

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM342149 (1-((2-(tert-butyl)-4-(3-chlorophenyl)thiazol-5-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medifron DBT Inc. US Patent | Assay Description The FLIPR protocol consists of 2 substance additions during a kinetic measurement. First the compounds to be tested (5 μM) are pipetted onto the... | US Patent US9771359 (2017) BindingDB Entry DOI: 10.7270/Q2CZ3984 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50100983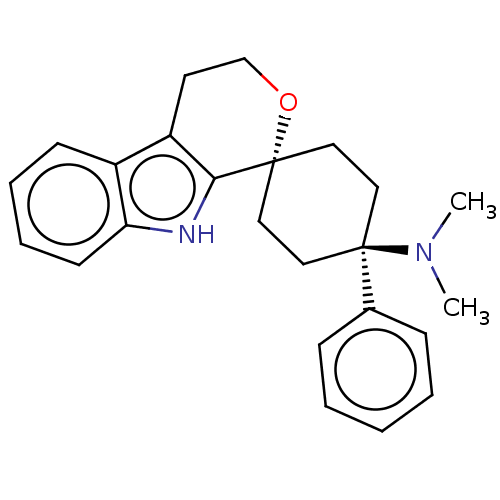 (CHEMBL3326224) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176564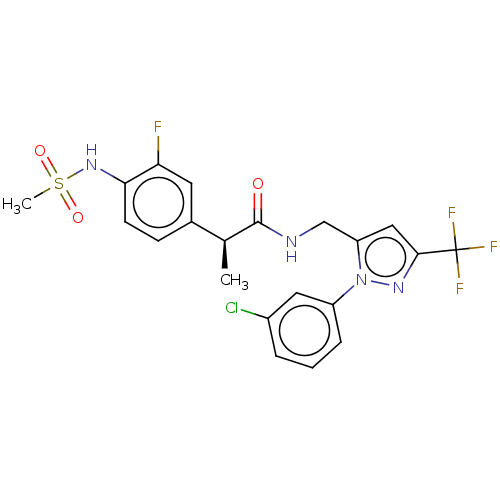 (US9120756, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | -59.4 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the vanilloid receptor 1 (VR1) can also be determined using the following assa... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176555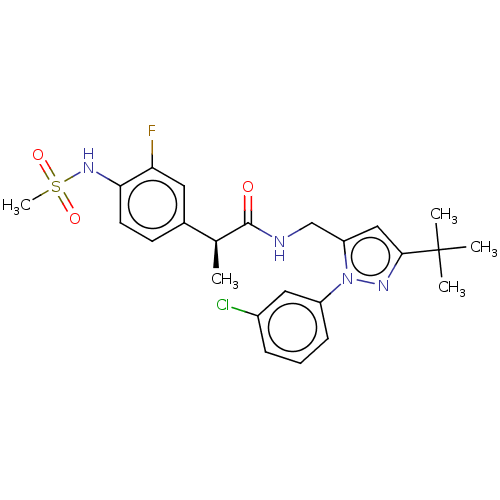 (US9120756, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | -59.4 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the vanilloid receptor 1 (VR1) can also be determined using the following assa... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM127310 (US8791268, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gruenenthal GmbH US Patent | Assay Description The FLIPR protocol consists of two substance additions. Initially, the compounds to be tested (10 uM) are pipeted onto the cells and the Ca2+ influx ... | US Patent US8791268 (2014) BindingDB Entry DOI: 10.7270/Q2BV7F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM176555 (US9120756, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the rat-species vanilloid receptor 1 (VR1/TRPV1) can be determined using the f... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM176564 (US9120756, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the rat-species vanilloid receptor 1 (VR1/TRPV1) can be determined using the f... