 Found 344 hits with Last Name = 'sarma' and Initial = 'k'
Found 344 hits with Last Name = 'sarma' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50165019
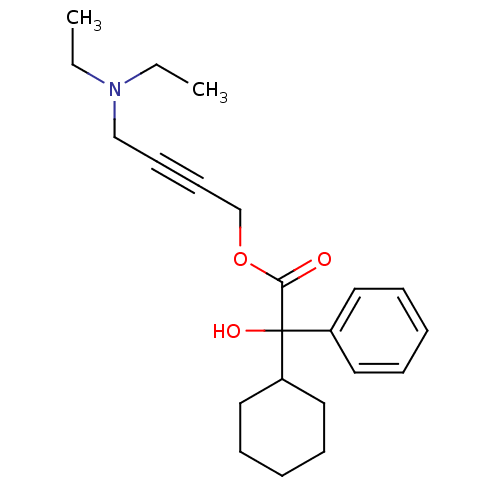
(4-(Diethylamino)-2-butynyl alpha-phenylcyclohexane...)Show InChI InChI=1S/C22H31NO3/c1-3-23(4-2)17-11-12-18-26-21(24)22(25,19-13-7-5-8-14-19)20-15-9-6-10-16-20/h5,7-8,13-14,20,25H,3-4,6,9-10,15-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M3 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50165017
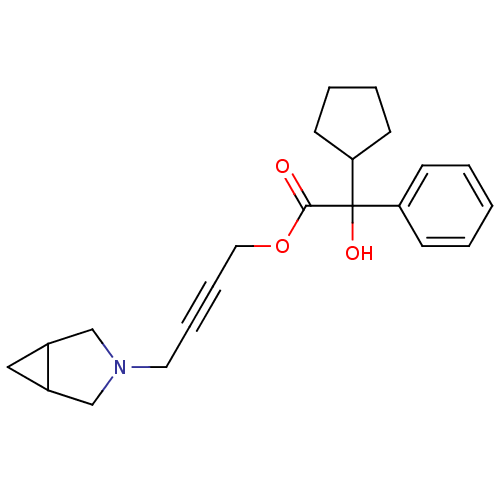
(CHEMBL192485 | Cyclopentyl-hydroxy-phenyl-acetic a...)Show InChI InChI=1S/C22H27NO3/c24-21(26-13-7-6-12-23-15-17-14-18(17)16-23)22(25,20-10-4-5-11-20)19-8-2-1-3-9-19/h1-3,8-9,17-18,20,25H,4-5,10-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Ki value for rat Muscarinic acetylcholine receptor M3 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50165017

(CHEMBL192485 | Cyclopentyl-hydroxy-phenyl-acetic a...)Show InChI InChI=1S/C22H27NO3/c24-21(26-13-7-6-12-23-15-17-14-18(17)16-23)22(25,20-10-4-5-11-20)19-8-2-1-3-9-19/h1-3,8-9,17-18,20,25H,4-5,10-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Ki value for rat Muscarinic acetylcholine receptor M3 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50165008

((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...)Show InChI InChI=1S/C22H31NO/c1-16(2)23(17(3)4)14-13-20(19-9-7-6-8-10-19)21-15-18(5)11-12-22(21)24/h6-12,15-17,20,24H,13-14H2,1-5H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50165019

(4-(Diethylamino)-2-butynyl alpha-phenylcyclohexane...)Show InChI InChI=1S/C22H31NO3/c1-3-23(4-2)17-11-12-18-26-21(24)22(25,19-13-7-5-8-14-19)20-15-9-6-10-16-20/h5,7-8,13-14,20,25H,3-4,6,9-10,15-18H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50165008

((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...)Show InChI InChI=1S/C22H31NO/c1-16(2)23(17(3)4)14-13-20(19-9-7-6-8-10-19)21-15-18(5)11-12-22(21)24/h6-12,15-17,20,24H,13-14H2,1-5H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.07 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M3 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50165012
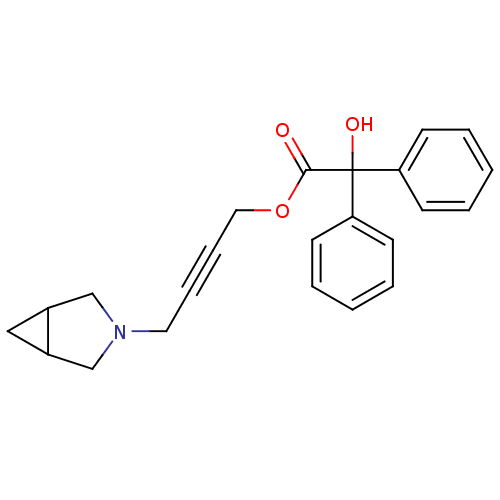
(CHEMBL192065 | Hydroxy-diphenyl-acetic acid 4-(3-a...)Show SMILES OC(C(=O)OCC#CCN1CC2CC2C1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C23H23NO3/c25-22(27-14-8-7-13-24-16-18-15-19(18)17-24)23(26,20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-6,9-12,18-19,26H,13-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M3 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50165012

