

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM50364378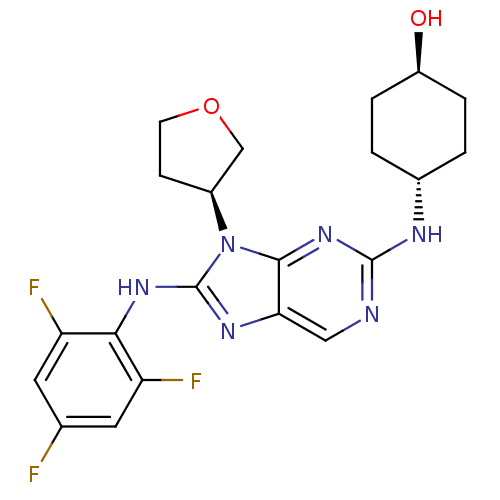 (CHEMBL1950289) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition of hexa-His-tagged JNK2 expressed in baculoviral system using GST-tagged cJun as substrate preincubated for 15 mins prior ATP addition mea... | Bioorg Med Chem Lett 22: 1433-8 (2012) Article DOI: 10.1016/j.bmcl.2011.12.027 BindingDB Entry DOI: 10.7270/Q2C829SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50364378 (CHEMBL1950289) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition of hexa-His-tagged JNK1 expressed in baculoviral system using GST-tagged c-Jun as substrate preincubated for 15 mins prior ATP addition me... | Bioorg Med Chem Lett 22: 1433-8 (2012) Article DOI: 10.1016/j.bmcl.2011.12.027 BindingDB Entry DOI: 10.7270/Q2C829SK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50005463 ((R)-1H-Indole-2-carboxylic acid (1-methyl-2-oxo-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Cholecystokinin-8 (125I-CCK-8) binding to Cholecystokinin type A receptor of rat pancreatic membranes | Bioorg Med Chem Lett 7: 169-174 (1997) Article DOI: 10.1016/S0960-894X(96)00609-9 BindingDB Entry DOI: 10.7270/Q27M07ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50070467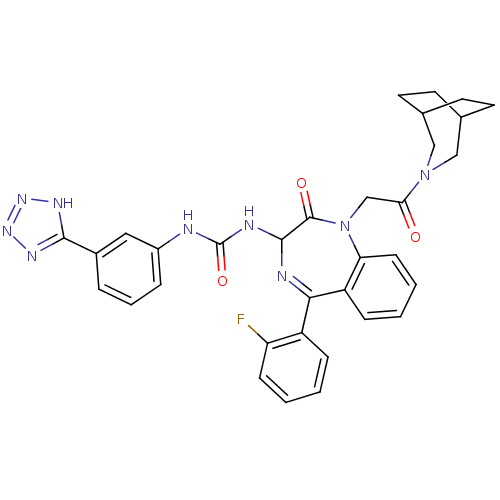 (1-[1-[2-(3-Aza-bicyclo[3.2.2]non-3-yl)-2-oxo-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of 124 I-CCK-8 binding at Cholecystokinin type B receptor on guinea pig cerebral cortical membranes | Bioorg Med Chem Lett 8: 1449-54 (1999) BindingDB Entry DOI: 10.7270/Q28P5ZMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50290398 (1-[1-[2-(3-Aza-bicyclo[3.2.2]non-3-yl)-2-oxo-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Cholecystokinin-8 binding to Cholecystokinin type B receptor of guinea pig cerebral cortical membranes | Bioorg Med Chem Lett 7: 169-174 (1997) Article DOI: 10.1016/S0960-894X(96)00609-9 BindingDB Entry DOI: 10.7270/Q27M07ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Mus musculus) | BDBM50247081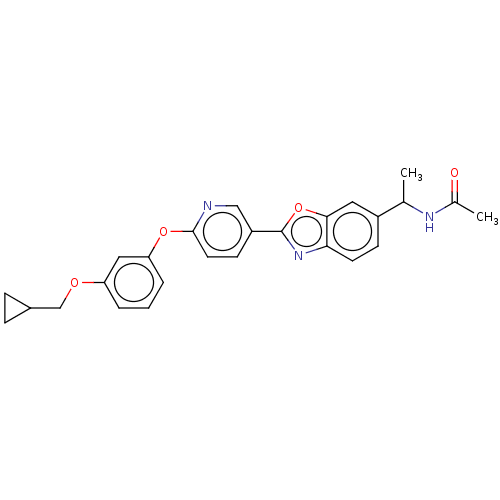 (CHEMBL4060253) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of mouse His-tagged ACC1 expressed in baculovirus infected SF9 cells using acetyl-CoA as substrate incubated for 60 