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50101099 (CHEMBL3326228) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM176554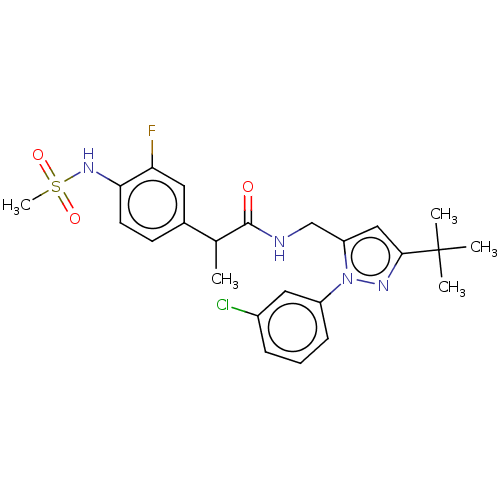 (US9120756, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the rat-species vanilloid receptor 1 (VR1/TRPV1) can be determined using the f... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50101306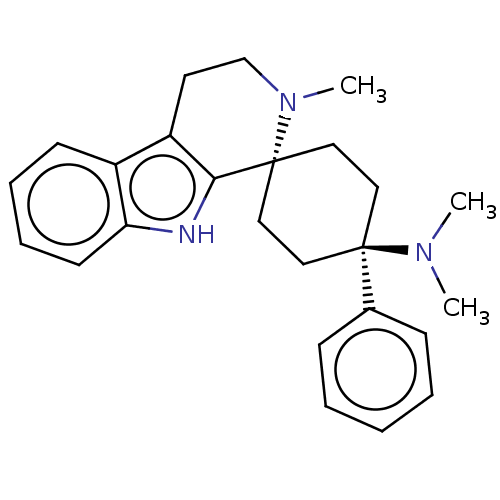 (CHEMBL3326229) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from human MOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50100983 (CHEMBL3326224) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from human MOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176562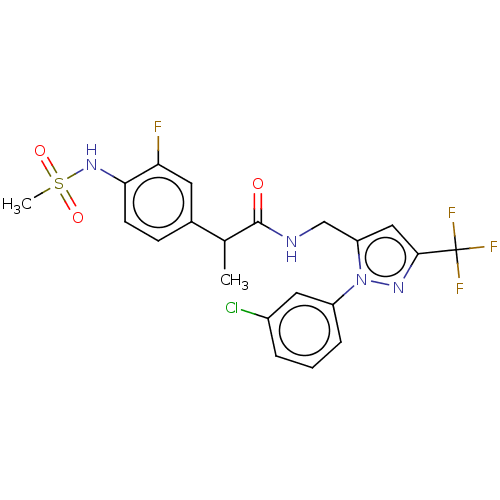 (US9120756, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -56.5 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the vanilloid receptor 1 (VR1) can also be determined using the following assa... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176561 (US9120756, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -56.5 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the vanilloid receptor 1 (VR1) can also be determined using the following assa... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176559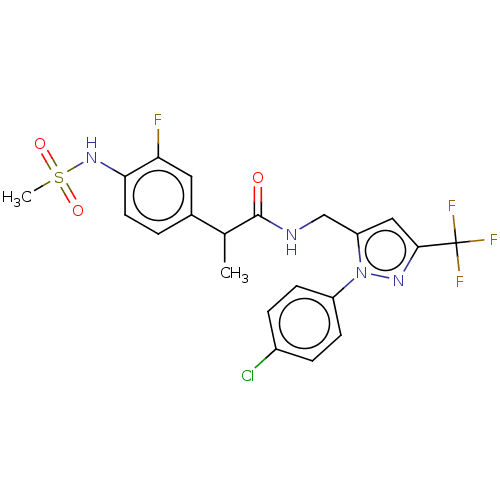 (US9120756, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -56.5 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the vanilloid receptor 1 (VR1) can also be determined using the following assa... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176554 (US9120756, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -56.5 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the vanilloid receptor 1 (VR1) can also be determined using the following assa... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM127312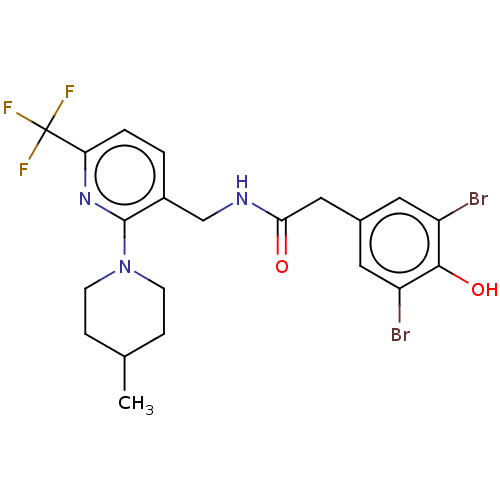 (US8791268, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gruenenthal GmbH US Patent | Assay Description The FLIPR protocol consists of two substance additions. Initially, the compounds to be tested (10 uM) are pipeted onto the cells and the Ca2+ influx ... | US Patent US8791268 (2014) BindingDB Entry DOI: 10.7270/Q2BV7F9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM342128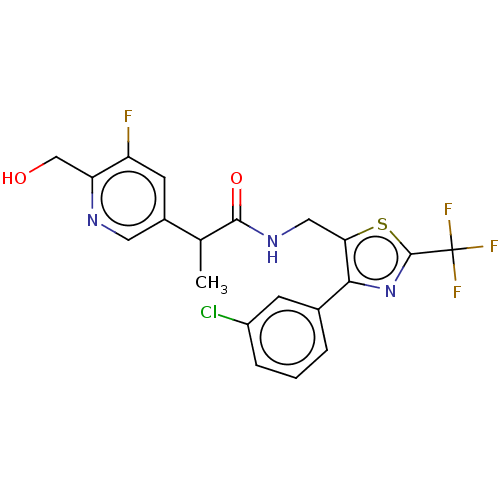 (N-((4-(3-chlorophenyl)-2-(trifluoromethyl)thiazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medifron DBT Inc. US Patent | Assay Description The FLIPR protocol consists of 2 substance additions during a kinetic measurement. First the compounds to be tested (5 μM) are pipetted onto the... | US Patent US9771359 (2017) BindingDB Entry DOI: 10.7270/Q2CZ3984 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50101088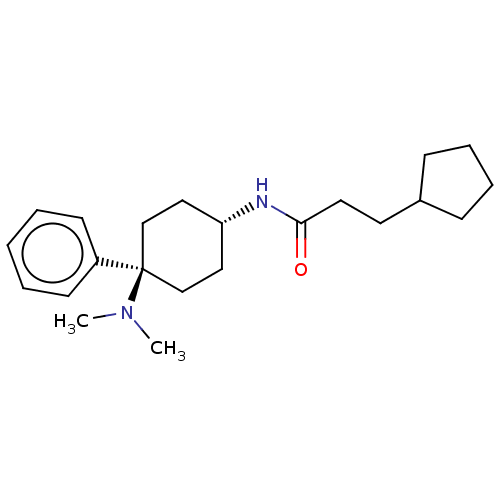 (CHEMBL3326220) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]Naloxone from human mu opioid receptor receptor expressed in CHO-K1 cells after 90 mins | ACS Med Chem Lett 5: 851-6 (2014) Article DOI: 10.1021/ml500116x BindingDB Entry DOI: 10.7270/Q28S4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50100983 (CHEMBL3326224) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells after 90 mins | ACS Med Chem Lett 5: 851-6 (2014) Article DOI: 10.1021/ml500116x BindingDB Entry DOI: 10.7270/Q28S4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50086063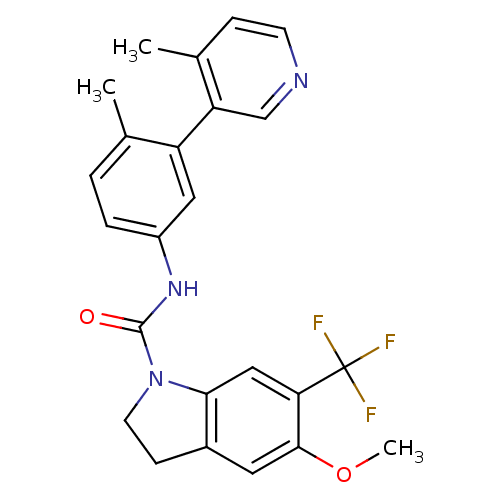 (5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards human cloned 5-hydroxytryptamine receptor 2C in HEK293 cells, using [3H]mesulergine as radioligand. | J Med Chem 43: 1123-34 (2000) Checked by Author BindingDB Entry DOI: 10.7270/Q23X85V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM176549 (US9120756, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the rat-species vanilloid receptor 1 (VR1/TRPV1) can be determined using the f... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50101152 (CHEMBL3326232) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from human MOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50101099 (CHEMBL3326228) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from human MOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50217990 (CHEMBL54560) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards human cloned 5-hydroxytryptamine 2C receptor of HEK293 cells by displacement of [3H]mesulergine | Bioorg Med Chem Lett 10: 1863-6 (2000) BindingDB Entry DOI: 10.7270/Q2X63Q4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM176561 (US9120756, 23) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the rat-species vanilloid receptor 1 (VR1/TRPV1) can be determined using the f... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50086073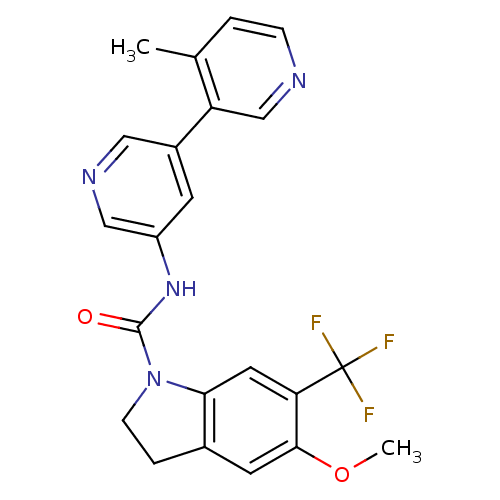 (5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards human cloned 5-hydroxytryptamine receptor 2C in HEK293 cells, using [3H]mesulergine as radioligand. | J Med Chem 43: 1123-34 (2000) Checked by Author BindingDB Entry DOI: 10.7270/Q23X85V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Rattus norvegicus (rat)) | BDBM176562 (US9120756, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the rat-species vanilloid receptor 1 (VR1/TRPV1) can be determined using the f... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM342122 (N-((4-(3-chlorophenyl)-2-(trifluoromethyl)thiazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medifron DBT Inc. US Patent | Assay Description The FLIPR protocol consists of 2 substance additions during a kinetic measurement. First the compounds to be tested (5 μM) are pipetted onto the... | US Patent US9771359 (2017) BindingDB Entry DOI: 10.7270/Q2CZ3984 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176566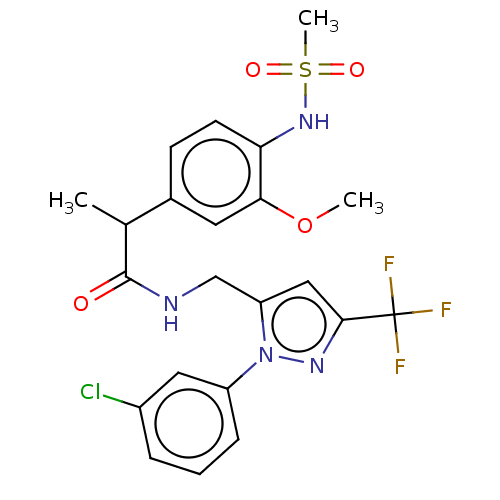 (US9120756, 28) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | -55.8 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the vanilloid receptor 1 (VR1) can also be determined using the following assa... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50101091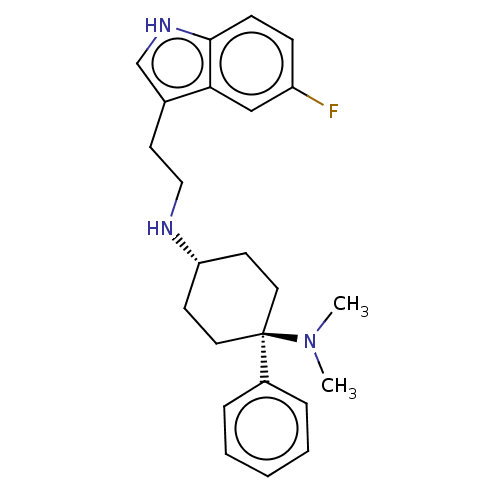 (CHEMBL3326223) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells after 90 mins | ACS Med Chem Lett 5: 851-6 (2014) Article DOI: 10.1021/ml500116x BindingDB Entry DOI: 10.7270/Q28S4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50101096 (CHEMBL3325961) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from human MOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50101088 (CHEMBL3326220) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells after 90 mins | ACS Med Chem Lett 5: 851-6 (2014) Article DOI: 10.1021/ml500116x BindingDB Entry DOI: 10.7270/Q28S4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50100991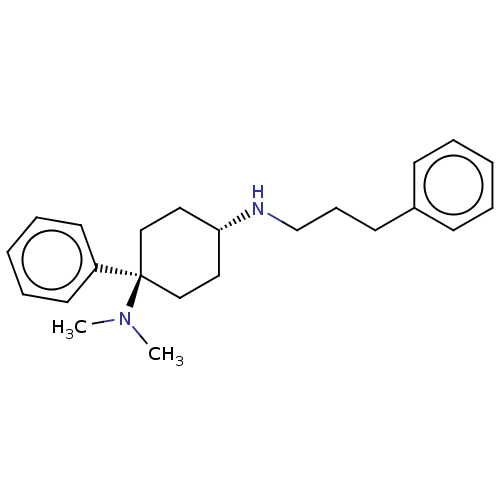 (CHEMBL3325879) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells after 90 mins | ACS Med Chem Lett 5: 851-6 (2014) Article DOI: 10.1021/ml500116x BindingDB Entry DOI: 10.7270/Q28S4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50086064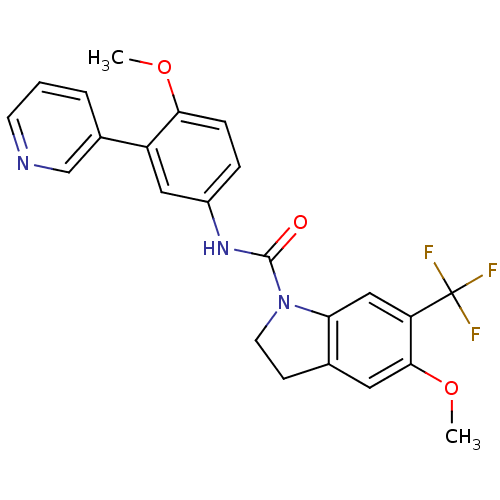 (5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards human cloned 5-hydroxytryptamine receptor 2C in HEK293 cells, using [3H]mesulergine as radioligand. | J Med Chem 43: 1123-34 (2000) Checked by Author BindingDB Entry DOI: 10.7270/Q23X85V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM176556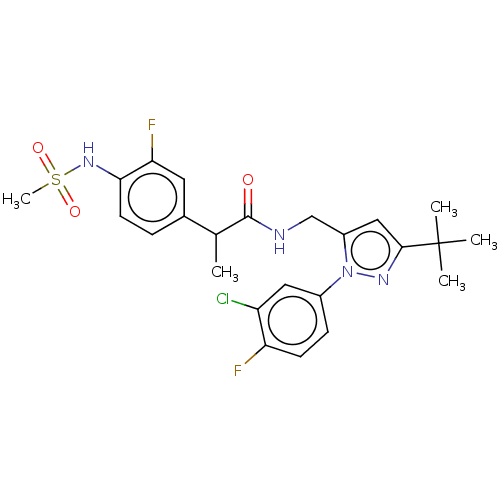 (US9120756, 18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -55.2 | n/a | n/a | n/a | n/a | n/a | n/a | 37 |