(CHEMBL192065 | Hydroxy-diphenyl-acetic acid 4-(3-a...)Show SMILES OC(C(=O)OCC#CCN1CC2CC2C1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C23H23NO3/c25-22(27-14-8-7-13-24-16-18-15-19(18)17-24)23(26,20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-6,9-12,18-19,26H,13-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50165016

(4-Methyl-2-[3-((1R,5S)-2-methyl-3-aza-bicyclo[3.1....)Show SMILES CC1[C@@H]2C[C@@H]2CN1CCC(c1ccccc1)c1cc(C)ccc1O Show InChI InChI=1S/C22H27NO/c1-15-8-9-22(24)21(12-15)19(17-6-4-3-5-7-17)10-11-23-14-18-13-20(18)16(23)2/h3-9,12,16,18-20,24H,10-11,13-14H2,1-2H3/t16?,18-,19?,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50165016

(4-Methyl-2-[3-((1R,5S)-2-methyl-3-aza-bicyclo[3.1....)Show SMILES CC1[C@@H]2C[C@@H]2CN1CCC(c1ccccc1)c1cc(C)ccc1O Show InChI InChI=1S/C22H27NO/c1-15-8-9-22(24)21(12-15)19(17-6-4-3-5-7-17)10-11-23-14-18-13-20(18)16(23)2/h3-9,12,16,18-20,24H,10-11,13-14H2,1-2H3/t16?,18-,19?,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50165010
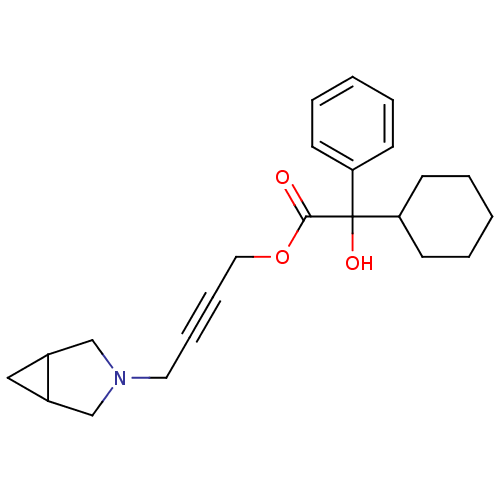
(CHEMBL192537 | Cyclohexyl-hydroxy-phenyl-acetic ac...)Show SMILES OC(C1CCCCC1)(C(=O)OCC#CCN1CC2CC2C1)c1ccccc1 Show InChI InChI=1S/C23H29NO3/c25-22(27-14-8-7-13-24-16-18-15-19(18)17-24)23(26,20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1,3-4,9-10,18-19,21,26H,2,5-6,11-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M3 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50165017

(CHEMBL192485 | Cyclopentyl-hydroxy-phenyl-acetic a...)Show InChI InChI=1S/C22H27NO3/c24-21(26-13-7-6-12-23-15-17-14-18(17)16-23)22(25,20-10-4-5-11-20)19-8-2-1-3-9-19/h1-3,8-9,17-18,20,25H,4-5,10-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M3 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50165020
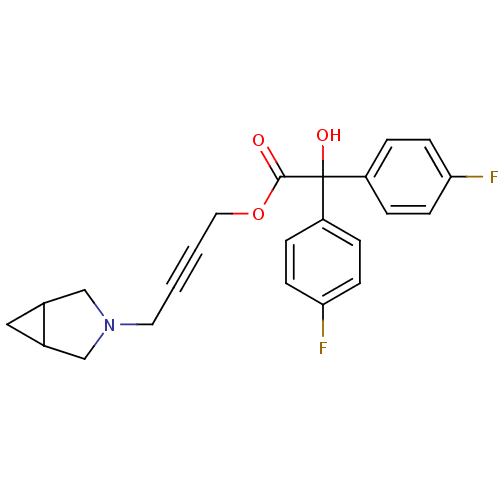
(Bis-(4-fluoro-phenyl)-hydroxy-acetic acid 4-(3-aza...)Show SMILES OC(C(=O)OCC#CCN1CC2CC2C1)(c1ccc(F)cc1)c1ccc(F)cc1 Show InChI InChI=1S/C23H21F2NO3/c24-20-7-3-18(4-8-20)23(28,19-5-9-21(25)10-6-19)22(27)29-12-2-1-11-26-14-16-13-17(16)15-26/h3-10,16-17,28H,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M3 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50165020

