 Found 752 hits with Last Name = 'saupe' and Initial = 'sm'
Found 752 hits with Last Name = 'saupe' and Initial = 'sm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50514081

(CHEMBL4471466)Show SMILES NCc1cccc(C[C@H](NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2cccc(c2)C(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)c1 |r| Show InChI InChI=1S/C37H42N6O6S/c38-22-28-11-4-10-27(19-28)21-33(35(44)41-23-26-15-17-30(18-16-26)34(39)40)42-36(45)32(14-6-9-25-7-2-1-3-8-25)43-50(48,49)24-29-12-5-13-31(20-29)37(46)47/h1-5,7-8,10-13,15-20,32-33,43H,6,9,14,21-24,38H2,(H3,39,40)(H,41,44)(H,42,45)(H,46,47)/t32-,33+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using S2302 as substrate by spectrophotometric method |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50514081

(CHEMBL4471466)Show SMILES NCc1cccc(C[C@H](NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2cccc(c2)C(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)c1 |r| Show InChI InChI=1S/C37H42N6O6S/c38-22-28-11-4-10-27(19-28)21-33(35(44)41-23-26-15-17-30(18-16-26)34(39)40)42-36(45)32(14-6-9-25-7-2-1-3-8-25)43-50(48,49)24-29-12-5-13-31(20-29)37(46)47/h1-5,7-8,10-13,15-20,32-33,43H,6,9,14,21-24,38H2,(H3,39,40)(H,41,44)(H,42,45)(H,46,47)/t32-,33+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using S2302 as substrate by spectrophotometric method |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532387
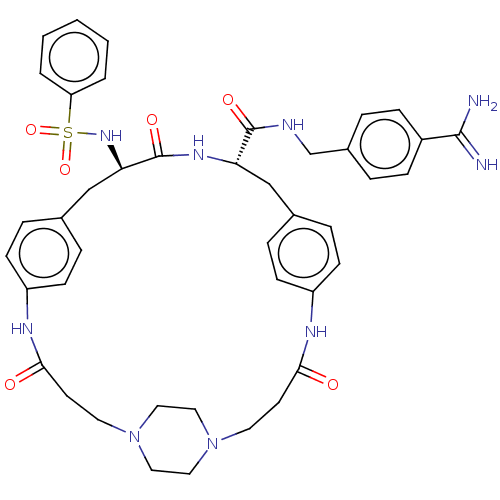
(CHEMBL4476141)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)c1ccccc1 |r,wU:49.47,wD:22.18,(24.52,-31.64,;25.32,-32.96,;24.57,-34.31,;26.87,-32.93,;28.27,-32.91,;27.9,-34.16,;27.86,-31.66,;25.46,-27.14,;26.25,-28.46,;25.5,-29.81,;27.8,-28.43,;29.21,-28.41,;28.84,-29.67,;28.8,-27.16,;25.54,-22.14,;26.33,-23.47,;25.58,-24.81,;27.88,-23.43,;29.28,-23.41,;28.92,-24.67,;28.88,-22.17,;8.51,-20.69,;8.47,-22.2,;8.42,-23.72,;7.44,-24.88,;5.94,-24.59,;4.95,-25.74,;5.46,-27.17,;4.71,-28.65,;5.45,-30.01,;4.64,-31.32,;6.99,-30.05,;7.73,-31.4,;9.27,-31.44,;10.07,-30.15,;11.59,-30.19,;12.3,-31.52,;11.51,-32.82,;9.99,-32.77,;13.84,-31.57,;14.66,-30.26,;16.2,-30.3,;16.93,-31.66,;16.96,-28.96,;15.17,-27.48,;15.54,-25.98,;14.43,-24.91,;12.94,-25.33,;12.39,-23.49,;12.44,-21.56,;12.48,-20.04,;11.1,-22.28,;9.81,-21.48,;9.85,-19.96,;13.72,-22.35,;13.68,-23.87,;15.06,-21.64,;16.35,-22.43,;17.69,-21.72,;18.98,-22.52,;20.32,-21.8,;20.36,-20.28,;19.06,-19.49,;17.73,-20.21,;21.7,-19.56,;23,-20.36,;21.74,-18.04,;12.56,-26.83,;13.68,-27.91,;6.96,-27.45,;7.94,-26.3,;7.17,-21.4,;5.81,-22.14,;6.57,-23.47,;5.04,-23.47,;4.5,-21.33,;4.54,-19.79,;3.23,-18.98,;1.87,-19.72,;1.83,-21.27,;3.15,-22.07,)| Show InChI InChI=1S/C42H49N9O6S.3C2HF3O2/c43-40(44)32-12-6-31(7-13-32)28-45-41(54)36-26-29-8-14-33(15-9-29)46-38(52)18-20-50-22-24-51(25-23-50)21-19-39(53)47-34-16-10-30(11-17-34)27-37(42(55)48-36)49-58(56,57)35-4-2-1-3-5-35;3*3-2(4,5)1(6)7/h1-17,36-37,49H,18-28H2,(H3,43,44)(H,45,54)(H,46,52)(H,47,53)(H,48,55);3*(H,6,7)/t36-,37+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532387

(CHEMBL4476141)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)c1ccccc1 |r,wU:49.47,wD:22.18,(24.52,-31.64,;25.32,-32.96,;24.57,-34.31,;26.87,-32.93,;28.27,-32.91,;27.9,-34.16,;27.86,-31.66,;25.46,-27.14,;26.25,-28.46,;25.5,-29.81,;27.8,-28.43,;29.21,-28.41,;28.84,-29.67,;28.8,-27.16,;25.54,-22.14,;26.33,-23.47,;25.58,-24.81,;27.88,-23.43,;29.28,-23.41,;28.92,-24.67,;28.88,-22.17,;8.51,-20.69,;8.47,-22.2,;8.42,-23.72,;7.44,-24.88,;5.94,-24.59,;4.95,-25.74,;5.46,-27.17,;4.71,-28.65,;5.45,-30.01,;4.64,-31.32,;6.99,-30.05,;7.73,-31.4,;9.27,-31.44,;10.07,-30.15,;11.59,-30.19,;12.3,-31.52,;11.51,-32.82,;9.99,-32.77,;13.84,-31.57,;14.66,-30.26,;16.2,-30.3,;16.93,-31.66,;16.96,-28.96,;15.17,-27.48,;15.54,-25.98,;14.43,-24.91,;12.94,-25.33,;12.39,-23.49,;12.44,-21.56,;12.48,-20.04,;11.1,-22.28,;9.81,-21.48,;9.85,-19.96,;13.72,-22.35,;13.68,-23.87,;15.06,-21.64,;16.35,-22.43,;17.69,-21.72,;18.98,-22.52,;20.32,-21.8,;20.36,-20.28,;19.06,-19.49,;17.73,-20.21,;21.7,-19.56,;23,-20.36,;21.74,-18.04,;12.56,-26.83,;13.68,-27.91,;6.96,-27.45,;7.94,-26.3,;7.17,-21.4,;5.81,-22.14,;6.57,-23.47,;5.04,-23.47,;4.5,-21.33,;4.54,-19.79,;3.23,-18.98,;1.87,-19.72,;1.83,-21.27,;3.15,-22.07,)| Show InChI InChI=1S/C42H49N9O6S.3C2HF3O2/c43-40(44)32-12-6-31(7-13-32)28-45-41(54)36-26-29-8-14-33(15-9-29)46-38(52)18-20-50-22-24-51(25-23-50)21-19-39(53)47-34-16-10-30(11-17-34)27-37(42(55)48-36)49-58(56,57)35-4-2-1-3-5-35;3*3-2(4,5)1(6)7/h1-17,36-37,49H,18-28H2,(H3,43,44)(H,45,54)(H,46,52)(H,47,53)(H,48,55);3*(H,6,7)/t36-,37+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50380619
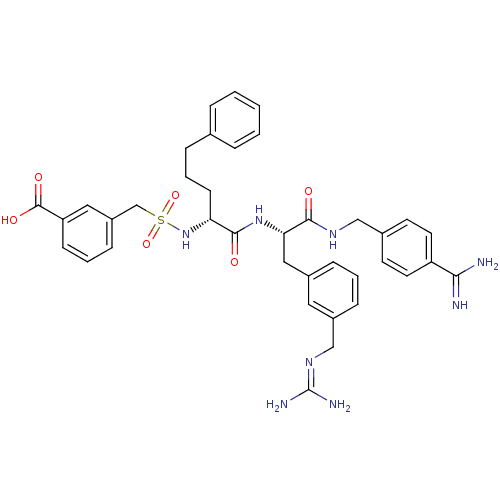
(CHEMBL2016865)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-c1cccc(-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]-c2ccccc2)-[#7]S(=O)(=O)[#6]-c2cccc(c2)-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-c2ccc(cc2)-[#6](-[#7])=[#7])c1 |r| Show InChI InChI=1S/C38H44N8O6S/c39-34(40)30-17-15-26(16-18-30)22-43-35(47)33(21-27-10-4-11-28(19-27)23-44-38(41)42)45-36(48)32(14-6-9-25-7-2-1-3-8-25)46-53(51,52)24-29-12-5-13-31(20-29)37(49)50/h1-5,7-8,10-13,15-20,32-33,46H,6,9,14,21-24H2,(H3,39,40)(H,43,47)(H,45,48)(H,49,50)(H4,41,42,44)/t32-,33+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of plasma kallikrein by dixon plot method |
J Med Chem 55: 1171-80 (2012)
Article DOI: 10.1021/jm2011996
BindingDB Entry DOI: 10.7270/Q26T0NN4 |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM50253952
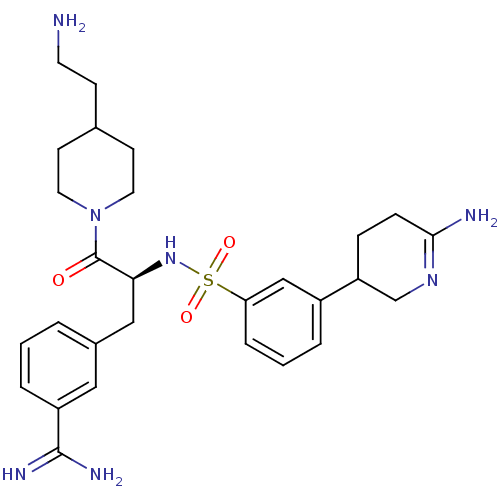
(3-{(S)-3-[4-(2-Amino-ethyl)-piperidin-1-yl]-2-[3-(...)Show SMILES NCCC1CCN(CC1)C(=O)[C@H](Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1cccc(c1)C1CCC(N)=NC1 |r,c:39| Show InChI InChI=1S/C28H39N7O3S/c29-12-9-19-10-13-35(14-11-19)28(36)25(16-20-3-1-5-22(15-20)27(31)32)34-39(37,38)24-6-2-4-21(17-24)23-7-8-26(30)33-18-23/h1-6,15,17,19,23,25,34H,7-14,16,18,29H2,(H2,30,33)(H3,31,32)/t23?,25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of matriptase (unknown origin) |
Bioorg Med Chem Lett 19: 67-73 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.019
BindingDB Entry DOI: 10.7270/Q2HH6JXF |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532389