mins followed by subst... | Bioorg Med Chem Lett 29: (2019) Article DOI: 10.1016/j.bmcl.2019.126749 BindingDB Entry DOI: 10.7270/Q2R214TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase (Candida albicans (Yeast)) | BDBM50100335 (CHEMBL2371681 | RO-09-3655 derivative) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta glucan synthase | Bioorg Med Chem Lett 11: 1273-6 (2001) BindingDB Entry DOI: 10.7270/Q2CN74FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50111656 (1-[2-(5-Chloro-2-oxo-benzothiazol-3-yl)-acetyl]-pi...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Tested for the inhibition of [125I]-PYY binding to HEK 293 cells stably expressed with human neuropeptide NPY Y5 receptor | Bioorg Med Chem Lett 12: 1171-5 (2002) BindingDB Entry DOI: 10.7270/Q26M365J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase (Candida albicans (Yeast)) | BDBM50100338 (CHEMBL267407 | RO-09-3655 derivative) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta glucan synthase | Bioorg Med Chem Lett 11: 1273-6 (2001) BindingDB Entry DOI: 10.7270/Q2CN74FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase (Candida albicans (Yeast)) | BDBM50100332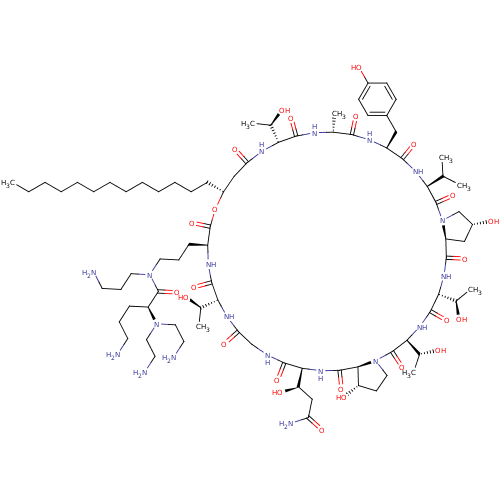 (CHEMBL415843 | RO-09-3655 derivative) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta glucan synthase | Bioorg Med Chem Lett 11: 1273-6 (2001) BindingDB Entry DOI: 10.7270/Q2CN74FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50290397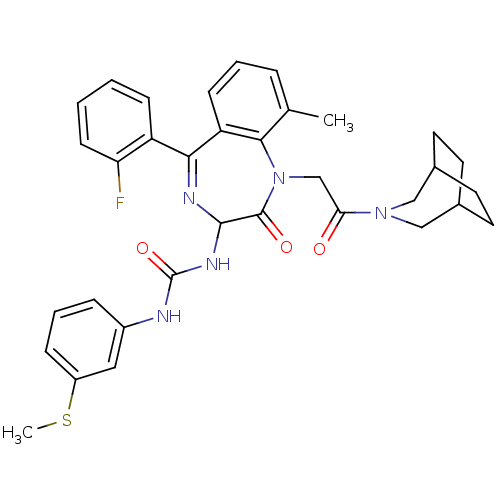 (1-[1-[2-(3-Aza-bicyclo[3.2.2]non-3-yl)-2-oxo-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Cholecystokinin-8 binding to Cholecystokinin type B receptor of guinea pig cerebral cortical membranes | Bioorg Med Chem Lett 7: 169-174 (1997) Article DOI: 10.1016/S0960-894X(96)00609-9 BindingDB Entry DOI: 10.7270/Q27M07ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50290396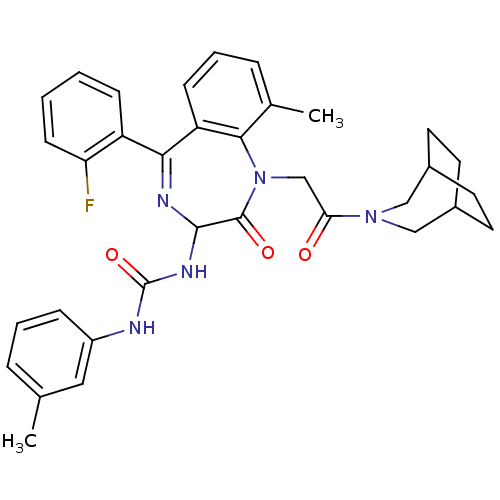 (1-[1-[2-(3-Aza-bicyclo[3.2.2]non-3-yl)-2-oxo-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Cholecystokinin-8 binding to Cholecystokinin type B receptor of guinea pig cerebral cortical membranes | Bioorg Med Chem Lett 7: 169-174 (1997) Article DOI: 10.1016/S0960-894X(96)00609-9 BindingDB Entry DOI: 10.7270/Q27M07ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50110571 (CHEMBL167991 | FR-230481 | Naphthalene-1-sulfonic ...