(Bis-(4-fluoro-phenyl)-hydroxy-acetic acid 4-(3-aza...)Show SMILES OC(C(=O)OCC#CCN1CC2CC2C1)(c1ccc(F)cc1)c1ccc(F)cc1 Show InChI InChI=1S/C23H21F2NO3/c24-20-7-3-18(4-8-20)23(28,19-5-9-21(25)10-6-19)22(27)29-12-2-1-11-26-14-16-13-17(16)15-26/h3-10,16-17,28H,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50165018
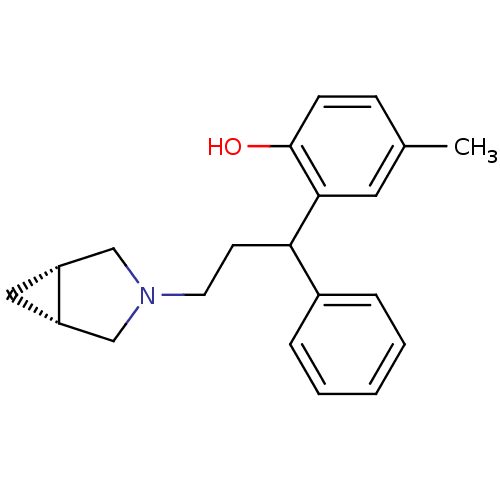
(2-((1R,5S)-3-3-Aza-bicyclo[3.1.0]hex-3-yl-1-phenyl...)Show SMILES Cc1ccc(O)c(c1)C(CCN1C[C@@H]2C[C@@H]2C1)c1ccccc1 Show InChI InChI=1S/C21H25NO/c1-15-7-8-21(23)20(11-15)19(16-5-3-2-4-6-16)9-10-22-13-17-12-18(17)14-22/h2-8,11,17-19,23H,9-10,12-14H2,1H3/t17-,18+,19? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M3 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50165010

(CHEMBL192537 | Cyclohexyl-hydroxy-phenyl-acetic ac...)Show SMILES OC(C1CCCCC1)(C(=O)OCC#CCN1CC2CC2C1)c1ccccc1 Show InChI InChI=1S/C23H29NO3/c25-22(27-14-8-7-13-24-16-18-15-19(18)17-24)23(26,20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1,3-4,9-10,18-19,21,26H,2,5-6,11-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50165016

(4-Methyl-2-[3-((1R,5S)-2-methyl-3-aza-bicyclo[3.1....)Show SMILES CC1[C@@H]2C[C@@H]2CN1CCC(c1ccccc1)c1cc(C)ccc1O Show InChI InChI=1S/C22H27NO/c1-15-8-9-22(24)21(12-15)19(17-6-4-3-5-7-17)10-11-23-14-18-13-20(18)16(23)2/h3-9,12,16,18-20,24H,10-11,13-14H2,1-2H3/t16?,18-,19?,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M3 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50165016

(4-Methyl-2-[3-((1R,5S)-2-methyl-3-aza-bicyclo[3.1....)Show SMILES CC1[C@@H]2C[C@@H]2CN1CCC(c1ccccc1)c1cc(C)ccc1O Show InChI InChI=1S/C22H27NO/c1-15-8-9-22(24)21(12-15)19(17-6-4-3-5-7-17)10-11-23-14-18-13-20(18)16(23)2/h3-9,12,16,18-20,24H,10-11,13-14H2,1-2H3/t16?,18-,19?,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M3 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402859
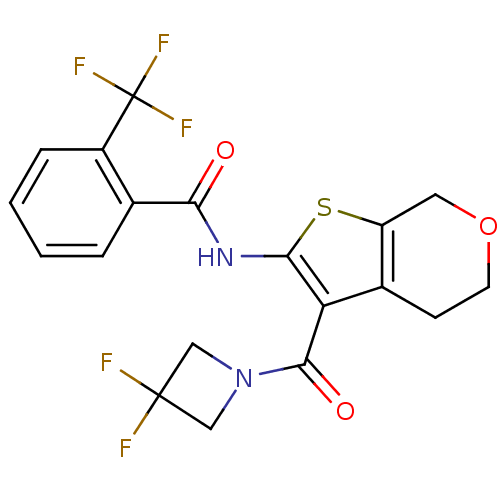
(CHEMBL2205591)Show SMILES FC(F)(F)c1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1 Show InChI InChI=1S/C19H15F5N2O3S/c20-18(21)8-26(9-18)17(28)14-11-5-6-29-7-13(11)30-16(14)25-15(27)10-3-1-2-4-12(10)19(22,23)24/h1-4H,5-9H2,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 81.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50165018

(2-((1R,5S)-3-3-Aza-bicyclo[3.1.0]hex-3-yl-1-phenyl...)Show SMILES Cc1ccc(O)c(c1)C(CCN1C[C@@H]2C[C@@H]2C1)c1ccccc1 Show InChI InChI=1S/C21H25NO/c1-15-7-8-21(23)20(11-15)19(16-5-3-2-4-6-16)9-10-22-13-17-12-18(17)14-22/h2-8,11,17-19,23H,9-10,12-14H2,1H3/t17-,18+,19? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50165022