(CHEMBL4458743)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)NC1CCCCC1 |r,wU:49.47,wD:22.18,(24.33,-27.66,;25.12,-28.98,;24.37,-30.34,;26.67,-28.95,;28.08,-28.93,;27.71,-30.19,;27.67,-27.68,;24.53,-22.53,;25.32,-23.86,;24.57,-25.21,;26.87,-23.83,;28.28,-23.8,;27.92,-25.06,;27.88,-22.56,;29.54,-25.73,;30.33,-27.05,;29.58,-28.41,;31.88,-27.02,;33.29,-27,;32.92,-28.26,;32.88,-25.75,;10.69,-19.49,;10.65,-21,;10.6,-22.53,;9.61,-23.69,;8.11,-23.4,;7.12,-24.55,;7.63,-25.99,;6.89,-27.47,;7.62,-28.82,;6.82,-30.14,;9.17,-28.87,;9.91,-30.22,;11.45,-30.27,;12.25,-28.97,;13.77,-29.01,;14.49,-30.35,;13.69,-31.64,;12.17,-31.59,;16.03,-30.39,;16.85,-29.07,;18.39,-29.12,;19.12,-30.48,;19.16,-27.77,;17.36,-26.29,;17.73,-24.79,;16.62,-23.72,;15.13,-24.14,;14.58,-22.3,;14.62,-20.36,;14.67,-18.84,;13.28,-21.08,;11.99,-20.29,;12.03,-18.76,;15.91,-21.16,;15.87,-22.68,;17.25,-20.44,;18.55,-21.24,;19.89,-20.52,;21.18,-21.33,;22.52,-20.6,;22.56,-19.09,;21.26,-18.29,;19.92,-19.01,;23.9,-18.36,;25.2,-19.16,;23.95,-16.83,;14.75,-25.65,;15.87,-26.72,;9.14,-26.26,;10.12,-25.11,;9.35,-20.21,;7.98,-20.94,;8.75,-22.28,;7.21,-22.27,;6.67,-20.13,;5.31,-20.87,;4,-20.07,;2.64,-20.8,;2.59,-22.34,;3.91,-23.16,;5.28,-22.42,)| Show InChI InChI=1S/C42H56N10O6S.3C2HF3O2/c43-40(44)32-12-6-31(7-13-32)28-45-41(55)36-26-29-8-14-33(15-9-29)46-38(53)18-20-51-22-24-52(25-23-51)21-19-39(54)47-34-16-10-30(11-17-34)27-37(42(56)48-36)50-59(57,58)49-35-4-2-1-3-5-35;3*3-2(4,5)1(6)7/h6-17,35-37,49-50H,1-5,18-28H2,(H3,43,44)(H,45,55)(H,46,53)(H,47,54)(H,48,56);3*(H,6,7)/t36-,37+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532389

(CHEMBL4458743)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)NC1CCCCC1 |r,wU:49.47,wD:22.18,(24.33,-27.66,;25.12,-28.98,;24.37,-30.34,;26.67,-28.95,;28.08,-28.93,;27.71,-30.19,;27.67,-27.68,;24.53,-22.53,;25.32,-23.86,;24.57,-25.21,;26.87,-23.83,;28.28,-23.8,;27.92,-25.06,;27.88,-22.56,;29.54,-25.73,;30.33,-27.05,;29.58,-28.41,;31.88,-27.02,;33.29,-27,;32.92,-28.26,;32.88,-25.75,;10.69,-19.49,;10.65,-21,;10.6,-22.53,;9.61,-23.69,;8.11,-23.4,;7.12,-24.55,;7.63,-25.99,;6.89,-27.47,;7.62,-28.82,;6.82,-30.14,;9.17,-28.87,;9.91,-30.22,;11.45,-30.27,;12.25,-28.97,;13.77,-29.01,;14.49,-30.35,;13.69,-31.64,;12.17,-31.59,;16.03,-30.39,;16.85,-29.07,;18.39,-29.12,;19.12,-30.48,;19.16,-27.77,;17.36,-26.29,;17.73,-24.79,;16.62,-23.72,;15.13,-24.14,;14.58,-22.3,;14.62,-20.36,;14.67,-18.84,;13.28,-21.08,;11.99,-20.29,;12.03,-18.76,;15.91,-21.16,;15.87,-22.68,;17.25,-20.44,;18.55,-21.24,;19.89,-20.52,;21.18,-21.33,;22.52,-20.6,;22.56,-19.09,;21.26,-18.29,;19.92,-19.01,;23.9,-18.36,;25.2,-19.16,;23.95,-16.83,;14.75,-25.65,;15.87,-26.72,;9.14,-26.26,;10.12,-25.11,;9.35,-20.21,;7.98,-20.94,;8.75,-22.28,;7.21,-22.27,;6.67,-20.13,;5.31,-20.87,;4,-20.07,;2.64,-20.8,;2.59,-22.34,;3.91,-23.16,;5.28,-22.42,)| Show InChI InChI=1S/C42H56N10O6S.3C2HF3O2/c43-40(44)32-12-6-31(7-13-32)28-45-41(55)36-26-29-8-14-33(15-9-29)46-38(53)18-20-51-22-24-52(25-23-51)21-19-39(54)47-34-16-10-30(11-17-34)27-37(42(56)48-36)50-59(57,58)49-35-4-2-1-3-5-35;3*3-2(4,5)1(6)7/h6-17,35-37,49-50H,1-5,18-28H2,(H3,43,44)(H,45,55)(H,46,53)(H,47,54)(H,48,56);3*(H,6,7)/t36-,37+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532389

(CHEMBL4458743)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)NC1CCCCC1 |r,wU:49.47,wD:22.18,(24.33,-27.66,;25.12,-28.98,;24.37,-30.34,;26.67,-28.95,;28.08,-28.93,;27.71,-30.19,;27.67,-27.68,;24.53,-22.53,;25.32,-23.86,;24.57,-25.21,;26.87,-23.83,;28.28,-23.8,;27.92,-25.06,;27.88,-22.56,;29.54,-25.73,;30.33,-27.05,;29.58,-28.41,;31.88,-27.02,;33.29,-27,;32.92,-28.26,;32.88,-25.75,;10.69,-19.49,;10.65,-21,;10.6,-22.53,;9.61,-23.69,;8.11,-23.4,;7.12,-24.55,;7.63,-25.99,;6.89,-27.47,;7.62,-28.82,;6.82,-30.14,;9.17,-28.87,;9.91,-30.22,;11.45,-30.27,;12.25,-28.97,;13.77,-29.01,;14.49,-30.35,;13.69,-31.64,;12.17,-31.59,;16.03,-30.39,;16.85,-29.07,;18.39,-29.12,;19.12,-30.48,;19.16,-27.77,;17.36,-26.29,;17.73,-24.79,;16.62,-23.72,;15.13,-24.14,;14.58,-22.3,;14.62,-20.36,;14.67,-18.84,;13.28,-21.08,;11.99,-20.29,;12.03,-18.76,;15.91,-21.16,;15.87,-22.68,;17.25,-20.44,;18.55,-21.24,;19.89,-20.52,;21.18,-21.33,;22.52,-20.6,;22.56,-19.09,;21.26,-18.29,;19.92,-19.01,;23.9,-18.36,;25.2,-19.16,;23.95,-16.83,;14.75,-25.65,;15.87,-26.72,;9.14,-26.26,;10.12,-25.11,;9.35,-20.21,;7.98,-20.94,;8.75,-22.28,;7.21,-22.27,;6.67,-20.13,;5.31,-20.87,;4,-20.07,;2.64,-20.8,;2.59,-22.34,;3.91,-23.16,;5.28,-22.42,)| Show InChI InChI=1S/C42H56N10O6S.3C2HF3O2/c43-40(44)32-12-6-31(7-13-32)28-45-41(55)36-26-29-8-14-33(15-9-29)46-38(53)18-20-51-22-24-52(25-23-51)21-19-39(54)47-34-16-10-30(11-17-34)27-37(42(56)48-36)50-59(57,58)49-35-4-2-1-3-5-35;3*3-2(4,5)1(6)7/h6-17,35-37,49-50H,1-5,18-28H2,(H3,43,44)(H,45,55)(H,46,53)(H,47,54)(H,48,56);3*(H,6,7)/t36-,37+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Binding affinity to human plasmin assessed as slow binding constant in presence of Mes-DArg-Phe-Arg-AMC after 20 mins by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532389

(CHEMBL4458743)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)NC1CCCCC1 |r,wU:49.47,wD:22.18,(24.33,-27.66,;25.12,-28.98,;24.37,-30.34,;26.67,-28.95,;28.08,-28.93,;27.71,-30.19,;27.67,-27.68,;24.53,-22.53,;25.32,-23.86,;24.57,-25.21,;26.87,-23.83,;28.28,-23.8,;27.92,-25.06,;27.88,-22.56,;29.54,-25.73,;30.33,-27.05,;29.58,-28.41,;31.88,-27.02,;33.29,-27,;32.92,-28.26,;32.88,-25.75,;10.69,-19.49,;10.65,-21,;10.6,-22.53,;9.61,-23.69,;8.11,-23.4,;7.12,-24.55,;7.63,-25.99,;6.89,-27.47,;7.62,-28.82,;6.82,-30.14,;9.17,-28.87,;9.91,-30.22,;11.45,-30.27,;12.25,-28.97,;13.77,-29.01,;14.49,-30.35,;13.69,-31.64,;12.17,-31.59,;16.03,-30.39,;16.85,-29.07,;18.39,-29.12,;19.12,-30.48,;19.16,-27.77,;17.36,-26.29,;17.73,-24.79,;16.62,-23.72,;15.13,-24.14,;14.58,-22.3,;14.62,-20.36,;14.67,-18.84,;13.28,-21.08,;11.99,-20.29,;12.03,-18.76,;15.91,-21.16,;15.87,-22.68,;17.25,-20.44,;18.55,-21.24,;19.89,-20.52,;21.18,-21.33,;22.52,-20.6,;22.56,-19.09,;21.26,-18.29,;19.92,-19.01,;23.9,-18.36,;25.2,-19.16,;23.95,-16.83,;14.75,-25.65,;15.87,-26.72,;9.14,-26.26,;10.12,-25.11,;9.35,-20.21,;7.98,-20.94,;8.75,-22.28,;7.21,-22.27,;6.67,-20.13,;5.31,-20.87,;4,-20.07,;2.64,-20.8,;2.59,-22.34,;3.91,-23.16,;5.28,-22.42,)| Show InChI InChI=1S/C42H56N10O6S.3C2HF3O2/c43-40(44)32-12-6-31(7-13-32)28-45-41(55)36-26-29-8-14-33(15-9-29)46-38(53)18-20-51-22-24-52(25-23-51)21-19-39(54)47-34-16-10-30(11-17-34)27-37(42(56)48-36)50-59(57,58)49-35-4-2-1-3-5-35;3*3-2(4,5)1(6)7/h6-17,35-37,49-50H,1-5,18-28H2,(H3,43,44)(H,45,55)(H,46,53)(H,47,54)(H,48,56);3*(H,6,7)/t36-,37+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Binding affinity to human plasmin assessed as slow binding constant in presence of Mes-DArg-Phe-Arg-AMC after 20 mins by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50425648
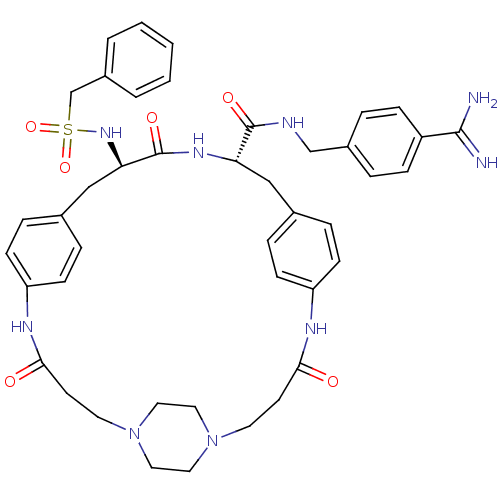
(CHEMBL2315243)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2Cc3ccc(NC(=O)CCN4CCN(CC4)CCC(=O)Nc4ccc(C[C@@H](NS(=O)(=O)Cc5ccccc5)C(=O)N2)cc4)cc3)cc1 |r,wU:38.39,wD:11.10,(46.28,-21.08,;46.25,-22.61,;47.58,-23.4,;44.91,-23.37,;44.89,-24.91,;43.54,-25.65,;42.23,-24.87,;40.88,-25.62,;39.56,-24.83,;38.22,-25.58,;38.2,-27.12,;36.89,-24.79,;35.55,-25.54,;35.54,-27.08,;34.2,-27.85,;34.19,-29.38,;35.52,-30.16,;35.62,-31.71,;36.95,-32.47,;38.28,-31.7,;36.96,-34.01,;35.64,-34.78,;34.31,-34.02,;32.98,-34.79,;31.65,-34.04,;31.65,-32.51,;32.96,-31.72,;34.3,-32.49,;30.32,-31.75,;30.3,-30.22,;28.97,-29.47,;27.65,-30.24,;28.95,-27.93,;30.28,-27.15,;30.26,-25.61,;31.59,-24.83,;32.93,-25.59,;34.25,-24.82,;34.24,-23.28,;32.9,-22.51,;31.57,-23.3,;30.79,-24.63,;32.33,-24.63,;30.23,-22.53,;28.9,-23.31,;27.57,-22.54,;26.24,-23.32,;26.23,-24.86,;27.57,-25.63,;28.91,-24.86,;35.57,-22.49,;35.56,-20.95,;36.91,-23.25,;32.95,-27.13,;31.63,-27.92,;36.86,-29.39,;36.86,-27.86,;42.24,-23.33,;43.58,-22.57,)| Show InChI InChI=1S/C43H51N9O6S/c44-41(45)34-12-6-32(7-13-34)28-46-42(55)37-26-30-8-14-35(15-9-30)47-39(53)18-20-51-22-24-52(25-23-51)21-19-40(54)48-36-16-10-31(11-17-36)27-38(43(56)49-37)50-59(57,58)29-33-4-2-1-3-5-33/h1-17,37-38,50H,18-29H2,(H3,44,45)(H,46,55)(H,47,53)(H,48,54)(H,49,56)/t37-,38+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin protease domain using Tos-Gly-Pro-Lys-pNA as substrate by micro plate reader analysis |
J Med Chem 56: 820-31 (2013)
Article DOI: 10.1021/jm3012917
BindingDB Entry DOI: 10.7270/Q2RB75W8 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532398

