 Found 1682 hits with Last Name = 'sato' and Initial = 'm'
Found 1682 hits with Last Name = 'sato' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50098965

(3-{2-[(Z)-4-Carbamimidoyl-benzoylimino]-3,4-dimeth...)Show SMILES Cc1c(CCC(O)=O)s\c(=N/C(=O)c2ccc(cc2)C(N)=N)n1C Show InChI InChI=1S/C16H18N4O3S/c1-9-12(7-8-13(21)22)24-16(20(9)2)19-15(23)11-5-3-10(4-6-11)14(17)18/h3-6H,7-8H2,1-2H3,(H3,17,18)(H,21,22)/b19-16- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co. Ltd.
Curated by ChEMBL
| Assay Description
Binding to [125I]- fibrinogen by washed platelet fibrinogen receptor |
Bioorg Med Chem Lett 11: 1031-5 (2001)
BindingDB Entry DOI: 10.7270/Q29G5M2G |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50055569
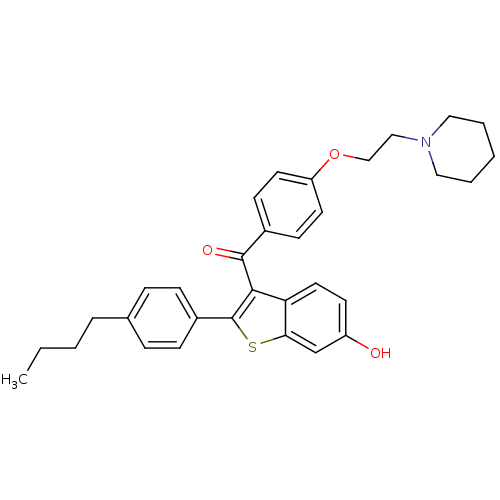
(CHEMBL46937 | [2-(4-Butyl-phenyl)-6-hydroxy-benzo[...)Show SMILES CCCCc1ccc(cc1)-c1sc2cc(O)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C32H35NO3S/c1-2-3-7-23-8-10-25(11-9-23)32-30(28-17-14-26(34)22-29(28)37-32)31(35)24-12-15-27(16-13-24)36-21-20-33-18-5-4-6-19-33/h8-17,22,34H,2-7,18-21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from ERalpha after 4 hrs by scintillation counting |
ACS Med Chem Lett 3: 207-210 (2012)
Article DOI: 10.1021/ml2002532
BindingDB Entry DOI: 10.7270/Q23F4QP6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50055569

(CHEMBL46937 | [2-(4-Butyl-phenyl)-6-hydroxy-benzo[...)Show SMILES CCCCc1ccc(cc1)-c1sc2cc(O)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C32H35NO3S/c1-2-3-7-23-8-10-25(11-9-23)32-30(28-17-14-26(34)22-29(28)37-32)31(35)24-12-15-27(16-13-24)36-21-20-33-18-5-4-6-19-33/h8-17,22,34H,2-7,18-21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha ligand binding domain |
ACS Med Chem Lett 3: 207-210 (2012)
Article DOI: 10.1021/ml2002532
BindingDB Entry DOI: 10.7270/Q23F4QP6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50181362
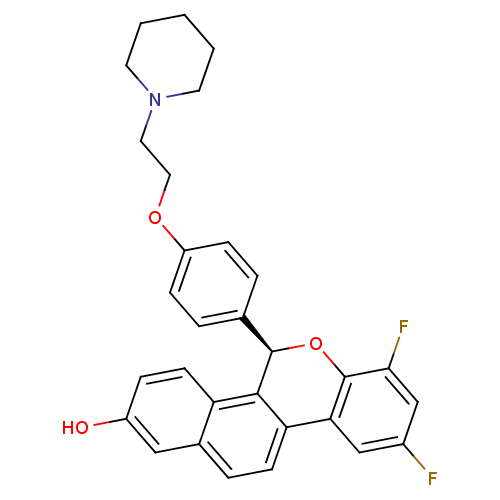
((R)-(+)-7,9-difluoro-5-[4-(2-piperidin-1-ylethoxy)...)Show SMILES Oc1ccc2c3[C@H](Oc4c(F)cc(F)cc4-c3ccc2c1)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C30H27F2NO3/c31-21-17-26-25-10-6-20-16-22(34)7-11-24(20)28(25)29(36-30(26)27(32)18-21)19-4-8-23(9-5-19)35-15-14-33-12-2-1-3-13-33/h4-11,16-18,29,34H,1-3,12-15H2/t29-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]17beta-estradiol from ERalpha |
J Med Chem 49: 843-6 (2006)
Article DOI: 10.1021/jm0509795
BindingDB Entry DOI: 10.7270/Q20R9Q60 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM19441