(4-Methyl-2-[3-((1R,5S)-1-methyl-3-aza-bicyclo[3.1....)Show SMILES Cc1ccc(O)c(c1)C(CCN1C[C@H]2C[C@@]2(C)C1)c1ccccc1 Show InChI InChI=1S/C22H27NO/c1-16-8-9-21(24)20(12-16)19(17-6-4-3-5-7-17)10-11-23-14-18-13-22(18,2)15-23/h3-9,12,18-19,24H,10-11,13-15H2,1-2H3/t18-,19?,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M3 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50165022

(4-Methyl-2-[3-((1R,5S)-1-methyl-3-aza-bicyclo[3.1....)Show SMILES Cc1ccc(O)c(c1)C(CCN1C[C@H]2C[C@@]2(C)C1)c1ccccc1 Show InChI InChI=1S/C22H27NO/c1-16-8-9-21(24)20(12-16)19(17-6-4-3-5-7-17)10-11-23-14-18-13-22(18,2)15-23/h3-9,12,18-19,24H,10-11,13-15H2,1-2H3/t18-,19?,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 221 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50165017

(CHEMBL192485 | Cyclopentyl-hydroxy-phenyl-acetic a...)Show InChI InChI=1S/C22H27NO3/c24-21(26-13-7-6-12-23-15-17-14-18(17)16-23)22(25,20-10-4-5-11-20)19-8-2-1-3-9-19/h1-3,8-9,17-18,20,25H,4-5,10-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50165017

(CHEMBL192485 | Cyclopentyl-hydroxy-phenyl-acetic a...)Show InChI InChI=1S/C22H27NO3/c24-21(26-13-7-6-12-23-15-17-14-18(17)16-23)22(25,20-10-4-5-11-20)19-8-2-1-3-9-19/h1-3,8-9,17-18,20,25H,4-5,10-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50165017

(CHEMBL192485 | Cyclopentyl-hydroxy-phenyl-acetic a...)Show InChI InChI=1S/C22H27NO3/c24-21(26-13-7-6-12-23-15-17-14-18(17)16-23)22(25,20-10-4-5-11-20)19-8-2-1-3-9-19/h1-3,8-9,17-18,20,25H,4-5,10-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402858