(CHEMBL4454130)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CN3CCN(CC3)CC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)NC1CCCCC1 |r,wU:47.45,wD:22.18,(24.55,-27.92,;25.35,-29.25,;24.6,-30.62,;26.92,-29.22,;28.34,-29.2,;27.97,-30.47,;27.93,-27.94,;24.76,-22.74,;25.56,-24.08,;24.8,-25.45,;27.13,-24.05,;28.55,-24.03,;28.18,-25.3,;28.14,-22.77,;29.81,-25.97,;30.61,-27.31,;29.86,-28.67,;32.18,-27.27,;33.6,-27.25,;33.23,-28.52,;33.19,-25.99,;11.22,-20.81,;11.22,-22.34,;11.22,-23.88,;10.13,-24.96,;8.64,-24.55,;7.55,-25.62,;7.94,-27.11,;7.07,-28.53,;7.73,-30.78,;6.3,-31.33,;8.92,-31.74,;10.46,-31.74,;11.22,-30.41,;12.76,-30.4,;13.52,-31.73,;12.76,-33.06,;11.23,-33.06,;15.06,-31.74,;16.28,-30.81,;17.7,-31.41,;16.71,-29.11,;16.5,-27.61,;14.98,-27.24,;14.54,-25.73,;15.63,-24.61,;15.03,-23.2,;15.22,-21.58,;15.22,-20.04,;13.88,-22.34,;12.55,-21.58,;12.55,-20.04,;16.54,-22.34,;16.54,-23.88,;17.87,-21.58,;19.2,-22.34,;20.53,-21.58,;21.86,-22.35,;23.19,-21.58,;23.19,-20.05,;21.85,-19.28,;20.52,-20.05,;24.52,-19.28,;25.85,-20.04,;24.52,-17.74,;17.15,-24.98,;17.59,-26.48,;9.43,-27.51,;10.51,-26.43,;9.89,-21.58,;8.54,-22.35,;9.31,-23.7,;7.76,-23.7,;7.19,-21.58,;5.84,-22.36,;4.48,-21.59,;3.14,-22.37,;3.13,-23.93,;4.49,-24.71,;5.84,-23.93,)| Show InChI InChI=1S/C40H52N10O6S.3C2HF3O2/c41-38(42)30-12-6-29(7-13-30)24-43-39(53)34-22-27-8-14-31(15-9-27)44-36(51)25-49-18-20-50(21-19-49)26-37(52)45-32-16-10-28(11-17-32)23-35(40(54)46-34)48-57(55,56)47-33-4-2-1-3-5-33;3*3-2(4,5)1(6)7/h6-17,33-35,47-48H,1-5,18-26H2,(H3,41,42)(H,43,53)(H,44,51)(H,45,52)(H,46,54);3*(H,6,7)/t34-,35+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532398

(CHEMBL4454130)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CN3CCN(CC3)CC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)NC1CCCCC1 |r,wU:47.45,wD:22.18,(24.55,-27.92,;25.35,-29.25,;24.6,-30.62,;26.92,-29.22,;28.34,-29.2,;27.97,-30.47,;27.93,-27.94,;24.76,-22.74,;25.56,-24.08,;24.8,-25.45,;27.13,-24.05,;28.55,-24.03,;28.18,-25.3,;28.14,-22.77,;29.81,-25.97,;30.61,-27.31,;29.86,-28.67,;32.18,-27.27,;33.6,-27.25,;33.23,-28.52,;33.19,-25.99,;11.22,-20.81,;11.22,-22.34,;11.22,-23.88,;10.13,-24.96,;8.64,-24.55,;7.55,-25.62,;7.94,-27.11,;7.07,-28.53,;7.73,-30.78,;6.3,-31.33,;8.92,-31.74,;10.46,-31.74,;11.22,-30.41,;12.76,-30.4,;13.52,-31.73,;12.76,-33.06,;11.23,-33.06,;15.06,-31.74,;16.28,-30.81,;17.7,-31.41,;16.71,-29.11,;16.5,-27.61,;14.98,-27.24,;14.54,-25.73,;15.63,-24.61,;15.03,-23.2,;15.22,-21.58,;15.22,-20.04,;13.88,-22.34,;12.55,-21.58,;12.55,-20.04,;16.54,-22.34,;16.54,-23.88,;17.87,-21.58,;19.2,-22.34,;20.53,-21.58,;21.86,-22.35,;23.19,-21.58,;23.19,-20.05,;21.85,-19.28,;20.52,-20.05,;24.52,-19.28,;25.85,-20.04,;24.52,-17.74,;17.15,-24.98,;17.59,-26.48,;9.43,-27.51,;10.51,-26.43,;9.89,-21.58,;8.54,-22.35,;9.31,-23.7,;7.76,-23.7,;7.19,-21.58,;5.84,-22.36,;4.48,-21.59,;3.14,-22.37,;3.13,-23.93,;4.49,-24.71,;5.84,-23.93,)| Show InChI InChI=1S/C40H52N10O6S.3C2HF3O2/c41-38(42)30-12-6-29(7-13-30)24-43-39(53)34-22-27-8-14-31(15-9-27)44-36(51)25-49-18-20-50(21-19-49)26-37(52)45-32-16-10-28(11-17-32)23-35(40(54)46-34)48-57(55,56)47-33-4-2-1-3-5-33;3*3-2(4,5)1(6)7/h6-17,33-35,47-48H,1-5,18-26H2,(H3,41,42)(H,43,53)(H,44,51)(H,45,52)(H,46,54);3*(H,6,7)/t34-,35+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50349355

(CHEMBL1809213)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6]-[#6@@H](-[#7]S(=O)(=O)[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6]-c1ccc(cc1)-[#6](-[#7])=[#7] |r| Show InChI InChI=1S/C27H38N8O4S/c28-24(29)21-13-11-19(12-14-21)17-33-25(36)23-10-6-16-35(23)26(37)22(9-4-5-15-32-27(30)31)34-40(38,39)18-20-7-2-1-3-8-20/h1-3,7-8,11-14,22-23,34H,4-6,9-10,15-18H2,(H3,28,29)(H,33,36)(H4,30,31,32)/t22-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 21: 4860-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.033
BindingDB Entry DOI: 10.7270/Q2ST7Q5M |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532398

(CHEMBL4454130)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CN3CCN(CC3)CC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)NC1CCCCC1 |r,wU:47.45,wD:22.18,(24.55,-27.92,;25.35,-29.25,;24.6,-30.62,;26.92,-29.22,;28.34,-29.2,;27.97,-30.47,;27.93,-27.94,;24.76,-22.74,;25.56,-24.08,;24.8,-25.45,;27.13,-24.05,;28.55,-24.03,;28.18,-25.3,;28.14,-22.77,;29.81,-25.97,;30.61,-27.31,;29.86,-28.67,;32.18,-27.27,;33.6,-27.25,;33.23,-28.52,;33.19,-25.99,;11.22,-20.81,;11.22,-22.34,;11.22,-23.88,;10.13,-24.96,;8.64,-24.55,;7.55,-25.62,;7.94,-27.11,;7.07,-28.53,;7.73,-30.78,;6.3,-31.33,;8.92,-31.74,;10.46,-31.74,;11.22,-30.41,;12.76,-30.4,;13.52,-31.73,;12.76,-33.06,;11.23,-33.06,;15.06,-31.74,;16.28,-30.81,;17.7,-31.41,;16.71,-29.11,;16.5,-27.61,;14.98,-27.24,;14.54,-25.73,;15.63,-24.61,;15.03,-23.2,;15.22,-21.58,;15.22,-20.04,;13.88,-22.34,;12.55,-21.58,;12.55,-20.04,;16.54,-22.34,;16.54,-23.88,;17.87,-21.58,;19.2,-22.34,;20.53,-21.58,;21.86,-22.35,;23.19,-21.58,;23.19,-20.05,;21.85,-19.28,;20.52,-20.05,;24.52,-19.28,;25.85,-20.04,;24.52,-17.74,;17.15,-24.98,;17.59,-26.48,;9.43,-27.51,;10.51,-26.43,;9.89,-21.58,;8.54,-22.35,;9.31,-23.7,;7.76,-23.7,;7.19,-21.58,;5.84,-22.36,;4.48,-21.59,;3.14,-22.37,;3.13,-23.93,;4.49,-24.71,;5.84,-23.93,)| Show InChI InChI=1S/C40H52N10O6S.3C2HF3O2/c41-38(42)30-12-6-29(7-13-30)24-43-39(53)34-22-27-8-14-31(15-9-27)44-36(51)25-49-18-20-50(21-19-49)26-37(52)45-32-16-10-28(11-17-32)23-35(40(54)46-34)48-57(55,56)47-33-4-2-1-3-5-33;3*3-2(4,5)1(6)7/h6-17,33-35,47-48H,1-5,18-26H2,(H3,41,42)(H,43,53)(H,44,51)(H,45,52)(H,46,54);3*(H,6,7)/t34-,35+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Binding affinity to human plasmin assessed as slow binding constant in presence of Mes-DArg-Phe-Arg-AMC after 20 mins by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532398