(2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...)Show SMILES Oc1ccc(cc1)-c1sc2cc(O)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H27NO4S/c30-21-8-4-20(5-9-21)28-26(24-13-10-22(31)18-25(24)34-28)27(32)19-6-11-23(12-7-19)33-17-16-29-14-2-1-3-15-29/h4-13,18,30-31H,1-3,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from ERalpha after 4 hrs by scintillation counting |
ACS Med Chem Lett 3: 207-210 (2012)
Article DOI: 10.1021/ml2002532
BindingDB Entry DOI: 10.7270/Q23F4QP6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM19441

(2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...)Show SMILES Oc1ccc(cc1)-c1sc2cc(O)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H27NO4S/c30-21-8-4-20(5-9-21)28-26(24-13-10-22(31)18-25(24)34-28)27(32)19-6-11-23(12-7-19)33-17-16-29-14-2-1-3-15-29/h4-13,18,30-31H,1-3,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha ligand binding domain |
ACS Med Chem Lett 3: 207-210 (2012)
Article DOI: 10.1021/ml2002532
BindingDB Entry DOI: 10.7270/Q23F4QP6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM20608
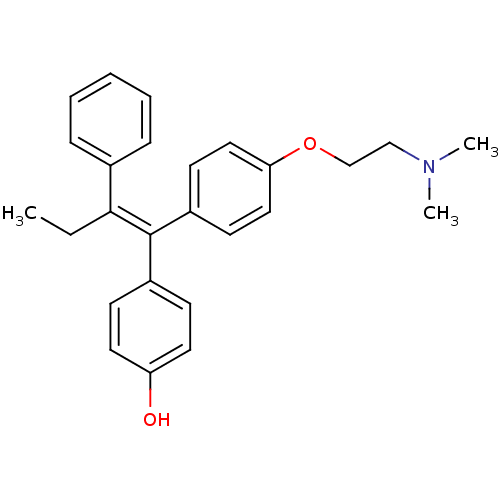
(4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO2/c1-4-25(20-8-6-5-7-9-20)26(21-10-14-23(28)15-11-21)22-12-16-24(17-13-22)29-19-18-27(2)3/h5-17,28H,4,18-19H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from ERalpha after 4 hrs by scintillation counting |
ACS Med Chem Lett 3: 207-210 (2012)
Article DOI: 10.1021/ml2002532
BindingDB Entry DOI: 10.7270/Q23F4QP6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor
(Homo sapiens (Human)) | BDBM19441

(2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...)Show SMILES Oc1ccc(cc1)-c1sc2cc(O)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H27NO4S/c30-21-8-4-20(5-9-21)28-26(24-13-10-22(31)18-25(24)34-28)27(32)19-6-11-23(12-7-19)33-17-16-29-14-2-1-3-15-29/h4-13,18,30-31H,1-3,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha (unknown origin) |
Bioorg Med Chem 24: 759-67 (2016)
Article DOI: 10.1016/j.bmc.2015.12.045
BindingDB Entry DOI: 10.7270/Q2GX4FJB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50181362

((R)-(+)-7,9-difluoro-5-[4-(2-piperidin-1-ylethoxy)...)Show SMILES Oc1ccc2c3[C@H](Oc4c(F)cc(F)cc4-c3ccc2c1)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C30H27F2NO3/c31-21-17-26-25-10-6-20-16-22(34)7-11-24(20)28(25)29(36-30(26)27(32)18-21)19-4-8-23(9-5-19)35-15-14-33-12-2-1-3-13-33/h4-11,16-18,29,34H,1-3,12-15H2/t29-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]17beta-estradiol from ERbeta |
J Med Chem 49: 843-6 (2006)
Article DOI: 10.1021/jm0509795
BindingDB Entry DOI: 10.7270/Q20R9Q60 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM20607
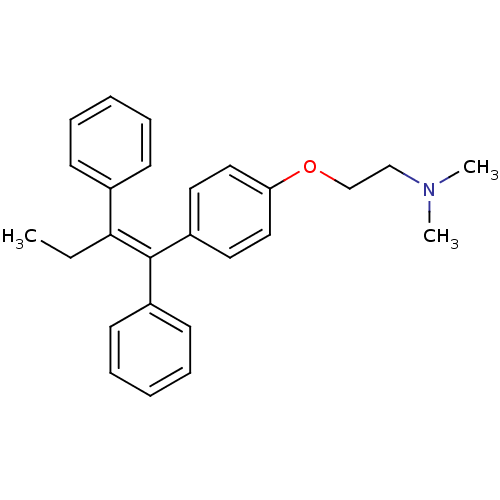
((2-{4-[(1Z)-1,2-diphenylbut-1-en-1-yl]phenoxy}ethy...)Show SMILES CC\C(=C(/c1ccccc1)c1ccc(OCCN(C)C)cc1)c1ccccc1 Show InChI InChI=1S/C26H29NO/c1-4-25(21-11-7-5-8-12-21)26(22-13-9-6-10-14-22)23-15-17-24(18-16-23)28-20-19-27(2)3/h5-18H,4,19-20H2,1-3H3/b26-25- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from ERbeta after 4 hrs by scintillation counting |
ACS Med Chem Lett 3: 207-210 (2012)
Article DOI: 10.1021/ml2002532
BindingDB Entry DOI: 10.7270/Q23F4QP6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM20628
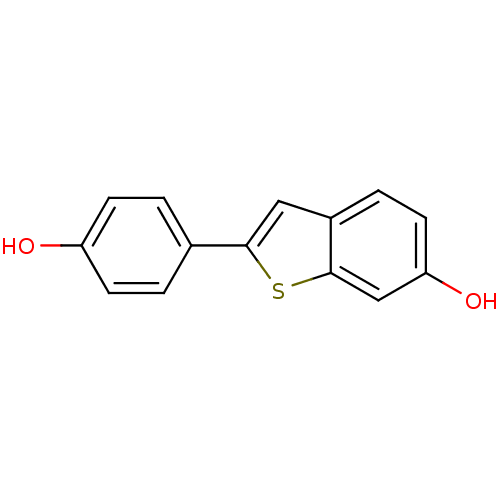
(2-(4-hydroxyphenyl)-1-benzothiophen-6-ol | CHEMBL4...)Show InChI InChI=1S/C14H10O2S/c15-11-4-1-9(2-5-11)13-7-10-3-6-12(16)8-14(10)17-13/h1-8,15-16H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to ERbeta (unknown origin) |
Bioorg Med Chem 24: 759-67 (2016)
Article DOI: 10.1016/j.bmc.2015.12.045
BindingDB Entry DOI: 10.7270/Q2GX4FJB |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM19441