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of 125 I-PYY binding to human Neuropeptide Y receptor type 5 | Bioorg Med Chem Lett 12: 799-802 (2002) BindingDB Entry DOI: 10.7270/Q2M044R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247130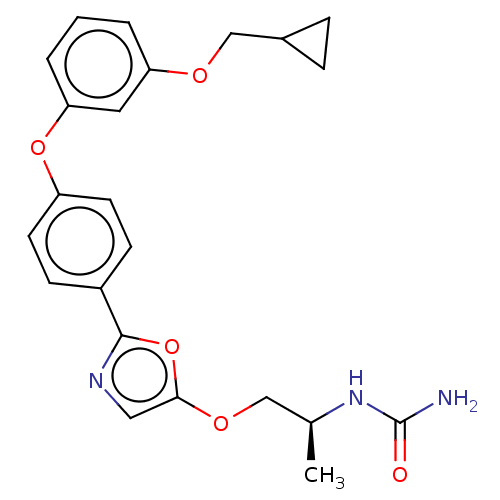 (CHEMBL4073202) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50111644 (1-[2-(5-Chloro-2-oxo-benzothiazol-3-yl)-acetyl]-pi...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of total specific binding of [125I]- -PYY to HEK 293 cells transfected with the Human NPY-Y5 cDNA (compound prepared by para... | Bioorg Med Chem Lett 12: 1171-5 (2002) BindingDB Entry DOI: 10.7270/Q26M365J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50111645 (1-[2-(5-Chloro-2-oxo-benzothiazol-3-yl)-acetyl]-pi...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of total specific binding of [125I]- -PYY to HEK 293 cells transfected with Human NPY-Y5 cDNA (compound prepared by manual s... | Bioorg Med Chem Lett 12: 1171-5 (2002) BindingDB Entry DOI: 10.7270/Q26M365J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase (Candida albicans (Yeast)) | BDBM50100333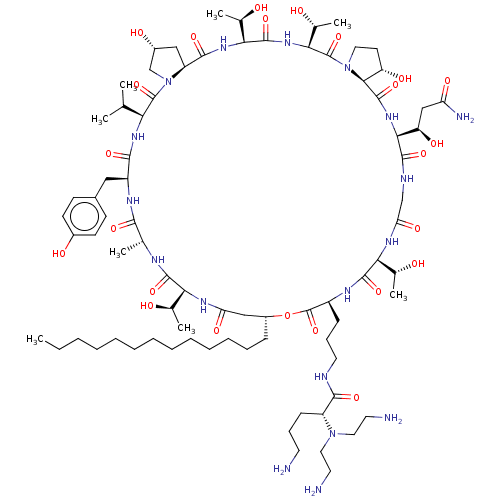 (CHEMBL2371765 | RO-09-3655 derivative) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta glucan synthase | Bioorg Med Chem Lett 11: 1273-6 (2001) BindingDB Entry DOI: 10.7270/Q2CN74FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50070464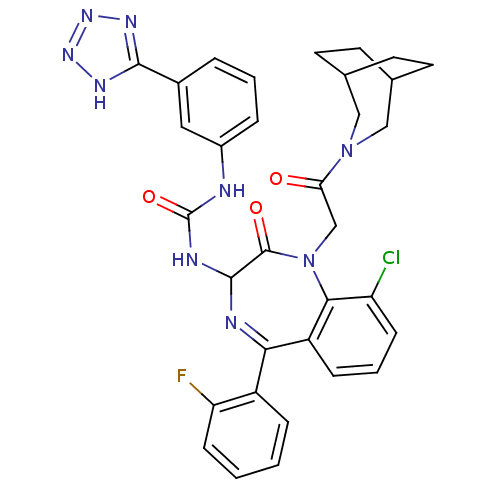 (1-[1-[2-(3-Aza-bicyclo[3.2.2]non-3-yl)-2-oxo-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of 124 I-CCK-8 binding at CCK-B receptorCholecystokinin type B receptorcerebral cortical membranes | Bioorg Med Chem Lett 8: 1449-54 (1999) BindingDB Entry DOI: 10.7270/Q28P5ZMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (RAT) | BDBM50290400 (1H-Indole-2-carboxylic acid [(S)-1-(2-fluoro-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Cholecystokinin-8 (125I-CCK-8) binding to Cholecystokinin type A receptor of rat pancreatic membranes | Bioorg Med Chem Lett 7: 169-174 (1997) Article DOI: 10.1016/S0960-894X(96)00609-9 BindingDB Entry DOI: 10.7270/Q27M07ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50070466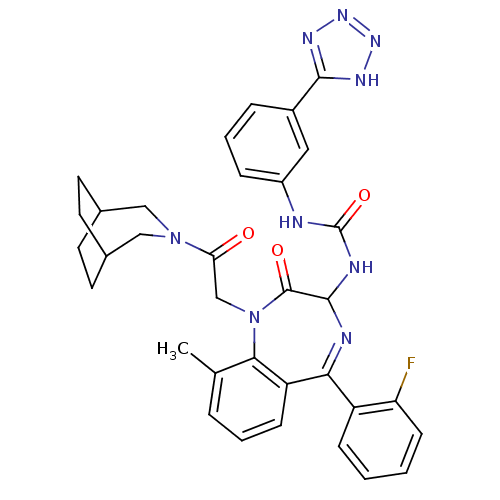 (1-[1-[2-(3-Aza-bicyclo[3.2.2]non-3-yl)-2-oxo-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of 124 I-CCK-8 binding at Cholecystokinin type B receptor on guinea pig cerebral cortical membranes | Bioorg Med Chem Lett 8: 1449-54 (1999) BindingDB Entry DOI: 10.7270/Q28P5ZMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50070466 (1-[1-[2-(3-Aza-bicyclo[3.2.2]non-3-yl)-2-oxo-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Cholecystokinin-8 binding to Cholecystokinin type A receptor of rat pancreatic membranes | Bioorg Med Chem Lett 7: 169-174 (1997) Article DOI: 10.1016/S0960-894X(96)00609-9 BindingDB Entry DOI: 10.7270/Q27M07ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50111645 (1-[2-(5-Chloro-2-oxo-benzothiazol-3-yl)-acetyl]-pi...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of total specific binding of [125I]- -PYY to HEK 293 cells transfected with the Human NPY-Y5 cDNA (compound prepared by para... | Bioorg Med Chem Lett 12: 1171-5 (2002) BindingDB Entry DOI: 10.7270/Q26M365J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50110562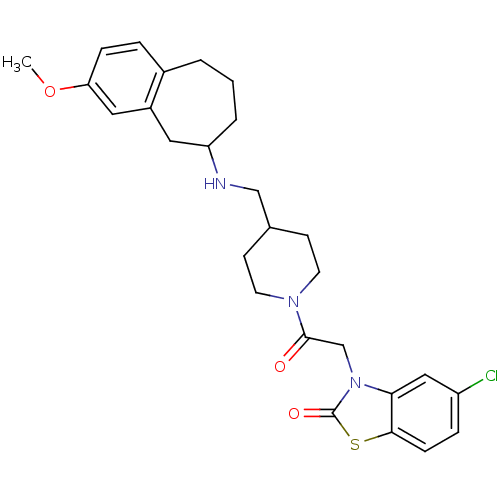 (5-Chloro-3-(2-{4-[(3-methoxy-6,7,8,9-tetrahydro-5H...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of 125 I-PYY binding to human Neuropeptide Y receptor type 5 | Bioorg Med Chem Lett 12: 799-802 (2002) BindingDB Entry DOI: 10.7270/Q2M044R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50111646 (1-[2-(5-Chloro-2-oxo-benzothiazol-3-yl)-acetyl]-pi...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of total specific binding of [125I]- -PYY to HEK 293 cells transfected with the Human NPY-Y5 cDNA (compound prepared by para... | Bioorg Med Chem Lett 12: 1171-5 (2002) BindingDB Entry DOI: 10.7270/Q26M365J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247081 (CHEMBL4060253) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of ACC1 in human HCT116 cells assessed as reduction of [14C]acetate uptake preincubated for 60 mins followed by addition of [14C]acetate a... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50070465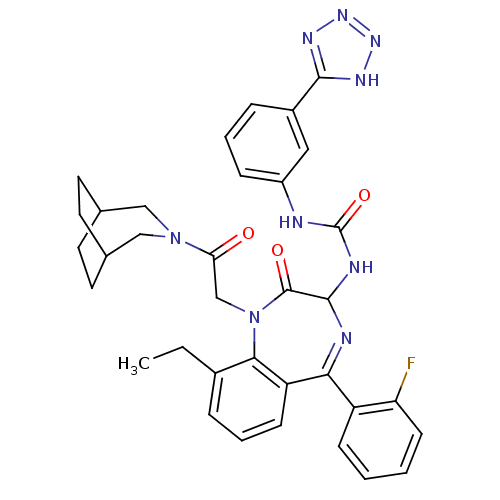 (1-[1-[2-(3-Aza-bicyclo[3.2.2]non-3-yl)-2-oxo-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of 124 I-CCK-8 binding at Cholecystokinin type B receptor on guinea pig cerebral cortical membranes | Bioorg Med Chem Lett 8: 1449-54 (1999) BindingDB Entry DOI: 10.7270/Q28P5ZMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase (Candida albicans (Yeast)) | BDBM50100331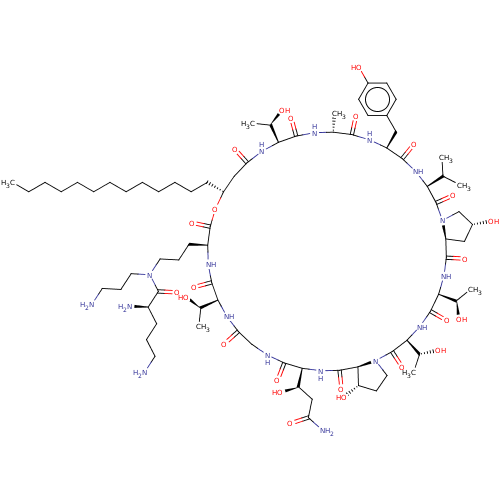 (CHEMBL2371715 | RO-09-3655 derivative) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta glucan synthase | Bioorg Med Chem Lett 11: 1273-6 (2001) BindingDB Entry DOI: 10.7270/Q2CN74FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50290401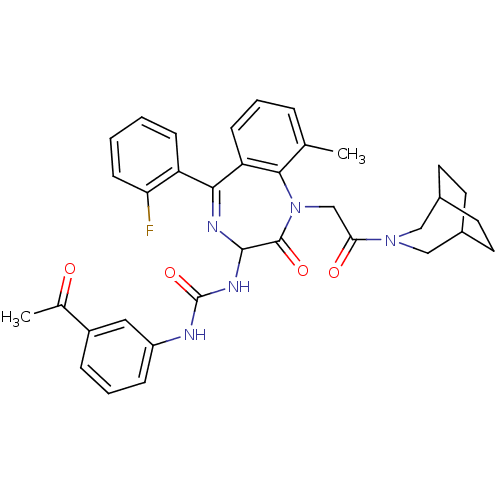 (1-(3-Acetyl-phenyl)-3-[1-[2-(3-aza-bicyclo[3.2.2]n...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Cholecystokinin-8 binding to Cholecystokinin type B receptor of guinea pig cerebral cortical membranes | Bioorg Med Chem Lett 7: 169-174 (1997) Article DOI: 10.1016/S0960-894X(96)00609-9 BindingDB Entry DOI: 10.7270/Q27M07ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50111647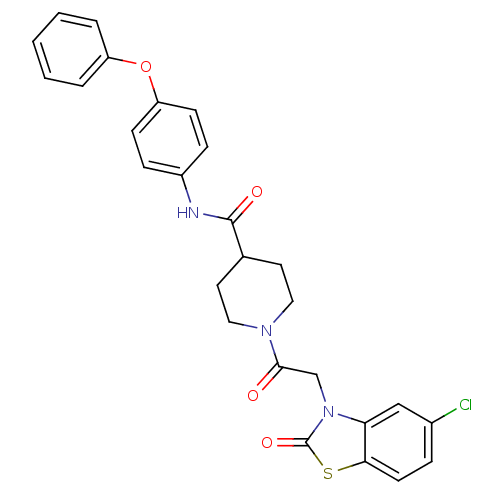 (1-[2-(5-Chloro-2-oxo-benzothiazol-3-yl)-acetyl]-pi...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of total specific binding of [125I]- -PYY to HEK 293 cells transfected with the Human NPY-Y5 cDNA (compound prepared by para... | Bioorg Med Chem Lett 12: 1171-5 (2002) BindingDB Entry DOI: 10.7270/Q26M365J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50111641 (1-[2-(5-Chloro-2-oxo-benzothiazol-3-yl)-acetyl]-pi...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of total specific binding of [125I]- -PYY to HEK 293 cells transfected with Human NPY-Y5 cDNA (compound prepared by manual s... | Bioorg Med Chem Lett 12: 1171-5 (2002) BindingDB Entry DOI: 10.7270/Q26M365J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase (Candida albicans (Yeast)) | BDBM50100329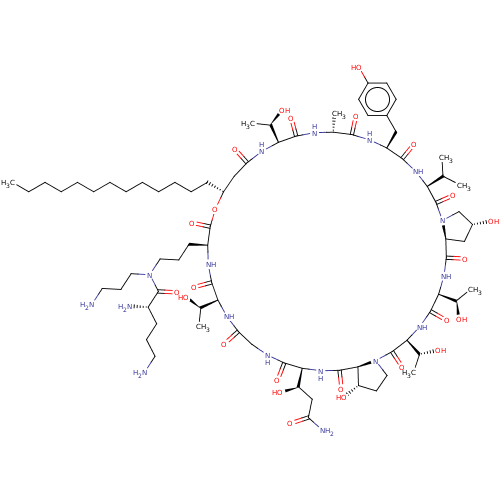 (CHEMBL2371727 | RO-09-3655 derivative) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta glucan synthase | Bioorg Med Chem Lett 11: 1273-6 (2001) BindingDB Entry DOI: 10.7270/Q2CN74FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase (Candida albicans (Yeast)) | BDBM50100337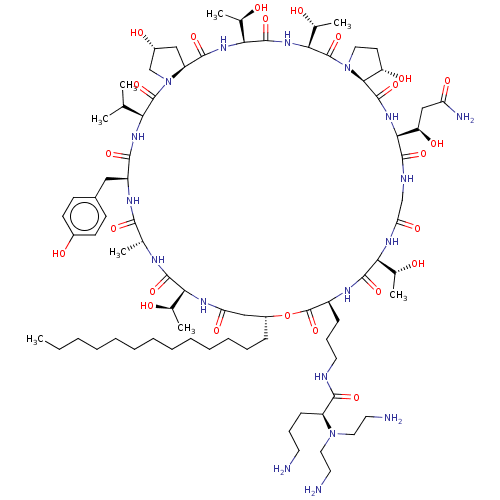 (CHEMBL2371766 | RO-09-3655 derivative) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta glucan synthase | Bioorg Med Chem Lett 11: 1273-6 (2001) BindingDB Entry DOI: 10.7270/Q2CN74FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247112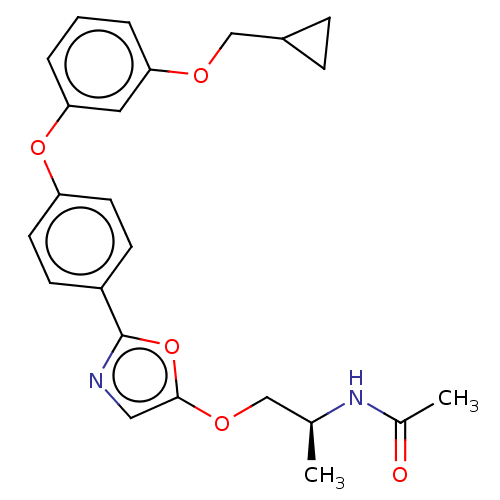 (CHEMBL4085633) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Homo sapiens (Human)) | BDBM50247114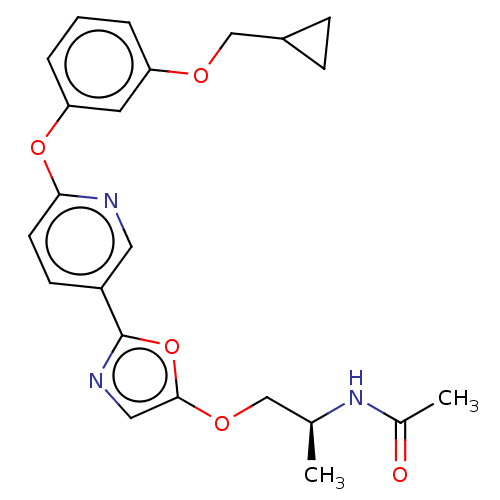 (CHEMBL4086127) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC1 expressed in SF-9 cells preincubated for 60 mins followed by addition of substrate solution containing acetyl-Co... | J Med Chem 61: 1098-1117 (2018) Article DOI: 10.1021/acs.jmedchem.7b01547 BindingDB Entry DOI: 10.7270/Q2HQ429W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50290399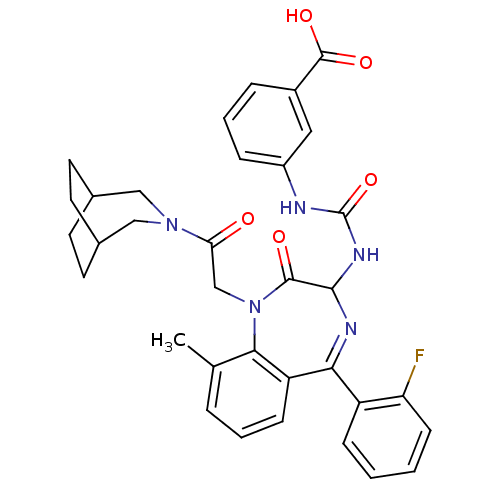 (3-{3-[1-[2-(3-Aza-bicyclo[3.2.2]non-3-yl)-2-oxo-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Cholecystokinin-8 binding to Cholecystokinin type B receptor of guinea pig cerebral cortical membranes | Bioorg Med Chem Lett 7: 169-174 (1997) Article DOI: 10.1016/S0960-894X(96)00609-9 BindingDB Entry DOI: 10.7270/Q27M07ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50110567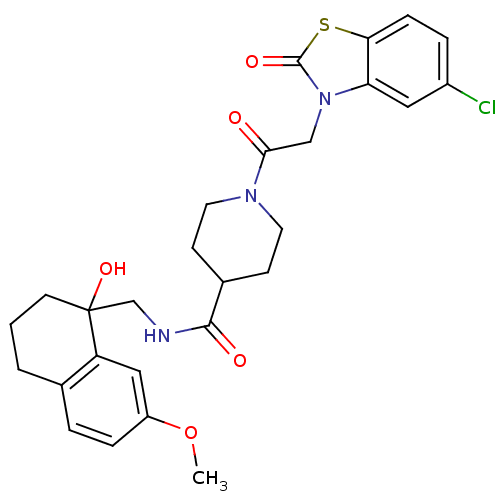 (1-[2-(5-Chloro-2-oxo-benzothiazol-3-yl)-acetyl]-pi...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of 125 I-PYY binding to human Neuropeptide Y receptor type 5 | Bioorg Med Chem Lett 12: 799-802 (2002) BindingDB Entry DOI: 10.7270/Q2M044R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50578357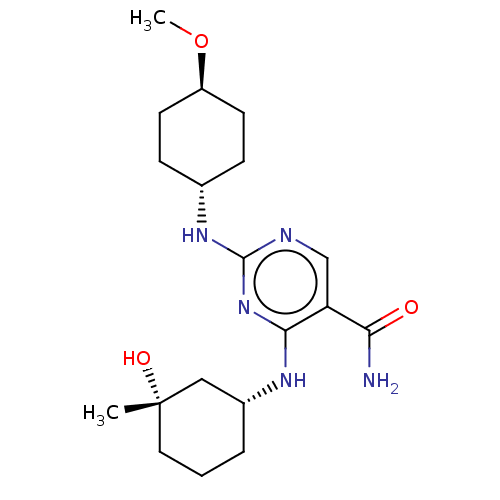 (CHEMBL4856984) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50064106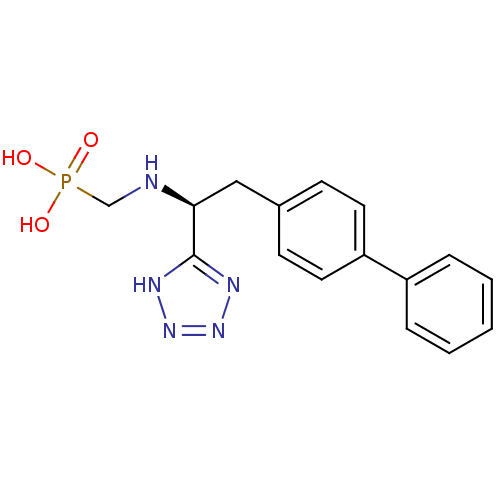 (CGS-26303 | CHEMBL290698 | {[(R)-2-Biphenyl-4-yl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research Curated by ChEMBL | Assay Description In vitro inhibition of rat neutral endopeptidase | J Med Chem 43: 488-504 (2000) BindingDB Entry DOI: 10.7270/Q21G0MZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (Homo sapiens (Human)) | BDBM50290402 (1-[1-[2-(3-Aza-bicyclo[3.2.2]non-3-yl)-2-oxo-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [125I]-Cholecystokinin-8 binding to Cholecystokinin type B receptor of guinea pig cerebral cortical membranes | Bioorg Med Chem Lett 7: 169-174 (1997) Article DOI: 10.1016/S0960-894X(96)00609-9 BindingDB Entry DOI: 