(CHEMBL4454130)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CN3CCN(CC3)CC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)NC1CCCCC1 |r,wU:47.45,wD:22.18,(24.55,-27.92,;25.35,-29.25,;24.6,-30.62,;26.92,-29.22,;28.34,-29.2,;27.97,-30.47,;27.93,-27.94,;24.76,-22.74,;25.56,-24.08,;24.8,-25.45,;27.13,-24.05,;28.55,-24.03,;28.18,-25.3,;28.14,-22.77,;29.81,-25.97,;30.61,-27.31,;29.86,-28.67,;32.18,-27.27,;33.6,-27.25,;33.23,-28.52,;33.19,-25.99,;11.22,-20.81,;11.22,-22.34,;11.22,-23.88,;10.13,-24.96,;8.64,-24.55,;7.55,-25.62,;7.94,-27.11,;7.07,-28.53,;7.73,-30.78,;6.3,-31.33,;8.92,-31.74,;10.46,-31.74,;11.22,-30.41,;12.76,-30.4,;13.52,-31.73,;12.76,-33.06,;11.23,-33.06,;15.06,-31.74,;16.28,-30.81,;17.7,-31.41,;16.71,-29.11,;16.5,-27.61,;14.98,-27.24,;14.54,-25.73,;15.63,-24.61,;15.03,-23.2,;15.22,-21.58,;15.22,-20.04,;13.88,-22.34,;12.55,-21.58,;12.55,-20.04,;16.54,-22.34,;16.54,-23.88,;17.87,-21.58,;19.2,-22.34,;20.53,-21.58,;21.86,-22.35,;23.19,-21.58,;23.19,-20.05,;21.85,-19.28,;20.52,-20.05,;24.52,-19.28,;25.85,-20.04,;24.52,-17.74,;17.15,-24.98,;17.59,-26.48,;9.43,-27.51,;10.51,-26.43,;9.89,-21.58,;8.54,-22.35,;9.31,-23.7,;7.76,-23.7,;7.19,-21.58,;5.84,-22.36,;4.48,-21.59,;3.14,-22.37,;3.13,-23.93,;4.49,-24.71,;5.84,-23.93,)| Show InChI InChI=1S/C40H52N10O6S.3C2HF3O2/c41-38(42)30-12-6-29(7-13-30)24-43-39(53)34-22-27-8-14-31(15-9-27)44-36(51)25-49-18-20-50(21-19-49)26-37(52)45-32-16-10-28(11-17-32)23-35(40(54)46-34)48-57(55,56)47-33-4-2-1-3-5-33;3*3-2(4,5)1(6)7/h6-17,33-35,47-48H,1-5,18-26H2,(H3,41,42)(H,43,53)(H,44,51)(H,45,52)(H,46,54);3*(H,6,7)/t34-,35+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Binding affinity to human plasmin assessed as slow binding constant in presence of Mes-DArg-Phe-Arg-AMC after 20 mins by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532394
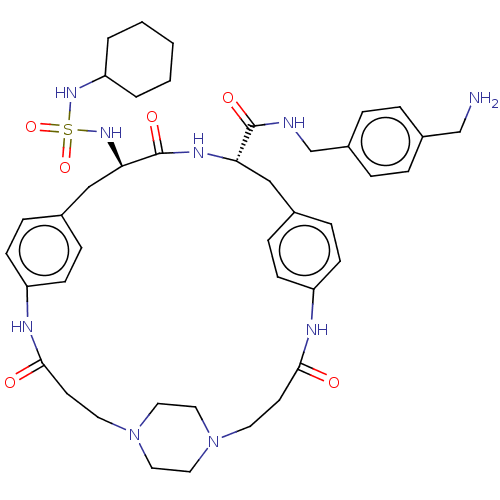
(CHEMBL4553652)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@@]([H])(NS(=O)(=O)NC4CCCCC4)C(=O)N1)cc3)cc2)C(=O)NCc1ccc(CN)cc1 |r,wU:22.18,wD:49.47,(26.65,-31.51,;27.44,-32.83,;26.69,-34.18,;28.99,-32.8,;30.39,-32.77,;30.03,-34.03,;29.99,-31.53,;26.37,-21.81,;27.16,-23.14,;26.41,-24.48,;28.71,-23.1,;30.11,-23.08,;29.75,-24.34,;29.71,-21.84,;26.77,-26.97,;27.56,-28.29,;26.81,-29.64,;29.11,-28.26,;30.51,-28.23,;30.15,-29.49,;30.11,-26.99,;16.78,-18.49,;16.73,-20.01,;16.69,-21.94,;17.23,-23.78,;18.72,-23.35,;19.83,-24.42,;19.46,-25.92,;21.25,-27.4,;20.49,-28.74,;21.22,-30.1,;18.95,-28.69,;18.14,-30,;16.6,-29.96,;15.8,-31.25,;14.29,-31.2,;13.57,-29.88,;14.37,-28.59,;15.88,-28.62,;12.03,-29.84,;11.3,-28.48,;9.76,-28.44,;8.95,-29.76,;9.02,-27.09,;9.76,-25.62,;9.26,-24.18,;10.24,-23.04,;11.74,-23.32,;12.73,-22.17,;12.77,-20.65,;12.81,-19.14,;11.48,-19.85,;10.11,-20.59,;10.88,-21.92,;9.34,-21.91,;8.78,-19.82,;7.44,-20.59,;6.11,-19.82,;4.78,-20.58,;4.77,-22.13,;6.1,-22.9,;7.45,-22.13,;14.1,-19.93,;14.15,-18.41,;15.4,-20.73,;12.24,-24.74,;11.26,-25.89,;17.97,-26.35,;16.86,-25.28,;18.02,-20.8,;17.97,-22.32,;19.35,-20.09,;20.64,-20.88,;21.98,-20.17,;23.26,-20.97,;24.6,-20.25,;24.65,-18.73,;26,-18,;27.31,-18.81,;23.35,-17.93,;22.02,-18.65,)| Show InChI InChI=1S/C42H57N9O6S.3C2HF3O2/c43-28-32-6-8-33(9-7-32)29-44-41(54)37-26-30-10-14-34(15-11-30)45-39(52)18-20-50-22-24-51(25-23-50)21-19-40(53)46-35-16-12-31(13-17-35)27-38(42(55)47-37)49-58(56,57)48-36-4-2-1-3-5-36;3*3-2(4,5)1(6)7/h6-17,36-38,48-49H,1-5,18-29,43H2,(H,44,54)(H,45,52)(H,46,53)(H,47,55);3*(H,6,7)/t37-,38+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Binding affinity to human plasmin assessed as slow binding constant in presence of Mes-DArg-Phe-Arg-AMC after 20 mins by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532394

(CHEMBL4553652)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@@]([H])(NS(=O)(=O)NC4CCCCC4)C(=O)N1)cc3)cc2)C(=O)NCc1ccc(CN)cc1 |r,wU:22.18,wD:49.47,(26.65,-31.51,;27.44,-32.83,;26.69,-34.18,;28.99,-32.8,;30.39,-32.77,;30.03,-34.03,;29.99,-31.53,;26.37,-21.81,;27.16,-23.14,;26.41,-24.48,;28.71,-23.1,;30.11,-23.08,;29.75,-24.34,;29.71,-21.84,;26.77,-26.97,;27.56,-28.29,;26.81,-29.64,;29.11,-28.26,;30.51,-28.23,;30.15,-29.49,;30.11,-26.99,;16.78,-18.49,;16.73,-20.01,;16.69,-21.94,;17.23,-23.78,;18.72,-23.35,;19.83,-24.42,;19.46,-25.92,;21.25,-27.4,;20.49,-28.74,;21.22,-30.1,;18.95,-28.69,;18.14,-30,;16.6,-29.96,;15.8,-31.25,;14.29,-31.2,;13.57,-29.88,;14.37,-28.59,;15.88,-28.62,;12.03,-29.84,;11.3,-28.48,;9.76,-28.44,;8.95,-29.76,;9.02,-27.09,;9.76,-25.62,;9.26,-24.18,;10.24,-23.04,;11.74,-23.32,;12.73,-22.17,;12.77,-20.65,;12.81,-19.14,;11.48,-19.85,;10.11,-20.59,;10.88,-21.92,;9.34,-21.91,;8.78,-19.82,;7.44,-20.59,;6.11,-19.82,;4.78,-20.58,;4.77,-22.13,;6.1,-22.9,;7.45,-22.13,;14.1,-19.93,;14.15,-18.41,;15.4,-20.73,;12.24,-24.74,;11.26,-25.89,;17.97,-26.35,;16.86,-25.28,;18.02,-20.8,;17.97,-22.32,;19.35,-20.09,;20.64,-20.88,;21.98,-20.17,;23.26,-20.97,;24.6,-20.25,;24.65,-18.73,;26,-18,;27.31,-18.81,;23.35,-17.93,;22.02,-18.65,)| Show InChI InChI=1S/C42H57N9O6S.3C2HF3O2/c43-28-32-6-8-33(9-7-32)29-44-41(54)37-26-30-10-14-34(15-11-30)45-39(52)18-20-50-22-24-51(25-23-50)21-19-40(53)46-35-16-12-31(13-17-35)27-38(42(55)47-37)49-58(56,57)48-36-4-2-1-3-5-36;3*3-2(4,5)1(6)7/h6-17,36-38,48-49H,1-5,18-29,43H2,(H,44,54)(H,45,52)(H,46,53)(H,47,55);3*(H,6,7)/t37-,38+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Binding affinity to human plasmin assessed as slow binding constant in presence of Mes-DArg-Phe-Arg-AMC after 20 mins by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532391
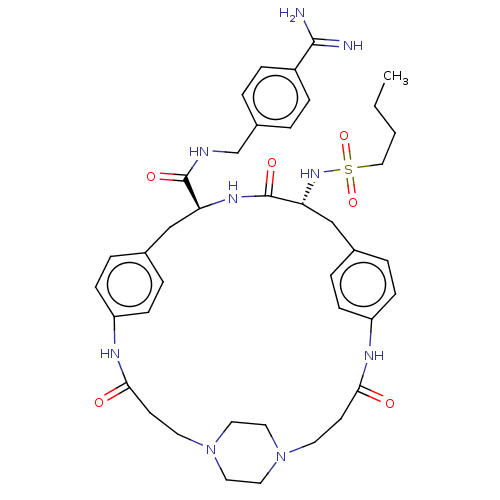
(CHEMBL4591922)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)CCCC |r,wU:49.47,wD:22.18,(25.66,-27.36,;26.46,-28.69,;25.7,-30.05,;28.02,-28.66,;29.44,-28.63,;29.07,-29.9,;29.03,-27.38,;25.74,-22.32,;26.54,-23.65,;25.78,-25.01,;28.1,-23.62,;29.52,-23.6,;29.15,-24.86,;29.11,-22.34,;26.34,-32.07,;27.14,-33.4,;26.39,-34.77,;28.71,-33.37,;30.12,-33.35,;29.75,-34.62,;29.71,-32.1,;11.88,-20.61,;11.84,-22.13,;11.79,-23.66,;10.8,-24.83,;9.29,-24.54,;8.29,-25.7,;8.8,-27.15,;8.05,-28.63,;8.79,-30,;7.98,-31.33,;10.35,-30.04,;11.09,-31.41,;12.65,-31.45,;13.45,-30.14,;14.98,-30.18,;15.7,-31.53,;14.9,-32.83,;13.37,-32.78,;17.26,-31.58,;18.07,-30.25,;19.63,-30.3,;20.37,-31.67,;20.4,-28.94,;18.59,-27.45,;18.96,-25.94,;17.84,-24.86,;16.34,-25.29,;15.79,-23.43,;15.84,-21.49,;15.88,-19.96,;14.49,-22.21,;13.18,-21.41,;13.23,-19.88,;17.13,-22.29,;17.09,-23.82,;18.48,-21.57,;19.78,-22.37,;21.13,-21.64,;22.43,-22.46,;23.78,-21.73,;23.83,-20.2,;22.52,-19.4,;21.17,-20.13,;25.17,-19.48,;26.48,-20.27,;25.22,-17.93,;15.97,-26.8,;17.09,-27.89,;10.32,-27.42,;11.3,-26.26,;10.53,-21.33,;9.16,-22.07,;9.93,-23.41,;8.38,-23.41,;7.84,-21.26,;6.47,-22,;5.14,-21.18,;3.77,-21.92,)| Show InChI InChI=1S/C40H53N9O6S.3C2HF3O2/c1-2-3-24-56(54,55)47-35-26-29-8-14-33(15-9-29)45-37(51)17-19-49-22-20-48(21-23-49)18-16-36(50)44-32-12-6-28(7-13-32)25-34(46-40(35)53)39(52)43-27-30-4-10-31(11-5-30)38(41)42;3*3-2(4,5)1(6)7/h4-15,34-35,47H,2-3,16-27H2,1H3,(H3,41,42)(H,43,52)(H,44,50)(H,45,51)(H,46,53);3*(H,6,7)/t34-,35+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532391

(CHEMBL4591922)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)CCCC |r,wU:49.47,wD:22.18,(25.66,-27.36,;26.46,-28.69,;25.7,-30.05,;28.02,-28.66,;29.44,-28.63,;29.07,-29.9,;29.03,-27.38,;25.74,-22.32,;26.54,-23.65,;25.78,-25.01,;28.1,-23.62,;29.52,-23.6,;29.15,-24.86,;29.11,-22.34,;26.34,-32.07,;27.14,-33.4,;26.39,-34.77,;28.71,-33.37,;30.12,-33.35,;29.75,-34.62,;29.71,-32.1,;11.88,-20.61,;11.84,-22.13,;11.79,-23.66,;10.8,-24.83,;9.29,-24.54,;8.29,-25.7,;8.8,-27.15,;8.05,-28.63,;8.79,-30,;7.98,-31.33,;10.35,-30.04,;11.09,-31.41,;12.65,-31.45,;13.45,-30.14,;14.98,-30.18,;15.7,-31.53,;14.9,-32.83,;13.37,-32.78,;17.26,-31.58,;18.07,-30.25,;19.63,-30.3,;20.37,-31.67,;20.4,-28.94,;18.59,-27.45,;18.96,-25.94,;17.84,-24.86,;16.34,-25.29,;15.79,-23.43,;15.84,-21.49,;15.88,-19.96,;14.49,-22.21,;13.18,-21.41,;13.23,-19.88,;17.13,-22.29,;17.09,-23.82,;18.48,-21.57,;19.78,-22.37,;21.13,-21.64,;22.43,-22.46,;23.78,-21.73,;23.83,-20.2,;22.52,-19.4,;21.17,-20.13,;25.17,-19.48,;26.48,-20.27,;25.22,-17.93,;15.97,-26.8,;17.09,-27.89,;10.32,-27.42,;11.3,-26.26,;10.53,-21.33,;9.16,-22.07,;9.93,-23.41,;8.38,-23.41,;7.84,-21.26,;6.47,-22,;5.14,-21.18,;3.77,-21.92,)| Show InChI InChI=1S/C40H53N9O6S.3C2HF3O2/c1-2-3-24-56(54,55)47-35-26-29-8-14-33(15-9-29)45-37(51)17-19-49-22-20-48(21-23-49)18-16-36(50)44-32-12-6-28(7-13-32)25-34(46-40(35)53)39(52)43-27-30-4-10-31(11-5-30)38(41)42;3*3-2(4,5)1(6)7/h4-15,34-35,47H,2-3,16-27H2,1H3,(H3,41,42)(H,43,52)(H,44,50)(H,45,51)(H,46,53);3*(H,6,7)/t34-,35+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50514081