(2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...)Show SMILES Oc1ccc(cc1)-c1sc2cc(O)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H27NO4S/c30-21-8-4-20(5-9-21)28-26(24-13-10-22(31)18-25(24)34-28)27(32)19-6-11-23(12-7-19)33-17-16-29-14-2-1-3-15-29/h4-13,18,30-31H,1-3,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from ERbeta after 4 hrs by scintillation counting |
ACS Med Chem Lett 3: 207-210 (2012)
Article DOI: 10.1021/ml2002532
BindingDB Entry DOI: 10.7270/Q23F4QP6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50123720
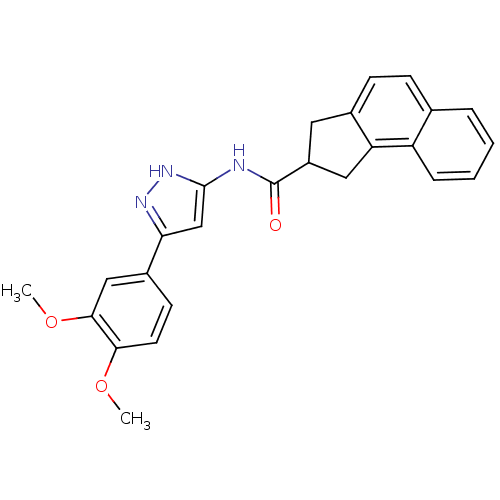
(2,3-Dihydro-1H-cyclopenta[a]naphthalene-2-carboxyl...)Show SMILES COc1ccc(cc1OC)-c1cc(NC(=O)C2Cc3ccc4ccccc4c3C2)[nH]n1 Show InChI InChI=1S/C25H23N3O3/c1-30-22-10-9-17(13-23(22)31-2)21-14-24(28-27-21)26-25(29)18-11-16-8-7-15-5-3-4-6-19(15)20(16)12-18/h3-10,13-14,18H,11-12H2,1-2H3,(H2,26,27,28,29) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells |
J Med Chem 46: 666-9 (2003)
Article DOI: 10.1021/jm025513q
BindingDB Entry DOI: 10.7270/Q2028QWP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50123724
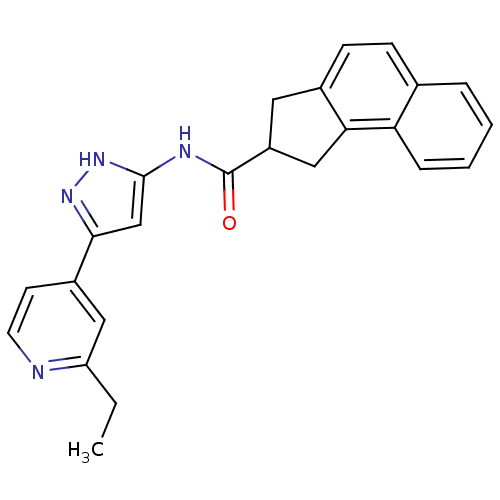
(2,3-Dihydro-1H-cyclopenta[a]naphthalene-2-carboxyl...)Show SMILES CCc1cc(ccn1)-c1cc(NC(=O)C2Cc3ccc4ccccc4c3C2)[nH]n1 Show InChI InChI=1S/C24H22N4O/c1-2-19-12-17(9-10-25-19)22-14-23(28-27-22)26-24(29)18-11-16-8-7-15-5-3-4-6-20(15)21(16)13-18/h3-10,12,14,18H,2,11,13H2,1H3,(H2,26,27,28,29) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells |
J Med Chem 46: 666-9 (2003)
Article DOI: 10.1021/jm025513q
BindingDB Entry DOI: 10.7270/Q2028QWP |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM20628