(CHEMBL2205592)Show SMILES FC(F)(F)Oc1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1 Show InChI InChI=1S/C19H15F5N2O4S/c20-18(21)8-26(9-18)17(28)14-11-5-6-29-7-13(11)31-16(14)25-15(27)10-3-1-2-4-12(10)30-19(22,23)24/h1-4H,5-9H2,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402864
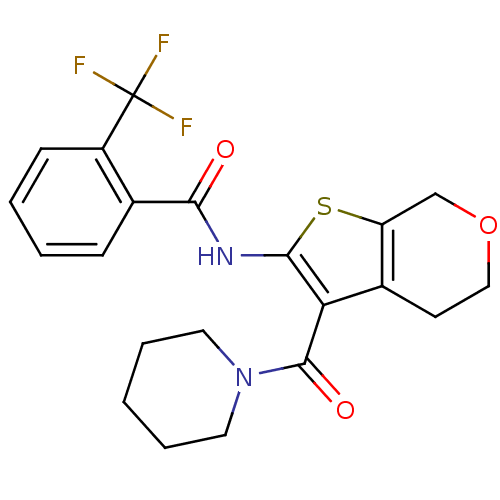
(CHEMBL2205615)Show SMILES FC(F)(F)c1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CCCCC1 Show InChI InChI=1S/C21H21F3N2O3S/c22-21(23,24)15-7-3-2-6-13(15)18(27)25-19-17(14-8-11-29-12-16(14)30-19)20(28)26-9-4-1-5-10-26/h2-3,6-7H,1,4-5,8-12H2,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 241 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50165024
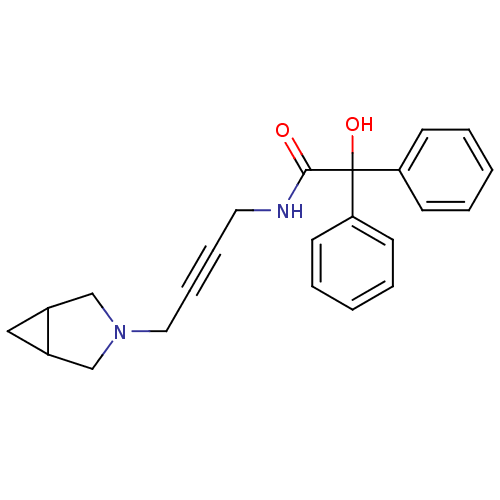
(CHEMBL195169 | N-[4-(3-Aza-bicyclo[3.1.0]hex-3-yl)...)Show SMILES OC(C(=O)NCC#CCN1CC2CC2C1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C23H24N2O2/c26-22(24-13-7-8-14-25-16-18-15-19(18)17-25)23(27,20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-6,9-12,18-19,27H,13-17H2,(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 291 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M3 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50380754
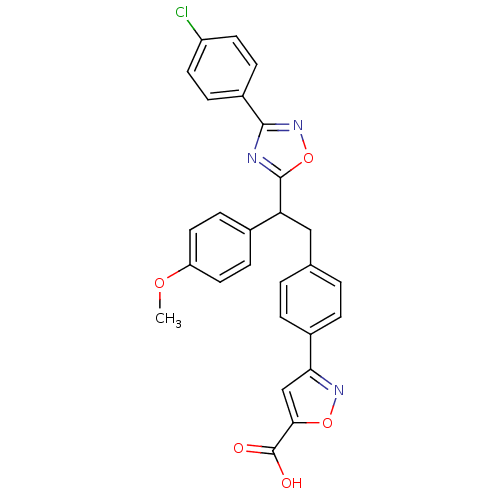
(CHEMBL2017853)Show SMILES COc1ccc(cc1)C(Cc1ccc(cc1)-c1cc(on1)C(O)=O)c1nc(no1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C27H20ClN3O5/c1-34-21-12-8-17(9-13-21)22(26-29-25(31-36-26)19-6-10-20(28)11-7-19)14-16-2-4-18(5-3-16)23-15-24(27(32)33)35-30-23/h2-13,15,22H,14H2,1H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... |
Bioorg Med Chem Lett 22: 2843-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.070
BindingDB Entry DOI: 10.7270/Q2FB53ZS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402863
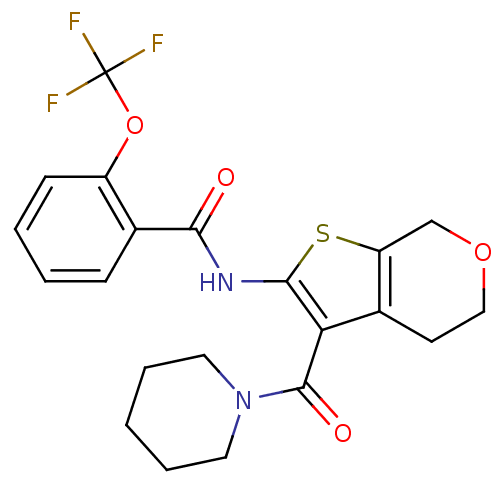
(CHEMBL2205616)Show SMILES FC(F)(F)Oc1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CCCCC1 Show InChI InChI=1S/C21H21F3N2O4S/c22-21(23,24)30-15-7-3-2-6-13(15)18(27)25-19-17(14-8-11-29-12-16(14)31-19)20(28)26-9-4-1-5-10-26/h2-3,6-7H,1,4-5,8-12H2,(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50165013

(CHEMBL194870 | N-[4-(3-Aza-bicyclo[3.1.0]hex-3-yl)...)Show SMILES OC(C(=O)NCC#CCN1CC2CC2C1)(c1ccc(F)cc1)c1ccc(F)cc1 Show InChI InChI=1S/C23H22F2N2O2/c24-20-7-3-18(4-8-20)23(29,19-5-9-21(25)10-6-19)22(28)26-11-1-2-12-27-14-16-13-17(16)15-27/h3-10,16-17,29H,11-15H2,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 366 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M3 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50380753
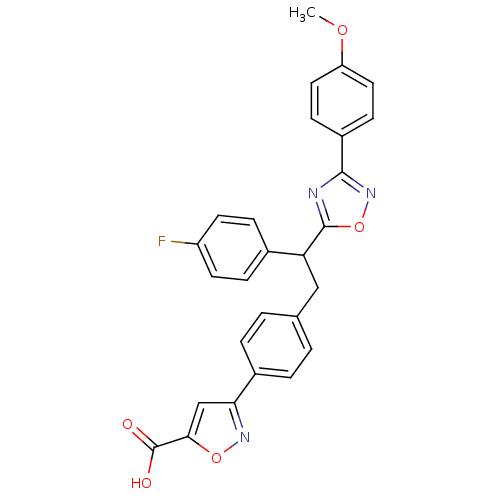
(CHEMBL2017852)Show SMILES COc1ccc(cc1)-c1noc(n1)C(Cc1ccc(cc1)-c1cc(on1)C(O)=O)c1ccc(F)cc1 Show InChI InChI=1S/C27H20FN3O5/c1-34-21-12-8-19(9-13-21)25-29-26(36-31-25)22(17-6-10-20(28)11-7-17)14-16-2-4-18(5-3-16)23-15-24(27(32)33)35-30-23/h2-13,15,22H,14H2,1H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... |
Bioorg Med Chem Lett 22: 2843-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.070
BindingDB Entry DOI: 10.7270/Q2FB53ZS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402857

