Human)) | CHEMBL5286335 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496280 (CHEMBL3127702) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor long form expressed in CHOK1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]... | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50496288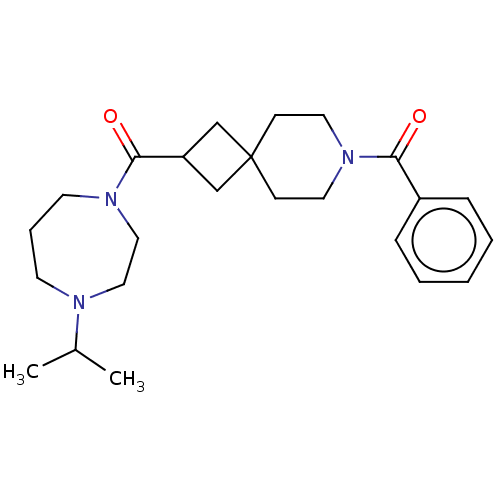 (CHEMBL3127687) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor long form expressed in CHOK1 cells assessed as inhibition of R-alpha-methylhistamine-induced [35S]... | J Med Chem 57: 733-58 (2014) Article DOI: 10.1021/jm4014828 BindingDB Entry DOI: 10.7270/Q2FT8Q13 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ubiquitin carboxyl-terminal hydrolase 21 (Homo sapiens (Human)) | CHEMBL5268828 | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL UniChem | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 428 total ) | Next | Last >> |