(CHEMBL4471466)Show SMILES NCc1cccc(C[C@H](NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2cccc(c2)C(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)c1 |r| Show InChI InChI=1S/C37H42N6O6S/c38-22-28-11-4-10-27(19-28)21-33(35(44)41-23-26-15-17-30(18-16-26)34(39)40)42-36(45)32(14-6-9-25-7-2-1-3-8-25)43-50(48,49)24-29-12-5-13-31(20-29)37(46)47/h1-5,7-8,10-13,15-20,32-33,43H,6,9,14,21-24,38H2,(H3,39,40)(H,41,44)(H,42,45)(H,46,47)/t32-,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of activated Protein C (unknown origin) |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50514081

(CHEMBL4471466)Show SMILES NCc1cccc(C[C@H](NC(=O)[C@@H](CCCc2ccccc2)NS(=O)(=O)Cc2cccc(c2)C(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)c1 |r| Show InChI InChI=1S/C37H42N6O6S/c38-22-28-11-4-10-27(19-28)21-33(35(44)41-23-26-15-17-30(18-16-26)34(39)40)42-36(45)32(14-6-9-25-7-2-1-3-8-25)43-50(48,49)24-29-12-5-13-31(20-29)37(46)47/h1-5,7-8,10-13,15-20,32-33,43H,6,9,14,21-24,38H2,(H3,39,40)(H,41,44)(H,42,45)(H,46,47)/t32-,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of activated Protein C (unknown origin) |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532383

(CHEMBL4571212)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CN3CCN(CC3)CC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)c1ccccc1 |r,wU:47.45,wD:22.18,(25.74,-27.44,;26.54,-28.78,;25.78,-30.14,;28.11,-28.75,;29.53,-28.72,;29.16,-29.99,;29.12,-27.47,;25.82,-22.39,;26.62,-23.73,;25.86,-25.09,;28.19,-23.69,;29.61,-23.67,;29.24,-24.94,;29.2,-22.41,;26.43,-32.17,;27.23,-33.51,;26.47,-34.87,;28.8,-33.48,;30.22,-33.45,;29.85,-34.72,;29.81,-32.2,;11.04,-20.93,;11.04,-22.46,;11.04,-23.99,;9.95,-25.08,;8.46,-24.66,;7.37,-25.74,;7.76,-27.22,;6.89,-28.65,;7.55,-30.89,;6.11,-31.45,;8.74,-31.86,;10.28,-31.86,;11.04,-30.52,;12.58,-30.52,;13.34,-31.85,;12.58,-33.18,;11.04,-33.17,;14.87,-31.85,;16.1,-30.93,;17.51,-31.53,;16.53,-29.23,;16.31,-27.73,;14.8,-27.35,;14.36,-25.85,;15.45,-24.72,;14.85,-23.32,;15.03,-21.69,;15.03,-20.15,;13.7,-22.46,;12.37,-21.69,;12.37,-20.15,;16.36,-22.46,;16.36,-23.99,;17.69,-21.69,;19.02,-22.46,;20.35,-21.69,;21.68,-22.47,;23.01,-21.7,;23.01,-20.17,;21.67,-19.4,;20.34,-20.17,;24.34,-19.4,;25.67,-20.16,;24.33,-17.85,;16.97,-25.1,;17.41,-26.59,;9.25,-27.63,;10.33,-26.55,;9.71,-21.69,;9.71,-20.13,;10.11,-18.61,;11.22,-19.72,;8.35,-19.35,;8.36,-17.8,;7.01,-17.02,;5.65,-17.8,;5.66,-19.37,;7.01,-20.14,)| Show InChI InChI=1S/C40H45N9O6S.3C2HF3O2/c41-38(42)30-12-6-29(7-13-30)24-43-39(52)34-22-27-8-14-31(15-9-27)44-36(50)25-48-18-20-49(21-19-48)26-37(51)45-32-16-10-28(11-17-32)23-35(40(53)46-34)47-56(54,55)33-4-2-1-3-5-33;3*3-2(4,5)1(6)7/h1-17,34-35,47H,18-26H2,(H3,41,42)(H,43,52)(H,44,50)(H,45,51)(H,46,53);3*(H,6,7)/t34-,35+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532383

(CHEMBL4571212)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CN3CCN(CC3)CC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NS(=O)(=O)c1ccccc1 |r,wU:47.45,wD:22.18,(25.74,-27.44,;26.54,-28.78,;25.78,-30.14,;28.11,-28.75,;29.53,-28.72,;29.16,-29.99,;29.12,-27.47,;25.82,-22.39,;26.62,-23.73,;25.86,-25.09,;28.19,-23.69,;29.61,-23.67,;29.24,-24.94,;29.2,-22.41,;26.43,-32.17,;27.23,-33.51,;26.47,-34.87,;28.8,-33.48,;30.22,-33.45,;29.85,-34.72,;29.81,-32.2,;11.04,-20.93,;11.04,-22.46,;11.04,-23.99,;9.95,-25.08,;8.46,-24.66,;7.37,-25.74,;7.76,-27.22,;6.89,-28.65,;7.55,-30.89,;6.11,-31.45,;8.74,-31.86,;10.28,-31.86,;11.04,-30.52,;12.58,-30.52,;13.34,-31.85,;12.58,-33.18,;11.04,-33.17,;14.87,-31.85,;16.1,-30.93,;17.51,-31.53,;16.53,-29.23,;16.31,-27.73,;14.8,-27.35,;14.36,-25.85,;15.45,-24.72,;14.85,-23.32,;15.03,-21.69,;15.03,-20.15,;13.7,-22.46,;12.37,-21.69,;12.37,-20.15,;16.36,-22.46,;16.36,-23.99,;17.69,-21.69,;19.02,-22.46,;20.35,-21.69,;21.68,-22.47,;23.01,-21.7,;23.01,-20.17,;21.67,-19.4,;20.34,-20.17,;24.34,-19.4,;25.67,-20.16,;24.33,-17.85,;16.97,-25.1,;17.41,-26.59,;9.25,-27.63,;10.33,-26.55,;9.71,-21.69,;9.71,-20.13,;10.11,-18.61,;11.22,-19.72,;8.35,-19.35,;8.36,-17.8,;7.01,-17.02,;5.65,-17.8,;5.66,-19.37,;7.01,-20.14,)| Show InChI InChI=1S/C40H45N9O6S.3C2HF3O2/c41-38(42)30-12-6-29(7-13-30)24-43-39(52)34-22-27-8-14-31(15-9-27)44-36(50)25-48-18-20-49(21-19-48)26-37(51)45-32-16-10-28(11-17-32)23-35(40(53)46-34)47-56(54,55)33-4-2-1-3-5-33;3*3-2(4,5)1(6)7/h1-17,34-35,47H,18-26H2,(H3,41,42)(H,43,52)(H,44,50)(H,45,51)(H,46,53);3*(H,6,7)/t34-,35+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50425654
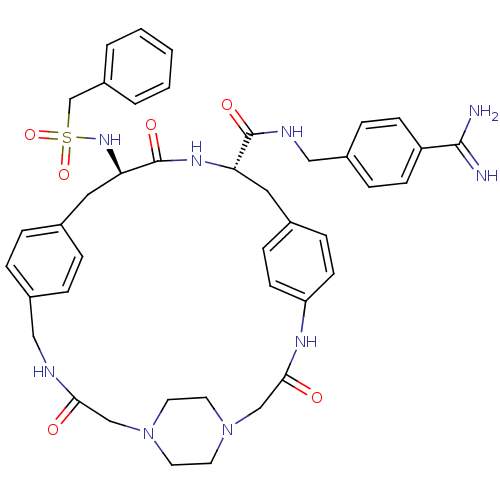
(CHEMBL2315236)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2Cc3ccc(NC(=O)CN4CCN(CC4)CC(=O)NCc4ccc(C[C@@H](NS(=O)(=O)Cc5ccccc5)C(=O)N2)cc4)cc3)cc1 |r,wU:37.38,wD:11.10,(75.05,-3.68,;75.03,-5.21,;76.35,-6,;73.69,-5.97,;73.66,-7.51,;72.31,-8.25,;71,-7.47,;69.65,-8.22,;68.34,-7.43,;66.99,-8.18,;66.97,-9.72,;65.67,-7.39,;64.32,-8.14,;64.31,-9.68,;62.97,-10.45,;62.96,-11.98,;64.39,-12.79,;63.08,-14.96,;63.13,-16.49,;64.47,-17.22,;61.82,-17.29,;60.47,-16.56,;59.16,-17.36,;57.82,-16.63,;57.78,-15.1,;59.08,-14.29,;60.43,-15.02,;56.43,-14.38,;56.42,-12.84,;55.08,-12.09,;57.74,-12.07,;57.73,-10.53,;59.05,-9.75,;59.04,-8.21,;60.36,-7.43,;61.7,-8.2,;63.03,-7.42,;63.01,-5.88,;61.67,-5.11,;60.34,-5.9,;59.57,-7.23,;61.11,-7.23,;59,-5.13,;57.68,-5.91,;56.34,-5.14,;55.01,-5.92,;55.01,-7.46,;56.34,-8.23,;57.68,-7.46,;64.34,-5.09,;64.33,-3.55,;65.68,-5.85,;61.72,-9.73,;60.4,-10.52,;65.63,-11.99,;65.64,-10.46,;71.01,-5.93,;72.35,-5.17,)| Show InChI InChI=1S/C42H49N9O6S/c43-40(44)34-14-10-32(11-15-34)25-46-41(54)36-22-30-12-16-35(17-13-30)47-39(53)27-51-20-18-50(19-21-51)26-38(52)45-24-31-8-6-29(7-9-31)23-37(42(55)48-36)49-58(56,57)28-33-4-2-1-3-5-33/h1-17,36-37,49H,18-28H2,(H3,43,44)(H,45,52)(H,46,54)(H,47,53)(H,48,55)/t36-,37+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin protease domain using Tos-Gly-Pro-Lys-pNA as substrate by micro plate reader analysis |
J Med Chem 56: 820-31 (2013)
Article DOI: 10.1021/jm3012917
BindingDB Entry DOI: 10.7270/Q2RB75W8 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532394