(CHEMBL2205593)Show SMILES Fc1cccc(c1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1)C(F)(F)F Show InChI InChI=1S/C19H14F6N2O3S/c20-11-3-1-2-10(19(23,24)25)14(11)15(28)26-16-13(9-4-5-30-6-12(9)31-16)17(29)27-7-18(21,22)8-27/h1-3H,4-8H2,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 444 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50380755
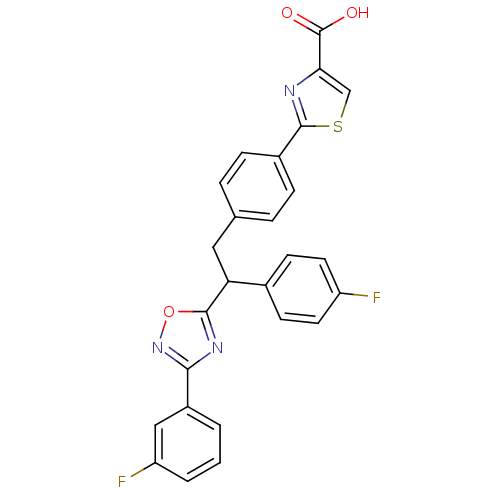
(CHEMBL2017854)Show SMILES OC(=O)c1csc(n1)-c1ccc(CC(c2nc(no2)-c2cccc(F)c2)c2ccc(F)cc2)cc1 Show InChI InChI=1S/C26H17F2N3O3S/c27-19-10-8-16(9-11-19)21(24-30-23(31-34-24)18-2-1-3-20(28)13-18)12-15-4-6-17(7-5-15)25-29-22(14-35-25)26(32)33/h1-11,13-14,21H,12H2,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... |
Bioorg Med Chem Lett 22: 2843-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.070
BindingDB Entry DOI: 10.7270/Q2FB53ZS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50380756
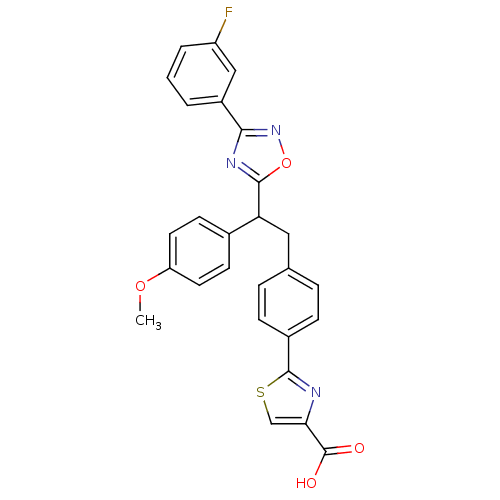
(CHEMBL2017855)Show SMILES COc1ccc(cc1)C(Cc1ccc(cc1)-c1nc(cs1)C(O)=O)c1nc(no1)-c1cccc(F)c1 Show InChI InChI=1S/C27H20FN3O4S/c1-34-21-11-9-17(10-12-21)22(25-30-24(31-35-25)19-3-2-4-20(28)14-19)13-16-5-7-18(8-6-16)26-29-23(15-36-26)27(32)33/h2-12,14-15,22H,13H2,1H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... |
Bioorg Med Chem Lett 22: 2843-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.070
BindingDB Entry DOI: 10.7270/Q2FB53ZS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM50402859

(CHEMBL2205591)Show SMILES FC(F)(F)c1ccccc1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1 Show InChI InChI=1S/C19H15F5N2O3S/c20-18(21)8-26(9-18)17(28)14-11-5-6-29-7-13(11)30-16(14)25-15(27)10-3-1-2-4-12(10)19(22,23)24/h1-4H,5-9H2,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 594 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50380751
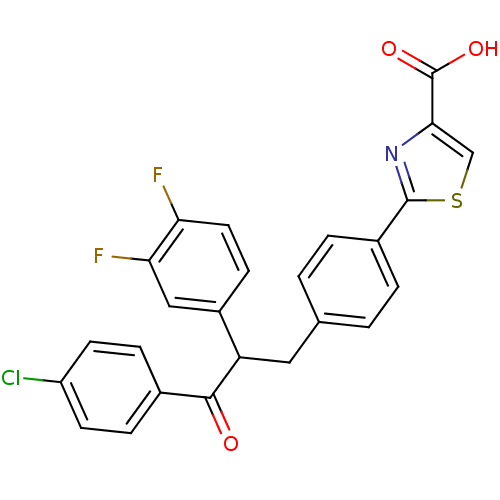
(CHEMBL2017850)Show SMILES OC(=O)c1csc(n1)-c1ccc(CC(C(=O)c2ccc(Cl)cc2)c2ccc(F)c(F)c2)cc1 Show InChI InChI=1S/C25H16ClF2NO3S/c26-18-8-5-15(6-9-18)23(30)19(17-7-10-20(27)21(28)12-17)11-14-1-3-16(4-2-14)24-29-22(13-33-24)25(31)32/h1-10,12-13,19H,11H2,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... |
Bioorg Med Chem Lett 22: 2843-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.070
BindingDB Entry DOI: 10.7270/Q2FB53ZS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Rattus norvegicus (Rat)) | BDBM50402857