Gruenenthal GmbH US Patent | Assay Description The agonistic or antagonistic effect of the substances to be tested on the vanilloid receptor 1 (VR1) can also be determined using the following assa... | US Patent US9120756 (2015) BindingDB Entry DOI: 10.7270/Q26D5RRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50086068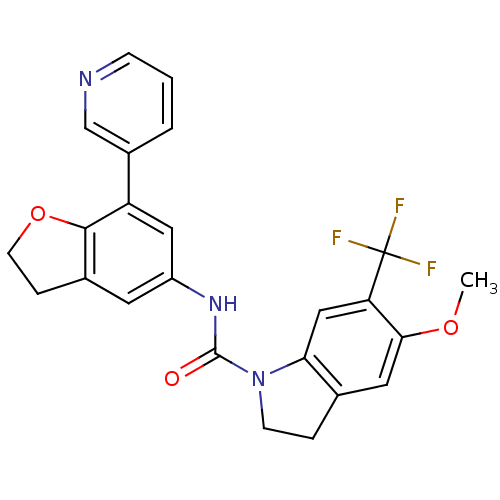 (5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards human cloned 5-hydroxytryptamine receptor 2C in HEK293 cells, using [3H]mesulergine as radioligand. | J Med Chem 43: 1123-34 (2000) Checked by Author BindingDB Entry DOI: 10.7270/Q23X85V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50217974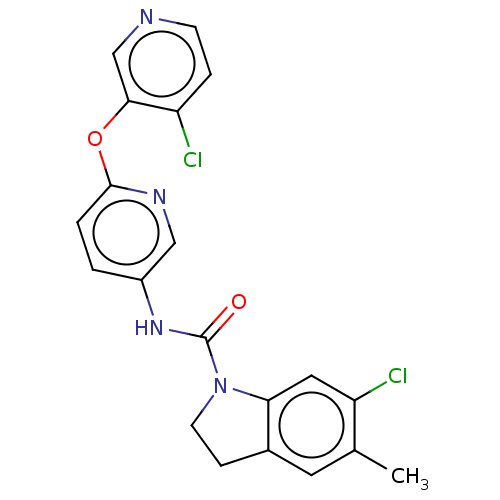 (CHEMBL55207) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards human cloned 5-hydroxytryptamine 2C receptor of HEK293 cells by displacement of [3H]mesulergine | Bioorg Med Chem Lett 10: 1863-6 (2000) BindingDB Entry DOI: 10.7270/Q2X63Q4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50101306 (CHEMBL3326229) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]nociceptin from human NOP receptor expressed in CHO-K1 cells by scintillation proximity assay | ACS Med Chem Lett 5: 857-62 (2014) Article DOI: 10.1021/ml500117c BindingDB Entry DOI: 10.7270/Q25140ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50100983 (CHEMBL3326224) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]Naloxone from human mu opioid receptor receptor expressed in CHO-K1 cells after 90 mins | ACS Med Chem Lett 5: 851-6 (2014) Article DOI: 10.1021/ml500116x BindingDB Entry DOI: 10.7270/Q28S4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM342117 (N-((2-(tert-butyl)-4-(3-chlorophenyl)thiazol-5-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medifron DBT Inc. US Patent | Assay Description The FLIPR protocol consists of 2 substance additions during a kinetic measurement. First the compounds to be tested (5 μM) are pipetted onto the... | US Patent US9771359 (2017) BindingDB Entry DOI: 10.7270/Q2CZ3984 