(CHEMBL4553652)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@@]([H])(NS(=O)(=O)NC4CCCCC4)C(=O)N1)cc3)cc2)C(=O)NCc1ccc(CN)cc1 |r,wU:22.18,wD:49.47,(26.65,-31.51,;27.44,-32.83,;26.69,-34.18,;28.99,-32.8,;30.39,-32.77,;30.03,-34.03,;29.99,-31.53,;26.37,-21.81,;27.16,-23.14,;26.41,-24.48,;28.71,-23.1,;30.11,-23.08,;29.75,-24.34,;29.71,-21.84,;26.77,-26.97,;27.56,-28.29,;26.81,-29.64,;29.11,-28.26,;30.51,-28.23,;30.15,-29.49,;30.11,-26.99,;16.78,-18.49,;16.73,-20.01,;16.69,-21.94,;17.23,-23.78,;18.72,-23.35,;19.83,-24.42,;19.46,-25.92,;21.25,-27.4,;20.49,-28.74,;21.22,-30.1,;18.95,-28.69,;18.14,-30,;16.6,-29.96,;15.8,-31.25,;14.29,-31.2,;13.57,-29.88,;14.37,-28.59,;15.88,-28.62,;12.03,-29.84,;11.3,-28.48,;9.76,-28.44,;8.95,-29.76,;9.02,-27.09,;9.76,-25.62,;9.26,-24.18,;10.24,-23.04,;11.74,-23.32,;12.73,-22.17,;12.77,-20.65,;12.81,-19.14,;11.48,-19.85,;10.11,-20.59,;10.88,-21.92,;9.34,-21.91,;8.78,-19.82,;7.44,-20.59,;6.11,-19.82,;4.78,-20.58,;4.77,-22.13,;6.1,-22.9,;7.45,-22.13,;14.1,-19.93,;14.15,-18.41,;15.4,-20.73,;12.24,-24.74,;11.26,-25.89,;17.97,-26.35,;16.86,-25.28,;18.02,-20.8,;17.97,-22.32,;19.35,-20.09,;20.64,-20.88,;21.98,-20.17,;23.26,-20.97,;24.6,-20.25,;24.65,-18.73,;26,-18,;27.31,-18.81,;23.35,-17.93,;22.02,-18.65,)| Show InChI InChI=1S/C42H57N9O6S.3C2HF3O2/c43-28-32-6-8-33(9-7-32)29-44-41(54)37-26-30-10-14-34(15-11-30)45-39(52)18-20-50-22-24-51(25-23-50)21-19-40(53)46-35-16-12-31(13-17-35)27-38(42(55)47-37)49-58(56,57)48-36-4-2-1-3-5-36;3*3-2(4,5)1(6)7/h6-17,36-38,48-49H,1-5,18-29,43H2,(H,44,54)(H,45,52)(H,46,53)(H,47,55);3*(H,6,7)/t37-,38+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532394

(CHEMBL4553652)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@@]([H])(NS(=O)(=O)NC4CCCCC4)C(=O)N1)cc3)cc2)C(=O)NCc1ccc(CN)cc1 |r,wU:22.18,wD:49.47,(26.65,-31.51,;27.44,-32.83,;26.69,-34.18,;28.99,-32.8,;30.39,-32.77,;30.03,-34.03,;29.99,-31.53,;26.37,-21.81,;27.16,-23.14,;26.41,-24.48,;28.71,-23.1,;30.11,-23.08,;29.75,-24.34,;29.71,-21.84,;26.77,-26.97,;27.56,-28.29,;26.81,-29.64,;29.11,-28.26,;30.51,-28.23,;30.15,-29.49,;30.11,-26.99,;16.78,-18.49,;16.73,-20.01,;16.69,-21.94,;17.23,-23.78,;18.72,-23.35,;19.83,-24.42,;19.46,-25.92,;21.25,-27.4,;20.49,-28.74,;21.22,-30.1,;18.95,-28.69,;18.14,-30,;16.6,-29.96,;15.8,-31.25,;14.29,-31.2,;13.57,-29.88,;14.37,-28.59,;15.88,-28.62,;12.03,-29.84,;11.3,-28.48,;9.76,-28.44,;8.95,-29.76,;9.02,-27.09,;9.76,-25.62,;9.26,-24.18,;10.24,-23.04,;11.74,-23.32,;12.73,-22.17,;12.77,-20.65,;12.81,-19.14,;11.48,-19.85,;10.11,-20.59,;10.88,-21.92,;9.34,-21.91,;8.78,-19.82,;7.44,-20.59,;6.11,-19.82,;4.78,-20.58,;4.77,-22.13,;6.1,-22.9,;7.45,-22.13,;14.1,-19.93,;14.15,-18.41,;15.4,-20.73,;12.24,-24.74,;11.26,-25.89,;17.97,-26.35,;16.86,-25.28,;18.02,-20.8,;17.97,-22.32,;19.35,-20.09,;20.64,-20.88,;21.98,-20.17,;23.26,-20.97,;24.6,-20.25,;24.65,-18.73,;26,-18,;27.31,-18.81,;23.35,-17.93,;22.02,-18.65,)| Show InChI InChI=1S/C42H57N9O6S.3C2HF3O2/c43-28-32-6-8-33(9-7-32)29-44-41(54)37-26-30-10-14-34(15-11-30)45-39(52)18-20-50-22-24-51(25-23-50)21-19-40(53)46-35-16-12-31(13-17-35)27-38(42(55)47-37)49-58(56,57)48-36-4-2-1-3-5-36;3*3-2(4,5)1(6)7/h6-17,36-38,48-49H,1-5,18-29,43H2,(H,44,54)(H,45,52)(H,46,53)(H,47,55);3*(H,6,7)/t37-,38+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532384
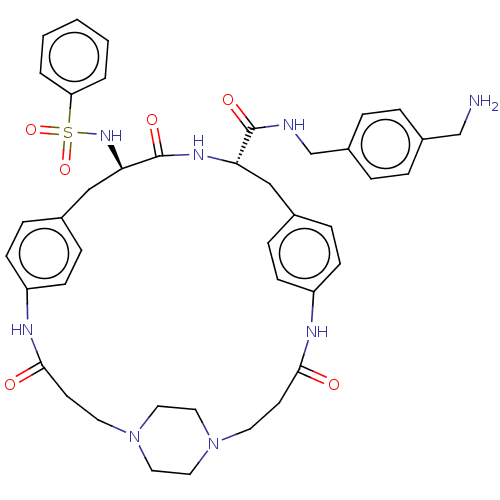
(CHEMBL4560508)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@@]([H])(NS(=O)(=O)c4ccccc4)C(=O)N1)cc3)cc2)C(=O)NCc1ccc(CN)cc1 |r,wU:22.18,wD:49.47,(26.65,-31.51,;27.44,-32.83,;26.69,-34.18,;28.99,-32.8,;30.39,-32.77,;30.03,-34.03,;29.99,-31.53,;26.37,-21.81,;27.16,-23.14,;26.41,-24.48,;28.71,-23.1,;30.11,-23.08,;29.75,-24.34,;29.71,-21.84,;26.77,-26.97,;27.56,-28.29,;26.81,-29.64,;29.11,-28.26,;30.51,-28.23,;30.15,-29.49,;30.11,-26.99,;16.78,-18.49,;16.73,-20.01,;16.69,-21.94,;17.23,-23.78,;18.72,-23.35,;19.83,-24.42,;19.46,-25.92,;21.25,-27.4,;20.49,-28.74,;21.22,-30.1,;18.95,-28.69,;18.14,-30,;16.6,-29.96,;15.8,-31.25,;14.29,-31.2,;13.57,-29.88,;14.37,-28.59,;15.88,-28.62,;12.03,-29.84,;11.3,-28.48,;9.76,-28.44,;8.95,-29.76,;9.02,-27.09,;9.76,-25.62,;9.26,-24.18,;10.24,-23.04,;11.74,-23.32,;12.73,-22.17,;12.77,-20.65,;12.81,-19.14,;11.48,-19.85,;10.11,-20.59,;10.88,-21.92,;9.34,-21.91,;8.78,-19.82,;8.78,-18.27,;7.44,-17.5,;6.1,-18.27,;6.11,-19.83,;7.45,-20.59,;14.1,-19.93,;14.15,-18.41,;15.4,-20.73,;12.24,-24.74,;11.26,-25.89,;17.97,-26.35,;16.86,-25.28,;18.02,-20.8,;17.97,-22.32,;19.35,-20.09,;20.64,-20.88,;21.98,-20.17,;23.26,-20.97,;24.6,-20.25,;24.65,-18.73,;26,-18,;27.31,-18.81,;23.35,-17.93,;22.02,-18.65,)| Show InChI InChI=1S/C42H50N8O6S.3C2HF3O2/c43-28-32-6-8-33(9-7-32)29-44-41(53)37-26-30-10-14-34(15-11-30)45-39(51)18-20-49-22-24-50(25-23-49)21-19-40(52)46-35-16-12-31(13-17-35)27-38(42(54)47-37)48-57(55,56)36-4-2-1-3-5-36;3*3-2(4,5)1(6)7/h1-17,37-38,48H,18-29,43H2,(H,44,53)(H,45,51)(H,46,52)(H,47,54);3*(H,6,7)/t37-,38+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Binding affinity to human plasmin assessed as slow binding constant in presence of Mes-DArg-Phe-Arg-AMC after 20 mins by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532384

(CHEMBL4560508)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@@]([H])(NS(=O)(=O)c4ccccc4)C(=O)N1)cc3)cc2)C(=O)NCc1ccc(CN)cc1 |r,wU:22.18,wD:49.47,(26.65,-31.51,;27.44,-32.83,;26.69,-34.18,;28.99,-32.8,;30.39,-32.77,;30.03,-34.03,;29.99,-31.53,;26.37,-21.81,;27.16,-23.14,;26.41,-24.48,;28.71,-23.1,;30.11,-23.08,;29.75,-24.34,;29.71,-21.84,;26.77,-26.97,;27.56,-28.29,;26.81,-29.64,;29.11,-28.26,;30.51,-28.23,;30.15,-29.49,;30.11,-26.99,;16.78,-18.49,;16.73,-20.01,;16.69,-21.94,;17.23,-23.78,;18.72,-23.35,;19.83,-24.42,;19.46,-25.92,;21.25,-27.4,;20.49,-28.74,;21.22,-30.1,;18.95,-28.69,;18.14,-30,;16.6,-29.96,;15.8,-31.25,;14.29,-31.2,;13.57,-29.88,;14.37,-28.59,;15.88,-28.62,;12.03,-29.84,;11.3,-28.48,;9.76,-28.44,;8.95,-29.76,;9.02,-27.09,;9.76,-25.62,;9.26,-24.18,;10.24,-23.04,;11.74,-23.32,;12.73,-22.17,;12.77,-20.65,;12.81,-19.14,;11.48,-19.85,;10.11,-20.59,;10.88,-21.92,;9.34,-21.91,;8.78,-19.82,;8.78,-18.27,;7.44,-17.5,;6.1,-18.27,;6.11,-19.83,;7.45,-20.59,;14.1,-19.93,;14.15,-18.41,;15.4,-20.73,;12.24,-24.74,;11.26,-25.89,;17.97,-26.35,;16.86,-25.28,;18.02,-20.8,;17.97,-22.32,;19.35,-20.09,;20.64,-20.88,;21.98,-20.17,;23.26,-20.97,;24.6,-20.25,;24.65,-18.73,;26,-18,;27.31,-18.81,;23.35,-17.93,;22.02,-18.65,)| Show InChI InChI=1S/C42H50N8O6S.3C2HF3O2/c43-28-32-6-8-33(9-7-32)29-44-41(53)37-26-30-10-14-34(15-11-30)45-39(51)18-20-49-22-24-50(25-23-49)21-19-40(52)46-35-16-12-31(13-17-35)27-38(42(54)47-37)48-57(55,56)36-4-2-1-3-5-36;3*3-2(4,5)1(6)7/h1-17,37-38,48H,18-29,43H2,(H,44,53)(H,45,51)(H,46,52)(H,47,54);3*(H,6,7)/t37-,38+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Binding affinity to human plasmin assessed as slow binding constant in presence of Mes-DArg-Phe-Arg-AMC after 20 mins by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM141387

(US8921319, 3)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2Cc3ccc(CNC(=O)Cc4cccc(CC(=O)NCc5ccc(C[C@@H](NS(=O)(=O)Cc6ccccc6)C(=O)N2)cc5)c4)cc3)cc1 |r| Show InChI InChI=1S/C45H47N7O6S/c46-43(47)38-19-17-34(18-20-38)28-50-44(55)39-22-30-9-13-32(14-10-30)26-48-41(53)24-36-7-4-8-37(21-36)25-42(54)49-27-33-15-11-31(12-16-33)23-40(45(56)51-39)52-59(57,58)29-35-5-2-1-3-6-35/h1-21,39-40,52H,22-29H2,(H3,46,47)(H,48,53)(H,49,54)(H,50,55)(H,51,56)/t39-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
The Medicines Company (Leipzig) GmbH
US Patent
| Assay Description
The inhibition constants for human plasmin (h plasmin), human plasma kallikrein (h PK), thrombin and factor Xa were determined in analogy to a previo... |
US Patent US8921319 (2014)
BindingDB Entry DOI: 10.7270/Q2RX99S6 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532384