(CHEMBL2205593)Show SMILES Fc1cccc(c1C(=O)Nc1sc2COCCc2c1C(=O)N1CC(F)(F)C1)C(F)(F)F Show InChI InChI=1S/C19H14F6N2O3S/c20-11-3-1-2-10(19(23,24)25)14(11)15(28)26-16-13(9-4-5-30-6-12(9)31-16)17(29)27-7-18(21,22)8-27/h1-3H,4-8H2,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from rat CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50165024

(CHEMBL195169 | N-[4-(3-Aza-bicyclo[3.1.0]hex-3-yl)...)Show SMILES OC(C(=O)NCC#CCN1CC2CC2C1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C23H24N2O2/c26-22(24-13-7-8-14-25-16-18-15-19(18)17-25)23(27,20-9-3-1-4-10-20)21-11-5-2-6-12-21/h1-6,9-12,18-19,27H,13-17H2,(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 639 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50165013

(CHEMBL194870 | N-[4-(3-Aza-bicyclo[3.1.0]hex-3-yl)...)Show SMILES OC(C(=O)NCC#CCN1CC2CC2C1)(c1ccc(F)cc1)c1ccc(F)cc1 Show InChI InChI=1S/C23H22F2N2O2/c24-20-7-3-18(4-8-20)23(29,19-5-9-21(25)10-6-19)22(28)26-11-1-2-12-27-14-16-13-17(16)15-27/h3-10,16-17,29H,11-15H2,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 736 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50380749
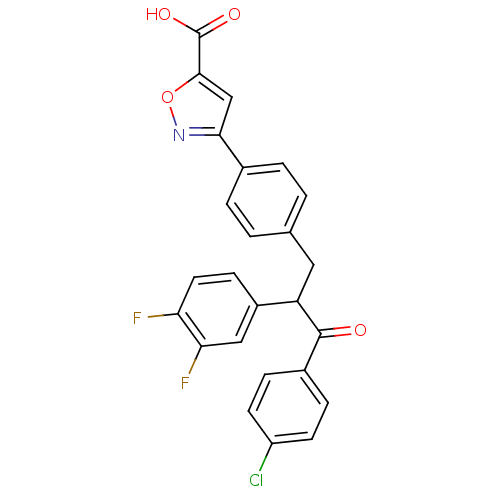
(CHEMBL2017848)Show SMILES OC(=O)c1cc(no1)-c1ccc(CC(C(=O)c2ccc(Cl)cc2)c2ccc(F)c(F)c2)cc1 Show InChI InChI=1S/C25H16ClF2NO4/c26-18-8-5-16(6-9-18)24(30)19(17-7-10-20(27)21(28)12-17)11-14-1-3-15(4-2-14)22-13-23(25(31)32)33-29-22/h1-10,12-13,19H,11H2,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... |
Bioorg Med Chem Lett 22: 2843-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.070
BindingDB Entry DOI: 10.7270/Q2FB53ZS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402860

(CHEMBL2205588)Show SMILES COC1CN(C1)C(=O)c1c(NC(=O)c2ccccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C20H19F3N2O4S/c1-28-11-8-25(9-11)19(27)16-13-6-7-29-10-15(13)30-18(16)24-17(26)12-4-2-3-5-14(12)20(21,22)23/h2-5,11H,6-10H2,1H3,(H,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 899 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402861
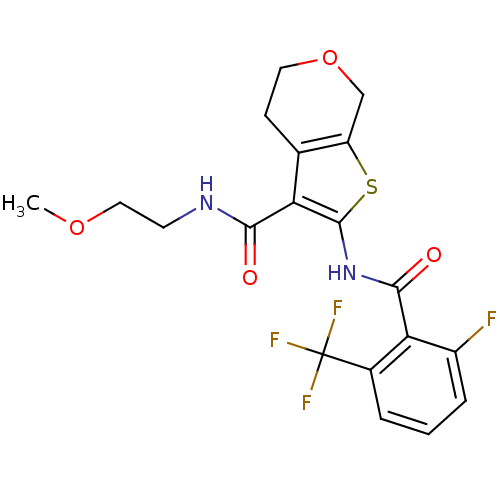
(CHEMBL2205584)Show SMILES COCCNC(=O)c1c(NC(=O)c2c(F)cccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C19H18F4N2O4S/c1-28-8-6-24-16(26)14-10-5-7-29-9-13(10)30-18(14)25-17(27)15-11(19(21,22)23)3-2-4-12(15)20/h2-4H,5-9H2,1H3,(H,24,26)(H,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 904 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50165014