(2-(4-hydroxyphenyl)-1-benzothiophen-6-ol | CHEMBL4...)Show InChI InChI=1S/C14H10O2S/c15-11-4-1-9(2-5-11)13-7-10-3-6-12(16)8-14(10)17-13/h1-8,15-16H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha (unknown origin) |
Bioorg Med Chem 24: 759-67 (2016)
Article DOI: 10.1016/j.bmc.2015.12.045
BindingDB Entry DOI: 10.7270/Q2GX4FJB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM19441

(2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...)Show SMILES Oc1ccc(cc1)-c1sc2cc(O)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H27NO4S/c30-21-8-4-20(5-9-21)28-26(24-13-10-22(31)18-25(24)34-28)27(32)19-6-11-23(12-7-19)33-17-16-29-14-2-1-3-15-29/h4-13,18,30-31H,1-3,14-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to ERbeta (unknown origin) |
Bioorg Med Chem 24: 759-67 (2016)
Article DOI: 10.1016/j.bmc.2015.12.045
BindingDB Entry DOI: 10.7270/Q2GX4FJB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50330441
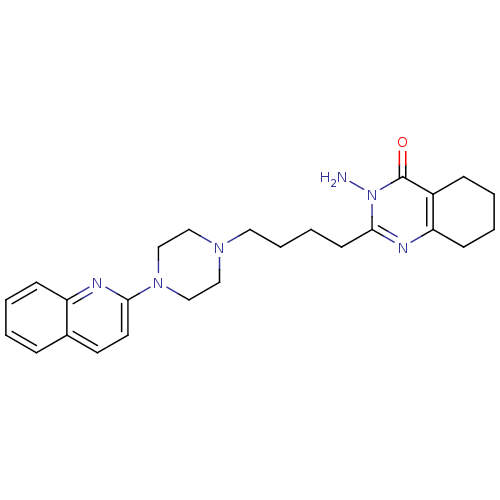
(3-amino-2-(4-(4-(quinolin-2-yl)piperazin-1-yl)buty...)Show SMILES Nn1c(CCCCN2CCN(CC2)c2ccc3ccccc3n2)nc2CCCCc2c1=O Show InChI InChI=1S/C25H32N6O/c26-31-24(28-22-10-4-2-8-20(22)25(31)32)11-5-6-14-29-15-17-30(18-16-29)23-13-12-19-7-1-3-9-21(19)27-23/h1,3,7,9,12-13H,2,4-6,8,10-11,14-18,26H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ASKA Pharmaceutical Co, Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3 receptor |
J Med Chem 53: 7549-63 (2010)
Article DOI: 10.1021/jm1002292
BindingDB Entry DOI: 10.7270/Q27082D1 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50181363
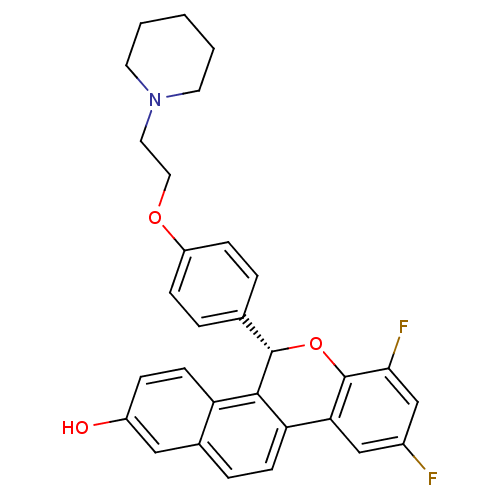
((S)-(-)-7,9-difluoro-5-[4-(2-piperidin-1-ylethoxy)...)Show SMILES Oc1ccc2c3[C@@H](Oc4c(F)cc(F)cc4-c3ccc2c1)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C30H27F2NO3/c31-21-17-26-25-10-6-20-16-22(34)7-11-24(20)28(25)29(36-30(26)27(32)18-21)19-4-8-23(9-5-19)35-15-14-33-12-2-1-3-13-33/h4-11,16-18,29,34H,1-3,12-15H2/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]17beta-estradiol from ERalpha |
J Med Chem 49: 843-6 (2006)
Article DOI: 10.1021/jm0509795
BindingDB Entry DOI: 10.7270/Q20R9Q60 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50123724