(CHEMBL4560508)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@@]([H])(NS(=O)(=O)c4ccccc4)C(=O)N1)cc3)cc2)C(=O)NCc1ccc(CN)cc1 |r,wU:22.18,wD:49.47,(26.65,-31.51,;27.44,-32.83,;26.69,-34.18,;28.99,-32.8,;30.39,-32.77,;30.03,-34.03,;29.99,-31.53,;26.37,-21.81,;27.16,-23.14,;26.41,-24.48,;28.71,-23.1,;30.11,-23.08,;29.75,-24.34,;29.71,-21.84,;26.77,-26.97,;27.56,-28.29,;26.81,-29.64,;29.11,-28.26,;30.51,-28.23,;30.15,-29.49,;30.11,-26.99,;16.78,-18.49,;16.73,-20.01,;16.69,-21.94,;17.23,-23.78,;18.72,-23.35,;19.83,-24.42,;19.46,-25.92,;21.25,-27.4,;20.49,-28.74,;21.22,-30.1,;18.95,-28.69,;18.14,-30,;16.6,-29.96,;15.8,-31.25,;14.29,-31.2,;13.57,-29.88,;14.37,-28.59,;15.88,-28.62,;12.03,-29.84,;11.3,-28.48,;9.76,-28.44,;8.95,-29.76,;9.02,-27.09,;9.76,-25.62,;9.26,-24.18,;10.24,-23.04,;11.74,-23.32,;12.73,-22.17,;12.77,-20.65,;12.81,-19.14,;11.48,-19.85,;10.11,-20.59,;10.88,-21.92,;9.34,-21.91,;8.78,-19.82,;8.78,-18.27,;7.44,-17.5,;6.1,-18.27,;6.11,-19.83,;7.45,-20.59,;14.1,-19.93,;14.15,-18.41,;15.4,-20.73,;12.24,-24.74,;11.26,-25.89,;17.97,-26.35,;16.86,-25.28,;18.02,-20.8,;17.97,-22.32,;19.35,-20.09,;20.64,-20.88,;21.98,-20.17,;23.26,-20.97,;24.6,-20.25,;24.65,-18.73,;26,-18,;27.31,-18.81,;23.35,-17.93,;22.02,-18.65,)| Show InChI InChI=1S/C42H50N8O6S.3C2HF3O2/c43-28-32-6-8-33(9-7-32)29-44-41(53)37-26-30-10-14-34(15-11-30)45-39(51)18-20-49-22-24-50(25-23-49)21-19-40(52)46-35-16-12-31(13-17-35)27-38(42(54)47-37)48-57(55,56)36-4-2-1-3-5-36;3*3-2(4,5)1(6)7/h1-17,37-38,48H,18-29,43H2,(H,44,53)(H,45,51)(H,46,52)(H,47,54);3*(H,6,7)/t37-,38+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532384

(CHEMBL4560508)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@@]([H])(NS(=O)(=O)c4ccccc4)C(=O)N1)cc3)cc2)C(=O)NCc1ccc(CN)cc1 |r,wU:22.18,wD:49.47,(26.65,-31.51,;27.44,-32.83,;26.69,-34.18,;28.99,-32.8,;30.39,-32.77,;30.03,-34.03,;29.99,-31.53,;26.37,-21.81,;27.16,-23.14,;26.41,-24.48,;28.71,-23.1,;30.11,-23.08,;29.75,-24.34,;29.71,-21.84,;26.77,-26.97,;27.56,-28.29,;26.81,-29.64,;29.11,-28.26,;30.51,-28.23,;30.15,-29.49,;30.11,-26.99,;16.78,-18.49,;16.73,-20.01,;16.69,-21.94,;17.23,-23.78,;18.72,-23.35,;19.83,-24.42,;19.46,-25.92,;21.25,-27.4,;20.49,-28.74,;21.22,-30.1,;18.95,-28.69,;18.14,-30,;16.6,-29.96,;15.8,-31.25,;14.29,-31.2,;13.57,-29.88,;14.37,-28.59,;15.88,-28.62,;12.03,-29.84,;11.3,-28.48,;9.76,-28.44,;8.95,-29.76,;9.02,-27.09,;9.76,-25.62,;9.26,-24.18,;10.24,-23.04,;11.74,-23.32,;12.73,-22.17,;12.77,-20.65,;12.81,-19.14,;11.48,-19.85,;10.11,-20.59,;10.88,-21.92,;9.34,-21.91,;8.78,-19.82,;8.78,-18.27,;7.44,-17.5,;6.1,-18.27,;6.11,-19.83,;7.45,-20.59,;14.1,-19.93,;14.15,-18.41,;15.4,-20.73,;12.24,-24.74,;11.26,-25.89,;17.97,-26.35,;16.86,-25.28,;18.02,-20.8,;17.97,-22.32,;19.35,-20.09,;20.64,-20.88,;21.98,-20.17,;23.26,-20.97,;24.6,-20.25,;24.65,-18.73,;26,-18,;27.31,-18.81,;23.35,-17.93,;22.02,-18.65,)| Show InChI InChI=1S/C42H50N8O6S.3C2HF3O2/c43-28-32-6-8-33(9-7-32)29-44-41(53)37-26-30-10-14-34(15-11-30)45-39(51)18-20-49-22-24-50(25-23-49)21-19-40(52)46-35-16-12-31(13-17-35)27-38(42(54)47-37)48-57(55,56)36-4-2-1-3-5-36;3*3-2(4,5)1(6)7/h1-17,37-38,48H,18-29,43H2,(H,44,53)(H,45,51)(H,46,52)(H,47,54);3*(H,6,7)/t37-,38+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50425656

(CHEMBL2315246)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2Cc3ccc(NC(=O)CN4CCCN(CC4)CC(=O)Nc4ccc(C[C@@H](NS(=O)(=O)Cc5ccccc5)C(=O)N2)cc4)cc3)cc1 |r| Show InChI InChI=1S/C42H49N9O6S/c43-40(44)33-13-7-31(8-14-33)25-45-41(54)36-23-29-9-15-34(16-10-29)46-38(52)26-50-19-4-20-51(22-21-50)27-39(53)47-35-17-11-30(12-18-35)24-37(42(55)48-36)49-58(56,57)28-32-5-2-1-3-6-32/h1-3,5-18,36-37,49H,4,19-28H2,(H3,43,44)(H,45,54)(H,46,52)(H,47,53)(H,48,55)/t36-,37+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin protease domain using Tos-Gly-Pro-Lys-pNA as substrate by micro plate reader analysis |
J Med Chem 56: 820-31 (2013)
Article DOI: 10.1021/jm3012917
BindingDB Entry DOI: 10.7270/Q2RB75W8 |
More data for this
Ligand-Target Pair | |
Trypsin
(Sus scrofa) | BDBM50425657
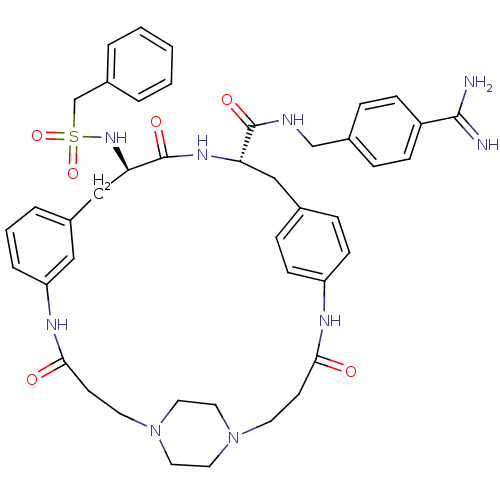
(CHEMBL2315245)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2Cc3ccc(NC(=O)CCN4CCN(CC4)CCC(=O)Nc4cccc(C[C@@H](NS(=O)(=O)Cc5ccccc5)C(=O)N2)c4)cc3)cc1 |r,wU:39.40,wD:11.10,(23.16,-38.31,;23.14,-39.85,;24.46,-40.63,;21.8,-40.6,;21.77,-42.14,;20.42,-42.88,;19.11,-42.1,;17.76,-42.85,;16.45,-42.06,;15.1,-42.81,;15.08,-44.35,;13.78,-42.03,;12.43,-42.78,;12.42,-44.32,;11.08,-45.08,;11.07,-46.62,;12.4,-47.39,;12.39,-48.93,;11.06,-49.69,;9.74,-48.91,;11.06,-51.22,;9.72,-51.98,;8.4,-51.21,;8.41,-49.68,;7.1,-48.91,;5.76,-49.66,;5.75,-51.2,;7.08,-51.98,;4.44,-48.89,;4.45,-47.35,;5.79,-46.6,;5.8,-45.06,;8.53,-46.68,;8.51,-45.15,;7.16,-44.39,;7.15,-42.84,;8.47,-42.07,;9.81,-42.83,;11.14,-42.05,;11.12,-40.51,;9.78,-39.75,;8.45,-40.54,;7.68,-41.86,;9.22,-41.86,;7.11,-39.77,;5.79,-40.55,;4.45,-39.78,;3.12,-40.55,;3.12,-42.1,;4.45,-42.87,;5.79,-42.1,;12.45,-39.72,;12.44,-38.18,;13.79,-40.48,;9.83,-44.36,;13.74,-46.63,;13.75,-45.09,;19.12,-40.56,;20.47,-39.81,)| Show InChI InChI=1S/C43H51N9O6S/c44-41(45)34-13-9-31(10-14-34)28-46-42(55)37-26-30-11-15-35(16-12-30)47-39(53)17-19-51-21-23-52(24-22-51)20-18-40(54)48-36-8-4-7-33(25-36)27-38(43(56)49-37)50-59(57,58)29-32-5-2-1-3-6-32/h1-16,25,37-38,50H,17-24,26-29H2,(H3,44,45)(H,46,55)(H,47,53)(H,48,54)(H,49,56)/t37-,38+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of pig trypsin using CH3SO2-D-Cha-Gly-Arg-pNA as substrate after 5 to 10 mins by micro plate reader analysis |
J Med Chem 56: 820-31 (2013)
Article DOI: 10.1021/jm3012917
BindingDB Entry DOI: 10.7270/Q2RB75W8 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50380619