10.7270/Q27M07ZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 8 (Homo sapiens (Human)) | BDBM50578360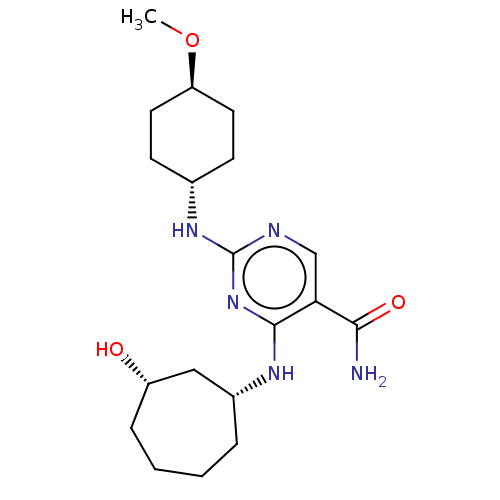 (CHEMBL4849353) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of JNK1 (unknown origin) assessed as reduction in phosphorylated ULight-labeled 4EBP1 peptide measured after 1 hr by time-resolved fluores... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01716 BindingDB Entry DOI: 10.7270/Q22Z19CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50111650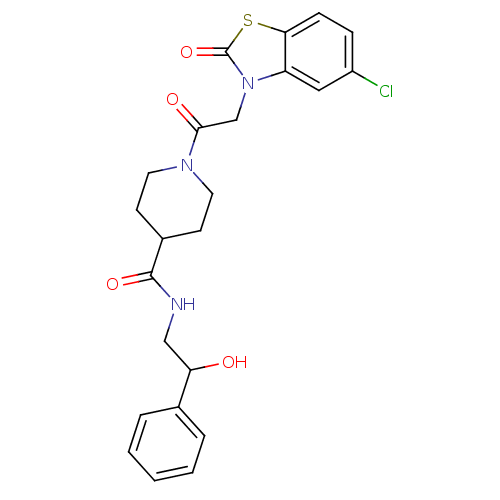 (1-[2-(5-Chloro-2-oxo-benzothiazol-3-yl)-acetyl]-pi...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of total specific binding of [125I]- -PYY to HEK 293 cells transfected with the Human NPY-Y5 cDNA (compound prepared by para... | Bioorg Med Chem Lett 12: 1171-5 (2002) BindingDB Entry DOI: 10.7270/Q26M365J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50110569 (1-[2-(5-Chloro-2-oxo-benzothiazol-3-yl)-acetyl]-pi...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of 125 I-PYY binding to human Neuropeptide Y receptor type 5 | Bioorg Med Chem Lett 12: 799-802 (2002) BindingDB Entry DOI: 10.7270/Q2M044R7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50267928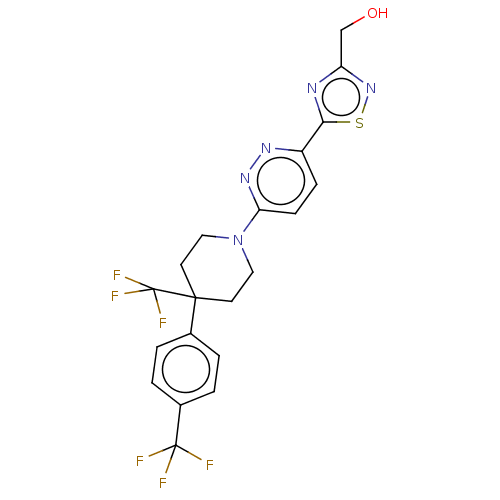 (CHEMBL4066506) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Division, Takeda Pharmaceutical Company Ltd., 26-1, Muraokahigashi 2-chome, Fujisawa, Kanagawa 251-8555, Japan. Electronic address: keisuke.imamura@takeda.com. Curated by ChEMBL | Assay Description Displacement of (5-(6-(10H-spiro(1-benzofuran-3,30-pyrrolidin)-10-yl)pyridazin-3-yl)-1,2,4-oxadiazol-3-yl)[3H2]methanol from SCD1 in human liver micr... | Bioorg Med Chem 25: 3768-3779 (2017) Article DOI: 10.1016/j.bmc.2017.05.016 BindingDB Entry DOI: 10.7270/Q2Z60RJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50111650 (1-[2-(5-Chloro-2-oxo-benzothiazol-3-yl)-acetyl]-pi...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of total specific binding of [125I]- -PYY to HEK 293 cells transfected with Human NPY-Y5 cDNA (compound prepared by manual s... | Bioorg Med Chem Lett 12: 1171-5 (2002) BindingDB Entry DOI: 10.7270/Q26M365J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50111649 (1-[2-(5-Chloro-2-oxo-benzothiazol-3-yl)-acetyl]-pi...