(2,3-Dihydro-1H-cyclopenta[a]naphthalene-2-carboxyl...)Show SMILES CCc1cc(ccn1)-c1cc(NC(=O)C2Cc3ccc4ccccc4c3C2)[nH]n1 Show InChI InChI=1S/C24H22N4O/c1-2-19-12-17(9-10-25-19)22-14-23(28-27-22)26-24(29)18-11-16-8-7-15-5-3-4-6-20(15)21(16)13-18/h3-10,12,14,18H,2,11,13H2,1H3,(H2,26,27,28,29) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells |
J Med Chem 46: 666-9 (2003)
Article DOI: 10.1021/jm025513q
BindingDB Entry DOI: 10.7270/Q2028QWP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50123724

(2,3-Dihydro-1H-cyclopenta[a]naphthalene-2-carboxyl...)Show SMILES CCc1cc(ccn1)-c1cc(NC(=O)C2Cc3ccc4ccccc4c3C2)[nH]n1 Show InChI InChI=1S/C24H22N4O/c1-2-19-12-17(9-10-25-19)22-14-23(28-27-22)26-24(29)18-11-16-8-7-15-5-3-4-6-20(15)21(16)13-18/h3-10,12,14,18H,2,11,13H2,1H3,(H2,26,27,28,29) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells |
J Med Chem 46: 666-9 (2003)
Article DOI: 10.1021/jm025513q
BindingDB Entry DOI: 10.7270/Q2028QWP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50073047
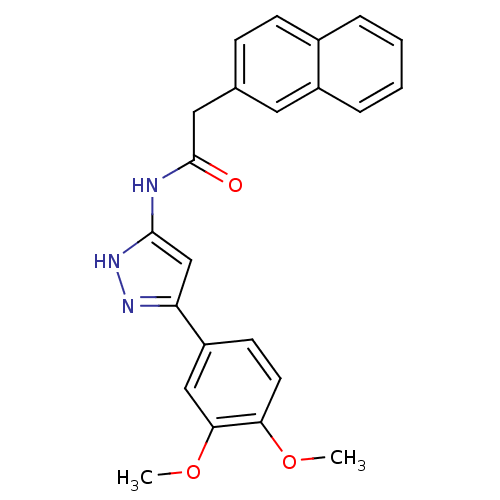
(CHEMBL291666 | N-[5-(3,4-Dimethoxy-phenyl)-1H-pyra...)Show SMILES COc1ccc(cc1OC)-c1cc(NC(=O)Cc2ccc3ccccc3c2)[nH]n1 Show InChI InChI=1S/C23H21N3O3/c1-28-20-10-9-18(13-21(20)29-2)19-14-22(26-25-19)24-23(27)12-15-7-8-16-5-3-4-6-17(16)11-15/h3-11,13-14H,12H2,1-2H3,(H2,24,25,26,27) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells |
J Med Chem 46: 666-9 (2003)
Article DOI: 10.1021/jm025513q
BindingDB Entry DOI: 10.7270/Q2028QWP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50123729
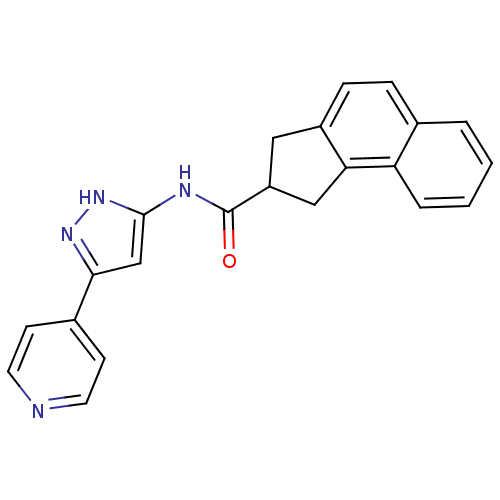
(2,3-Dihydro-1H-cyclopenta[a]naphthalene-2-carboxyl...)Show SMILES O=C(Nc1cc(n[nH]1)-c1ccncc1)C1Cc2ccc3ccccc3c2C1 Show InChI InChI=1S/C22H18N4O/c27-22(24-21-13-20(25-26-21)15-7-9-23-10-8-15)17-11-16-6-5-14-3-1-2-4-18(14)19(16)12-17/h1-10,13,17H,11-12H2,(H2,24,25,26,27) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells |
J Med Chem 46: 666-9 (2003)
Article DOI: 10.1021/jm025513q
BindingDB Entry DOI: 10.7270/Q2028QWP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50409778

(CHEMBL2110162)Show SMILES OC(=O)CCc1cccc(CC(=O)Nc2cc(n[nH]2)-c2ccc(Cl)cc2)c1 Show InChI InChI=1S/C20H18ClN3O3/c21-16-7-5-15(6-8-16)17-12-18(24-23-17)22-19(25)11-14-3-1-2-13(10-14)4-9-20(26)27/h1-3,5-8,10,12H,4,9,11H2,(H,26,27)(H2,22,23,24,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells |
J Med Chem 46: 666-9 (2003)
Article DOI: 10.1021/jm025513q
BindingDB Entry DOI: 10.7270/Q2028QWP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50123735