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50001275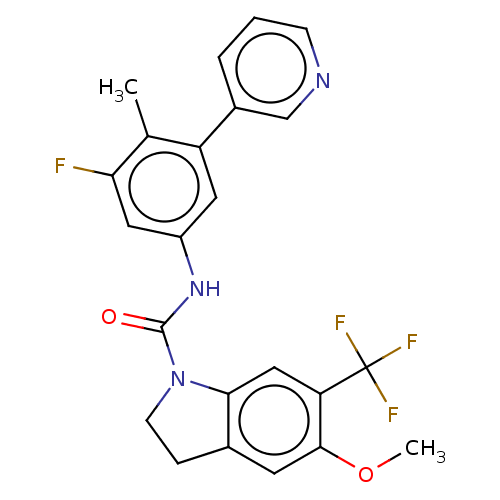 (CHEMBL280465) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards human cloned 5-hydroxytryptamine receptor 2C in HEK293 cells, using [3H]mesulergine as radioligand. | J Med Chem 43: 1123-34 (2000) Checked by Author BindingDB Entry DOI: 10.7270/Q23X85V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50086055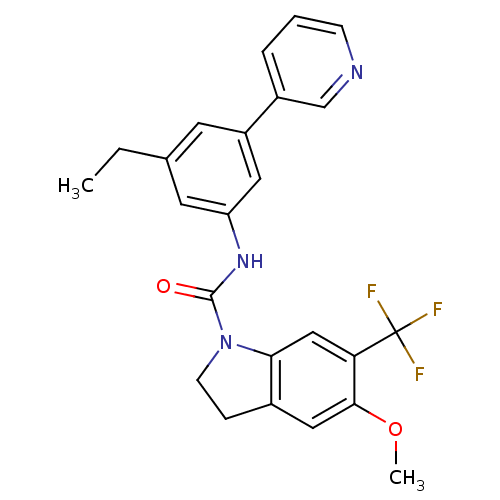 (5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards human cloned 5-hydroxytryptamine receptor 2C in HEK293 cells, using [3H]mesulergine as radioligand. | J Med Chem 43: 1123-34 (2000) Checked by Author BindingDB Entry DOI: 10.7270/Q23X85V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50086067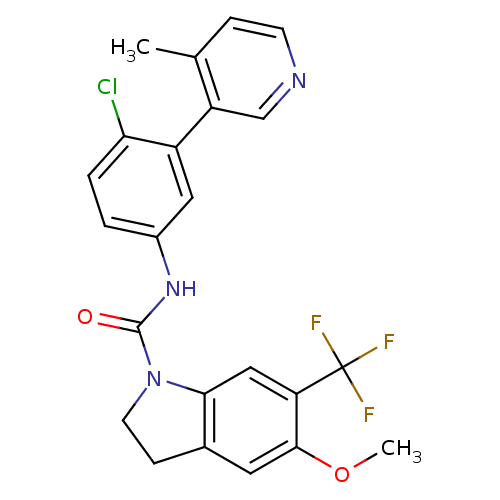 (5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards human cloned 5-hydroxytryptamine receptor 2C in HEK293 cells, using [3H]mesulergine as radioligand. | J Med Chem 43: 1123-34 (2000) Checked by Author BindingDB Entry DOI: 10.7270/Q23X85V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50086071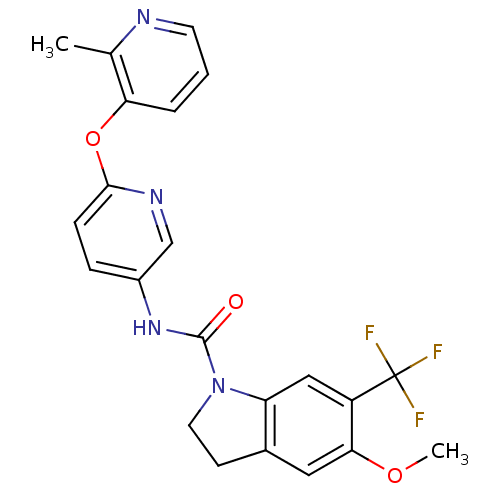 (5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards human cloned 5-hydroxytryptamine receptor 2C in HEK293 cells, using [3H]mesulergine as radioligand. | J Med Chem 43: 1123-34 (2000) Checked by Author BindingDB Entry DOI: 10.7270/Q23X85V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50086059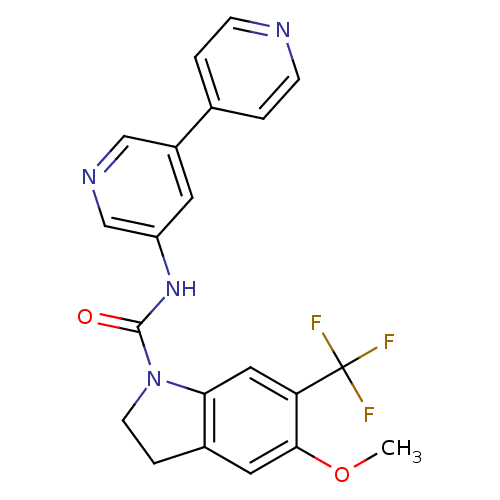 (5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards human cloned 5-hydroxytryptamine receptor 2C in HEK293 cells, using [3H]mesulergine as radioligand. | J Med Chem 43: 1123-34 (2000) Checked by Author BindingDB Entry DOI: 10.7270/Q23X85V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50086053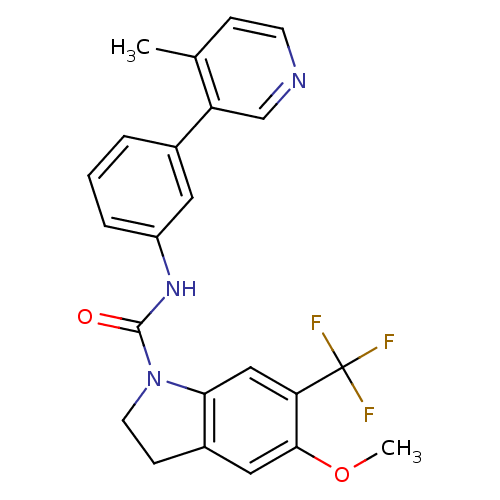 (5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards human cloned 5-hydroxytryptamine receptor 2C in HEK293 cells, using [3H]mesulergine as radioligand. | J Med Chem 43: 1123-34 (2000) Checked by Author BindingDB Entry DOI: 10.7270/Q23X85V5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50217991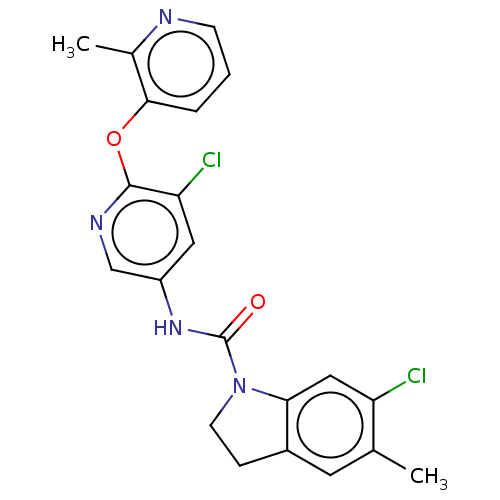 (CHEMBL301012) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards human cloned 5-hydroxytryptamine 2C receptor of HEK293 cells by displacement of [3H]mesulergine | Bioorg Med Chem Lett 10: 1863-6 (2000) BindingDB Entry DOI: 10.7270/Q2X63Q4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50086071 (5-Methoxy-6-trifluoromethyl-2,3-dihydro-indole-1-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity towards human cloned 5-hydroxytryptamine 2C receptor of HEK293 cells by displacement of [3H]mesulergine | Bioorg Med Chem Lett 10: 1863-6 (2000) BindingDB Entry DOI: 10.7270/Q2X63Q4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily V member 1 (Homo sapiens (Human)) | BDBM342119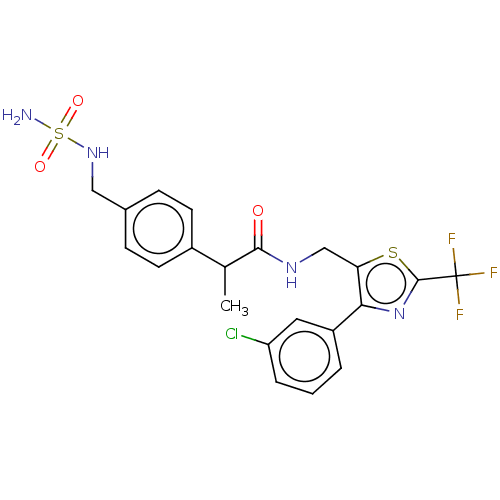 (N-((4-(3-chlorophenyl)-2-(trifluoromethyl)thiazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medifron DBT Inc. US Patent | Assay Description The FLIPR protocol consists of 2 substance additions during a kinetic measurement. First the compounds to be tested (5 μM) are pipetted onto the... | US Patent US9771359 (2017) BindingDB Entry DOI: 10.7270/Q2CZ3984 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50100993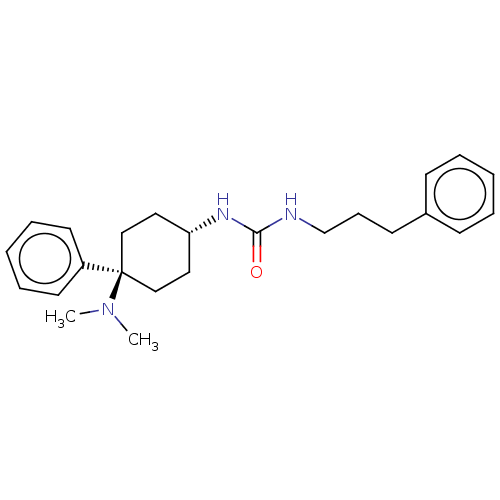 (CHEMBL3325881) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacokinetics Curated by ChEMBL | Assay Description Displacement of [3H]Naloxone from human mu opioid receptor receptor expressed in CHO-K1 cells after 90 mins | ACS Med Chem Lett 5: 851-6 (2014) Article DOI: 10.1021/ml500116x BindingDB Entry DOI: 10.7270/Q28S4RPM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1055 total ) | Next | Last >> |