(CHEMBL190459 | Cyclopentyl-hydroxy-(4-methoxy-phen...)Show SMILES COc1ccc(cc1)C(O)(C1CCCC1)C(=O)OCC#CCN1CC2CC2C1 Show InChI InChI=1S/C23H29NO4/c1-27-21-10-8-20(9-11-21)23(26,19-6-2-3-7-19)22(25)28-13-5-4-12-24-15-17-14-18(17)16-24/h8-11,17-19,26H,2-3,6-7,12-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 964 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M3 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50402862

(CHEMBL2205620)Show SMILES COCCNC(=O)c1c(NC(=O)c2ccccc2C(F)(F)F)sc2COCCc12 Show InChI InChI=1S/C19H19F3N2O4S/c1-27-9-7-23-17(26)15-12-6-8-28-10-14(12)29-18(15)24-16(25)11-4-2-3-5-13(11)19(20,21)22/h2-5H,6-10H2,1H3,(H,23,26)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 999 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed Pharma Pvt. Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP55,940 from human CB2 receptor after 60 mins by microscintillation counting analysis |
Bioorg Med Chem Lett 22: 7314-21 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.087
BindingDB Entry DOI: 10.7270/Q2QR4Z9V |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50165011

((1R,5S)-3-[3-(2-Benzyloxy-5-methyl-phenyl)-3-pheny...)Show SMILES CC1[C@@H]2C[C@@H]2CN1CCC(c1ccccc1)c1cc(C)ccc1OCc1ccccc1 Show InChI InChI=1S/C29H33NO/c1-21-13-14-29(31-20-23-9-5-3-6-10-23)28(17-21)26(24-11-7-4-8-12-24)15-16-30-19-25-18-27(25)22(30)2/h3-14,17,22,25-27H,15-16,18-20H2,1-2H3/t22?,25-,26?,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M3 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50165021

(2-[3-((1R,5S)-1,5-Dimethyl-3-aza-bicyclo[3.1.0]hex...)Show SMILES Cc1ccc(O)c(c1)C(CCN1C[C@@]2(C)C[C@@]2(C)C1)c1ccccc1 Show InChI InChI=1S/C23H29NO/c1-17-9-10-21(25)20(13-17)19(18-7-5-4-6-8-18)11-12-24-15-22(2)14-23(22,3)16-24/h4-10,13,19,25H,11-12,14-16H2,1-3H3/t19?,22-,23+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M3 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50165021

(2-[3-((1R,5S)-1,5-Dimethyl-3-aza-bicyclo[3.1.0]hex...)Show SMILES Cc1ccc(O)c(c1)C(CCN1C[C@@]2(C)C[C@@]2(C)C1)c1ccccc1 Show InChI InChI=1S/C23H29NO/c1-17-9-10-21(25)20(13-17)19(18-7-5-4-6-8-18)11-12-24-15-22(2)14-23(22,3)16-24/h4-10,13,19,25H,11-12,14-16H2,1-3H3/t19?,22-,23+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50165009

((1R,5S)-3-[3-(2-Benzyloxy-5-methyl-phenyl)-3-pheny...)Show SMILES Cc1ccc(OCc2ccccc2)c(c1)C(CCN1C[C@@H]2C[C@@H]2C1)c1ccccc1 Show InChI InChI=1S/C28H31NO/c1-21-12-13-28(30-20-22-8-4-2-5-9-22)27(16-21)26(23-10-6-3-7-11-23)14-15-29-18-24-17-25(24)19-29/h2-13,16,24-26H,14-15,17-20H2,1H3/t24-,25+,26? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for rat Muscarinic acetylcholine receptor M3 |
Bioorg Med Chem Lett 15: 2093-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.036
BindingDB Entry DOI: 10.7270/Q2DJ5F4X |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50380738

(CHEMBL2017836)Show SMILES OC(=O)c1cc(no1)-c1ccc(CC(C(=O)c2ccccc2)c2ccccc2)cc1 Show InChI InChI=1S/C25H19NO4/c27-24(20-9-5-2-6-10-20)21(18-7-3-1-4-8-18)15-17-11-13-19(14-12-17)22-16-23(25(28)29)30-26-22/h1-14,16,21H,15H2,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Advinus Therapeutics Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PTP1B expressed in Escherichia coli using para-nitrophenyl phosphate as substrate preincubated for 30 mins followed b... |
Bioorg Med Chem Lett 22: 2843-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.070
BindingDB Entry DOI: 10.7270/Q2FB53ZS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data