(CHEMBL160443 | Indan-2-carboxylic acid [5-(3,4-dim...)Show SMILES COc1ccc(cc1OC)-c1cc(NC(=O)C2Cc3ccccc3C2)[nH]n1 Show InChI InChI=1S/C21H21N3O3/c1-26-18-8-7-15(11-19(18)27-2)17-12-20(24-23-17)22-21(25)16-9-13-5-3-4-6-14(13)10-16/h3-8,11-12,16H,9-10H2,1-2H3,(H2,22,23,24,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells |
J Med Chem 46: 666-9 (2003)
Article DOI: 10.1021/jm025513q
BindingDB Entry DOI: 10.7270/Q2028QWP |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50055569

(CHEMBL46937 | [2-(4-Butyl-phenyl)-6-hydroxy-benzo[...)Show SMILES CCCCc1ccc(cc1)-c1sc2cc(O)ccc2c1C(=O)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C32H35NO3S/c1-2-3-7-23-8-10-25(11-9-23)32-30(28-17-14-26(34)22-29(28)37-32)31(35)24-12-15-27(16-13-24)36-21-20-33-18-5-4-6-19-33/h8-17,22,34H,2-7,18-21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]estradiol from ERbeta after 4 hrs by scintillation counting |
ACS Med Chem Lett 3: 207-210 (2012)
Article DOI: 10.1021/ml2002532
BindingDB Entry DOI: 10.7270/Q23F4QP6 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50181363

((S)-(-)-7,9-difluoro-5-[4-(2-piperidin-1-ylethoxy)...)Show SMILES Oc1ccc2c3[C@@H](Oc4c(F)cc(F)cc4-c3ccc2c1)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C30H27F2NO3/c31-21-17-26-25-10-6-20-16-22(34)7-11-24(20)28(25)29(36-30(26)27(32)18-21)19-4-8-23(9-5-19)35-15-14-33-12-2-1-3-13-33/h4-11,16-18,29,34H,1-3,12-15H2/t29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]17beta-estradiol from ERbeta |
J Med Chem 49: 843-6 (2006)
Article DOI: 10.1021/jm0509795
BindingDB Entry DOI: 10.7270/Q20R9Q60 |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50123717

(CHEMBL435036 | Indan-2-carboxylic acid [5-(4-chlor...)Show SMILES Clc1ccc(cc1)-c1cc(NC(=O)C2Cc3ccccc3C2)[nH]n1 Show InChI InChI=1S/C19H16ClN3O/c20-16-7-5-12(6-8-16)17-11-18(23-22-17)21-19(24)15-9-13-3-1-2-4-14(13)10-15/h1-8,11,15H,9-10H2,(H2,21,22,23,24) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells |
J Med Chem 46: 666-9 (2003)
Article DOI: 10.1021/jm025513q
BindingDB Entry DOI: 10.7270/Q2028QWP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50123726
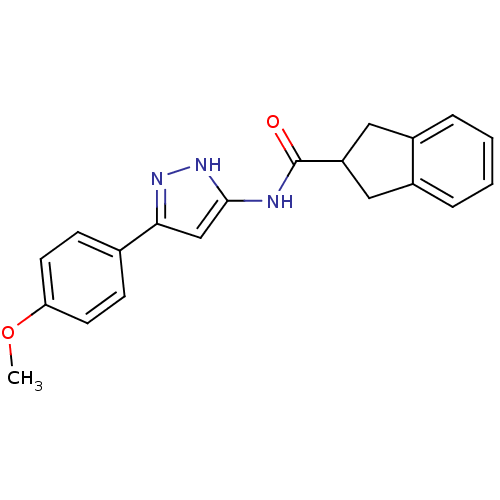
(CHEMBL159070 | Indan-2-carboxylic acid [5-(4-metho...)Show SMILES COc1ccc(cc1)-c1cc(NC(=O)C2Cc3ccccc3C2)[nH]n1 Show InChI InChI=1S/C20H19N3O2/c1-25-17-8-6-13(7-9-17)18-12-19(23-22-18)21-20(24)16-10-14-4-2-3-5-15(14)11-16/h2-9,12,16H,10-11H2,1H3,(H2,21,22,23,24) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells |
J Med Chem 46: 666-9 (2003)
Article DOI: 10.1021/jm025513q
BindingDB Entry DOI: 10.7270/Q2028QWP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50123727
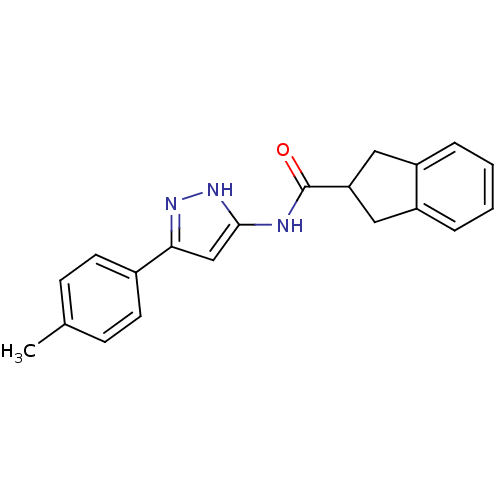
(CHEMBL158759 | Indan-2-carboxylic acid (5-p-tolyl-...)Show SMILES Cc1ccc(cc1)-c1cc(NC(=O)C2Cc3ccccc3C2)[nH]n1 Show InChI InChI=1S/C20H19N3O/c1-13-6-8-14(9-7-13)18-12-19(23-22-18)21-20(24)17-10-15-4-2-3-5-16(15)11-17/h2-9,12,17H,10-11H2,1H3,(H2,21,22,23,24) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells |
J Med Chem 46: 666-9 (2003)
Article DOI: 10.1021/jm025513q
BindingDB Entry DOI: 10.7270/Q2028QWP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
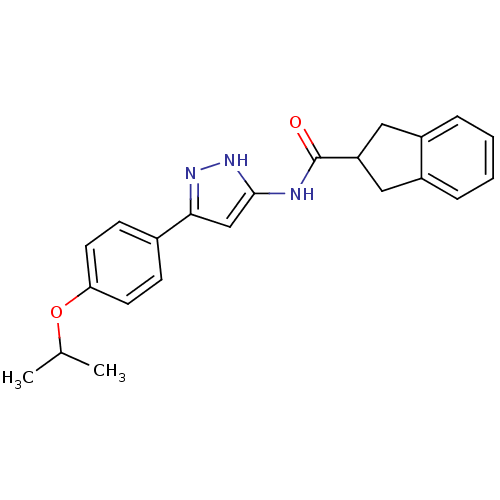
(Homo sapiens (Human)) | BDBM50123728
(CHEMBL160722 | Indan-2-carboxylic acid [5-(4-isopr...)Show SMILES CC(C)Oc1ccc(cc1)-c1cc(NC(=O)C2Cc3ccccc3C2)[nH]n1 Show InChI InChI=1S/C22H23N3O2/c1-14(2)27-19-9-7-15(8-10-19)20-13-21(25-24-20)23-22(26)18-11-16-5-3-4-6-17(16)12-18/h3-10,13-14,18H,11-12H2,1-2H3,(H2,23,24,25,26) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells |
J Med Chem 46: 666-9 (2003)
Article DOI: 10.1021/jm025513q
BindingDB Entry DOI: 10.7270/Q2028QWP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50123734