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of total specific binding of [125I]- -PYY to HEK 293 cells transfected with Human NPY-Y5 cDNA (compound prepared by manual s... | Bioorg Med Chem Lett 12: 1171-5 (2002) BindingDB Entry DOI: 10.7270/Q26M365J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase (Candida albicans (Yeast)) | BDBM50366679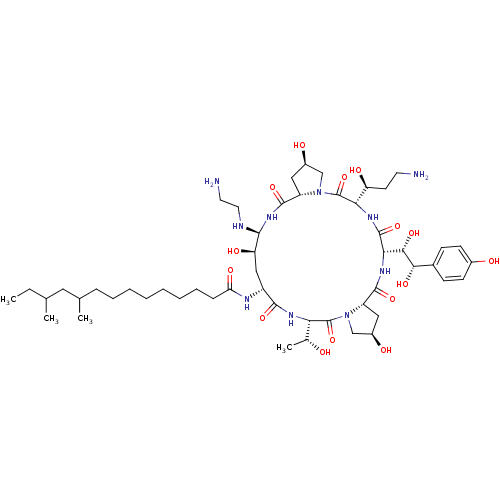 (CHEMBL1793852 | MK-991) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta glucan synthase | Bioorg Med Chem Lett 11: 1273-6 (2001) BindingDB Entry DOI: 10.7270/Q2CN74FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase (Candida albicans (Yeast)) | BDBM50100336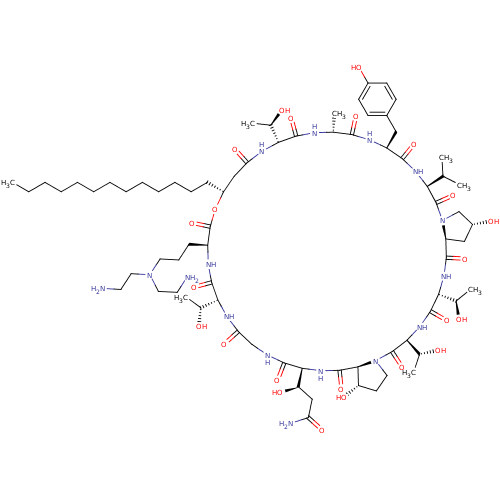 (CHEMBL405487 | RO-09-3655 derivative) | UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Roche Research Center Curated by ChEMBL | Assay Description In vitro inhibitory activity against Beta glucan synthase | Bioorg Med Chem Lett 11: 1273-6 (2001) BindingDB Entry DOI: 10.7270/Q2CN74FQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50111651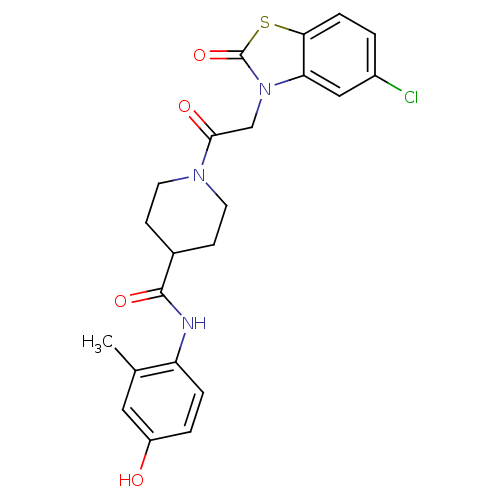 (1-[2-(5-Chloro-2-oxo-benzothiazol-3-yl)-acetyl]-pi...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of total specific binding of [125I]- -PYY to HEK 293 cells transfected with Human NPY-Y5 cDNA (compound prepared by manual s... | Bioorg Med Chem Lett 12: 1171-5 (2002) BindingDB Entry DOI: 10.7270/Q26M365J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50111641 (1-[2-(5-Chloro-2-oxo-benzothiazol-3-yl)-acetyl]-pi...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of total specific binding of [125I]- -PYY to HEK 293 cells transfected with Human NPY-Y5 cDNA (compound prepared by manual s... | Bioorg Med Chem Lett 12: 1171-5 (2002) BindingDB Entry DOI: 10.7270/Q26M365J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50111654 (1-[2-(5-Chloro-2-oxo-benzothiazol-3-yl)-acetyl]-pi...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory concentration of total specific binding of [125I]- -PYY to HEK 293 cells transfected with the Human NPY-Y5 cDNA (compound prepared by para... | Bioorg Med Chem Lett 12: 1171-5 (2002) BindingDB Entry DOI: 10.7270/Q26M365J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3377 total ) | Next | Last >> |