(CHEMBL158940 | Indan-2-carboxylic acid [5-(3-chlor...)Show SMILES Clc1cccc(c1)-c1cc(NC(=O)C2Cc3ccccc3C2)[nH]n1 Show InChI InChI=1S/C19H16ClN3O/c20-16-7-3-6-14(10-16)17-11-18(23-22-17)21-19(24)15-8-12-4-1-2-5-13(12)9-15/h1-7,10-11,15H,8-9H2,(H2,21,22,23,24) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells |
J Med Chem 46: 666-9 (2003)
Article DOI: 10.1021/jm025513q
BindingDB Entry DOI: 10.7270/Q2028QWP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50123732

(CHEMBL161398 | Indan-2-carboxylic acid (5-phenyl-1...)Show InChI InChI=1S/C19H17N3O/c23-19(16-10-14-8-4-5-9-15(14)11-16)20-18-12-17(21-22-18)13-6-2-1-3-7-13/h1-9,12,16H,10-11H2,(H2,20,21,22,23) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells |
J Med Chem 46: 666-9 (2003)
Article DOI: 10.1021/jm025513q
BindingDB Entry DOI: 10.7270/Q2028QWP |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50123730

(CHEMBL157588 | Indan-2-carboxylic acid [5-(2-chlor...)Show InChI InChI=1S/C19H16ClN3O/c20-16-8-4-3-7-15(16)17-11-18(23-22-17)21-19(24)14-9-12-5-1-2-6-13(12)10-14/h1-8,11,14H,9-10H2,(H2,21,22,23,24) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-PYY binding to human recombinant Neuropeptide Y receptor type 5 in LMtk-cells |
J Med Chem 46: 666-9 (2003)
Article DOI: 10.1021/jm025513q
BindingDB Entry DOI: 10.7270/Q2028QWP |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50013804
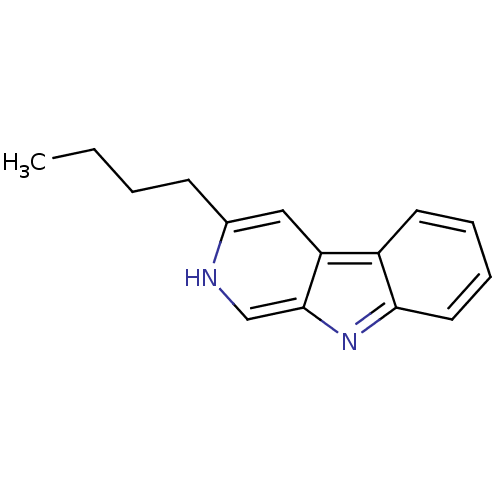
(10,13-Dimethyl-hexadecahydro-cyclopenta[a]phenanth...)Show InChI InChI=1S/C15H16N2/c1-2-3-6-11-9-13-12-7-4-5-8-14(12)17-15(13)10-16-11/h4-5,7-10,16H,2-3,6H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Toho University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant IDO assessed as inhibition of indoleamine 2,3-dioxygenase to kynurenine conversion after 60 mins by HPLC analysis |
Bioorg Med Chem 21: 1159-65 (2013)
Article DOI: 10.1016/j.bmc.2012.12.028
BindingDB Entry DOI: 10.7270/Q2RX9DDH |
More data for this
Ligand-Target Pair | |
Protein-arginine deiminase type-1
(Homo sapiens (Human)) | BDBM50355656

(CHEMBL1910970)Show SMILES NC(CF)=NCCC[C@H](NC(=O)c1ccccc1C(O)=O)C(N)=O |r,w:4.4| Show InChI InChI=1S/C15H19FN4O4/c16-8-12(17)19-7-3-6-11(13(18)21)20-14(22)9-4-1-2-5-10(9)15(23)24/h1-2,4-5,11H,3,6-8H2,(H2,17,19)(H2,18,21)(H,20,22)(H,23,24)/t11-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Irreversible inhibition of PAD1 assessed as hydrolysis of benzoyl-L-arginine ethyl ester preincubated for 15 mins measured after 15 mins by fluoromet... |
J Med Chem 54: 6919-35 (2011)
Article DOI: 10.1021/jm2008985
BindingDB Entry DOI: 10.7270/Q24F1R4M |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50500651
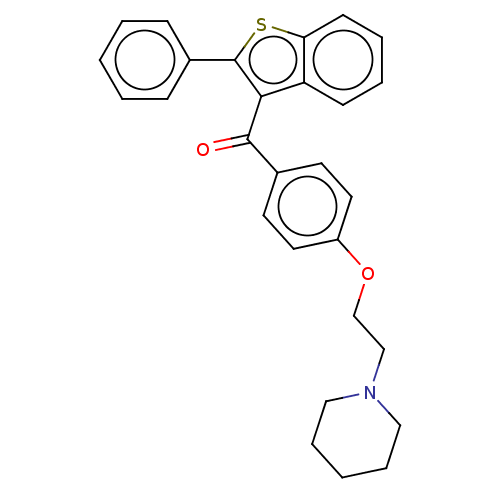
(CHEMBL1189544)Show SMILES O=C(c1c(sc2ccccc12)-c1ccccc1)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H27NO2S/c30-27(21-13-15-23(16-14-21)31-20-19-29-17-7-2-8-18-29)26-24-11-5-6-12-25(24)32-28(26)22-9-3-1-4-10-22/h1,3-6,9-16H,2,7-8,17-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to ERbeta (unknown origin) |
Bioorg Med Chem 24: 759-67 (2016)
Article DOI: 10.1016/j.bmc.2015.12.045
BindingDB Entry DOI: 10.7270/Q2GX4FJB |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50500651

(CHEMBL1189544)Show SMILES O=C(c1c(sc2ccccc12)-c1ccccc1)c1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H27NO2S/c30-27(21-13-15-23(16-14-21)31-20-19-29-17-7-2-8-18-29)26-24-11-5-6-12-25(24)32-28(26)22-9-3-1-4-10-22/h1,3-6,9-16H,2,7-8,17-20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to ERalpha (unknown origin) |
Bioorg Med Chem 24: 759-67 (2016)
Article DOI: 10.1016/j.bmc.2015.12.045
BindingDB Entry DOI: 10.7270/Q2GX4FJB |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 2
(RAT) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(RAT) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(MOUSE) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-9
(RAT) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Dual specificity calcium/calmodulin-dependent 3',5'-cyclic nucleotide phosphodiesterase 1A
(BOVINE) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Rattus norvegicus (Rat)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Rattus norvegicus (rat)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 1
(RAT) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member B1 [K65Q]
(Bos taurus (Cattle)) | BDBM86694

(N'-(3-Chloro-4-morpholinophenyl)-N-hydroxyform...)Show InChI InChI=1S/C11H14ClN3O2/c12-10-7-9(13-8-14-16)1-2-11(10)15-3-5-17-6-4-15/h1-2,7-8,16H,3-6H2,(H,13,14) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Taisho Pharmaceutical Co., Ltd
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 314: 77-85 (2005)
Article DOI: 10.1124/jpet.105.083964
BindingDB Entry DOI: 10.7270/Q2MW2FQQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data