(CHEMBL2016865)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-c1cccc(-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]-c2ccccc2)-[#7]S(=O)(=O)[#6]-c2cccc(c2)-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-c2ccc(cc2)-[#6](-[#7])=[#7])c1 |r| Show InChI InChI=1S/C38H44N8O6S/c39-34(40)30-17-15-26(16-18-30)22-43-35(47)33(21-27-10-4-11-28(19-27)23-44-38(41)42)45-36(48)32(14-6-9-25-7-2-1-3-8-25)46-53(51,52)24-29-12-5-13-31(20-29)37(49)50/h1-5,7-8,10-13,15-20,32-33,46H,6,9,14,21-24H2,(H3,39,40)(H,43,47)(H,45,48)(H,49,50)(H4,41,42,44)/t32-,33+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of activated protein C by dixon plot method |
J Med Chem 55: 1171-80 (2012)
Article DOI: 10.1021/jm2011996
BindingDB Entry DOI: 10.7270/Q26T0NN4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50425649
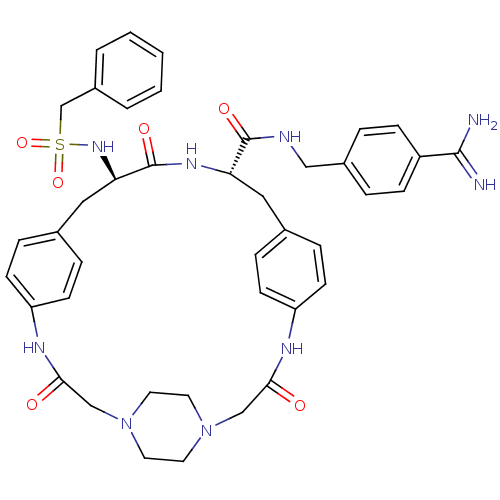
(CHEMBL2315239)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2Cc3ccc(NC(=O)CN4CCN(CC4)CC(=O)Nc4ccc(C[C@@H](NS(=O)(=O)Cc5ccccc5)C(=O)N2)cc4)cc3)cc1 |r,wU:36.37,wD:11.10,(23.09,-3.13,;23.07,-4.67,;24.39,-5.45,;21.73,-5.42,;21.7,-6.96,;20.35,-7.7,;19.04,-6.92,;17.69,-7.67,;16.38,-6.89,;15.03,-7.63,;15.01,-9.17,;13.71,-6.85,;12.36,-7.6,;12.35,-9.14,;11.01,-9.9,;11,-11.44,;12.33,-12.22,;12.32,-13.75,;10.99,-14.51,;10.98,-16.04,;9.67,-13.73,;8.33,-14.49,;7.02,-13.72,;5.69,-14.47,;5.67,-16,;6.99,-16.78,;8.33,-16.03,;4.33,-16.75,;5.78,-11.52,;7.12,-12.28,;5.77,-9.99,;7.09,-9.21,;7.08,-7.67,;8.4,-6.89,;9.74,-7.65,;11.07,-6.87,;11.05,-5.33,;9.71,-4.57,;8.38,-5.36,;7.6,-6.68,;9.15,-6.68,;7.04,-4.59,;5.72,-5.37,;4.38,-4.6,;3.05,-5.37,;3.05,-6.92,;4.38,-7.69,;5.72,-6.92,;12.38,-4.54,;12.37,-3,;13.72,-5.31,;9.76,-9.18,;8.44,-9.97,;13.67,-11.45,;13.68,-9.91,;19.05,-5.39,;20.39,-4.63,)| Show InChI InChI=1S/C41H47N9O6S/c42-39(43)32-12-6-30(7-13-32)24-44-40(53)35-22-28-8-14-33(15-9-28)45-37(51)25-49-18-20-50(21-19-49)26-38(52)46-34-16-10-29(11-17-34)23-36(41(54)47-35)48-57(55,56)27-31-4-2-1-3-5-31/h1-17,35-36,48H,18-27H2,(H3,42,43)(H,44,53)(H,45,51)(H,46,52)(H,47,54)/t35-,36+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin protease domain using Tos-Gly-Pro-Lys-pNA as substrate by micro plate reader analysis |
J Med Chem 56: 820-31 (2013)
Article DOI: 10.1021/jm3012917
BindingDB Entry DOI: 10.7270/Q2RB75W8 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM141391
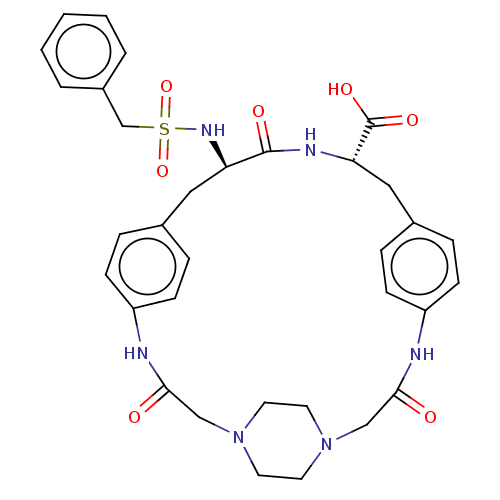
(US8921319, 15)Show SMILES OC(=O)[C@@H]1Cc2ccc(NC(=O)CN3CCN(CC3)CC(=O)Nc3ccc(C[C@@H](NS(=O)(=O)Cc4ccccc4)C(=O)N1)cc3)cc2 |r,wU:28.29,wD:3.2,(3.08,3.56,;1.6,3.96,;1.2,5.44,;.51,2.87,;.91,1.38,;2.39,.98,;2.79,-.51,;4.28,-.9,;5.37,.18,;6.86,-.21,;7.58,-1.48,;9.07,-1.08,;6.81,-2.82,;5.55,-3.27,;4.22,-2.5,;2.89,-3.27,;2.89,-4.81,;4.22,-5.58,;5.55,-4.81,;1.4,-5.21,;.31,-4.12,;-1.02,-4.89,;.31,-2.58,;-.29,-.85,;-1.62,-1.62,;-2.96,-.85,;-2.96,.69,;-4.05,1.78,;-3.65,3.27,;-4.98,4.04,;-6.32,3.27,;-6.71,4.75,;-4.73,2.84,;-7.74,2.45,;-7.74,.91,;-9.07,.14,;-9.07,-1.4,;-7.74,-2.17,;-6.4,-1.4,;-6.4,.14,;-2.31,4.04,;-2.31,5.58,;-.98,3.27,;-1.62,1.46,;-.29,.69,;4.97,1.67,;3.48,2.07,)| Show InChI InChI=1S/C33H38N6O7S/c40-30-20-38-14-16-39(17-15-38)21-31(41)35-27-12-8-24(9-13-27)19-29(33(43)44)36-32(42)28(18-23-6-10-26(34-30)11-7-23)37-47(45,46)22-25-4-2-1-3-5-25/h1-13,28-29,37H,14-22H2,(H,34,40)(H,35,41)(H,36,42)(H,43,44)/t28-,29+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
The Medicines Company (Leipzig) GmbH
US Patent
| Assay Description
The inhibition constants for human plasmin (h plasmin), human plasma kallikrein (h PK), thrombin and factor Xa were determined in analogy to a previo... |
US Patent US8921319 (2014)
BindingDB Entry DOI: 10.7270/Q2RX99S6 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50425653

(CHEMBL2315237)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2Cc3ccc(NC(=O)CN4CCCN(CC4)CC(=O)NCc4ccc(C[C@@H](NS(=O)(=O)Cc5ccccc5)C(=O)N2)cc4)cc3)cc1 |r| Show InChI InChI=1S/C43H51N9O6S/c44-41(45)35-15-11-33(12-16-35)26-47-42(55)37-23-31-13-17-36(18-14-31)48-40(54)28-52-20-4-19-51(21-22-52)27-39(53)46-25-32-9-7-30(8-10-32)24-38(43(56)49-37)50-59(57,58)29-34-5-2-1-3-6-34/h1-3,5-18,37-38,50H,4,19-29H2,(H3,44,45)(H,46,53)(H,47,55)(H,48,54)(H,49,56)/t37-,38+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin protease domain using Tos-Gly-Pro-Lys-pNA as substrate by micro plate reader analysis |
J Med Chem 56: 820-31 (2013)
Article DOI: 10.1021/jm3012917
BindingDB Entry DOI: 10.7270/Q2RB75W8 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50380617
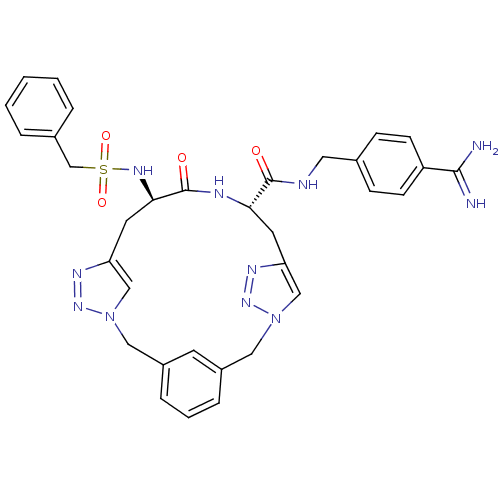
(CHEMBL2016873)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2Cc3cn(Cc4cccc(Cn5cc(C[C@@H](NS(=O)(=O)Cc6ccccc6)C(=O)N2)nn5)c4)nn3)cc1 |r| Show InChI InChI=1S/C33H35N11O4S/c34-31(35)26-11-9-22(10-12-26)16-36-32(45)29-14-27-19-43(41-38-27)17-24-7-4-8-25(13-24)18-44-20-28(39-42-44)15-30(33(46)37-29)40-49(47,48)21-23-5-2-1-3-6-23/h1-13,19-20,29-30,40H,14-18,21H2,(H3,34,35)(H,36,45)(H,37,46)/t29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of plasmin by dixon plot method |
J Med Chem 55: 1171-80 (2012)
Article DOI: 10.1021/jm2011996
BindingDB Entry DOI: 10.7270/Q26T0NN4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532406

(CHEMBL4545331)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NC(=O)OCc1ccccc1 |r,wU:49.47,wD:22.18,(28.26,-22.28,;29.05,-23.61,;28.3,-24.96,;30.6,-23.57,;32.01,-23.55,;31.64,-24.81,;31.6,-22.31,;22.31,-21.79,;23.1,-23.12,;22.35,-24.46,;24.65,-23.08,;26.06,-23.06,;25.69,-24.32,;25.65,-21.82,;23.44,-28.1,;24.23,-29.42,;23.48,-30.77,;25.78,-29.39,;27.19,-29.37,;26.82,-30.63,;26.78,-28.12,;8.28,-19.98,;8.23,-21.49,;8.19,-23.01,;7.2,-24.17,;5.7,-23.88,;4.72,-25.03,;5.22,-26.46,;4.48,-27.94,;5.22,-29.3,;4.41,-30.61,;6.76,-29.34,;7.5,-30.69,;9.04,-30.74,;9.83,-29.44,;11.35,-29.48,;12.07,-30.81,;11.27,-32.11,;9.76,-32.06,;13.61,-30.86,;14.42,-29.55,;15.97,-29.59,;16.7,-30.95,;16.73,-28.25,;14.94,-26.77,;15.3,-25.27,;14.19,-24.2,;12.71,-24.63,;12.16,-22.78,;12.2,-20.85,;12.25,-19.33,;10.87,-21.57,;9.57,-20.77,;9.62,-19.25,;13.49,-21.65,;13.44,-23.17,;14.83,-20.93,;16.12,-21.72,;17.46,-21.01,;18.75,-21.81,;20.09,-21.09,;20.13,-19.57,;18.83,-18.78,;17.5,-19.5,;21.47,-18.86,;22.76,-19.65,;21.51,-17.33,;12.33,-26.12,;13.44,-27.2,;6.73,-26.74,;7.7,-25.59,;6.94,-20.7,;5.58,-21.43,;5.54,-22.97,;4.27,-20.62,;4.31,-19.08,;2.99,-18.27,;3.03,-16.73,;1.72,-15.92,;.36,-16.66,;.32,-18.21,;1.64,-19.01,)| Show InChI InChI=1S/C44H51N9O6.3C2HF3O2/c45-41(46)34-12-6-32(7-13-34)28-47-42(56)37-26-30-8-14-35(15-9-30)48-39(54)18-20-52-22-24-53(25-23-52)21-19-40(55)49-36-16-10-31(11-17-36)27-38(43(57)50-37)51-44(58)59-29-33-4-2-1-3-5-33;3*3-2(4,5)1(6)7/h1-17,37-38H,18-29H2,(H3,45,46)(H,47,56)(H,48,54)(H,49,55)(H,50,57)(H,51,58);3*(H,6,7)/t37-,38+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532406

(CHEMBL4545331)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@]([H])(NC1=O)C(=O)NCc1ccc(cc1)C(N)=N)cc3)cc2)NC(=O)OCc1ccccc1 |r,wU:49.47,wD:22.18,(28.26,-22.28,;29.05,-23.61,;28.3,-24.96,;30.6,-23.57,;32.01,-23.55,;31.64,-24.81,;31.6,-22.31,;22.31,-21.79,;23.1,-23.12,;22.35,-24.46,;24.65,-23.08,;26.06,-23.06,;25.69,-24.32,;25.65,-21.82,;23.44,-28.1,;24.23,-29.42,;23.48,-30.77,;25.78,-29.39,;27.19,-29.37,;26.82,-30.63,;26.78,-28.12,;8.28,-19.98,;8.23,-21.49,;8.19,-23.01,;7.2,-24.17,;5.7,-23.88,;4.72,-25.03,;5.22,-26.46,;4.48,-27.94,;5.22,-29.3,;4.41,-30.61,;6.76,-29.34,;7.5,-30.69,;9.04,-30.74,;9.83,-29.44,;11.35,-29.48,;12.07,-30.81,;11.27,-32.11,;9.76,-32.06,;13.61,-30.86,;14.42,-29.55,;15.97,-29.59,;16.7,-30.95,;16.73,-28.25,;14.94,-26.77,;15.3,-25.27,;14.19,-24.2,;12.71,-24.63,;12.16,-22.78,;12.2,-20.85,;12.25,-19.33,;10.87,-21.57,;9.57,-20.77,;9.62,-19.25,;13.49,-21.65,;13.44,-23.17,;14.83,-20.93,;16.12,-21.72,;17.46,-21.01,;18.75,-21.81,;20.09,-21.09,;20.13,-19.57,;18.83,-18.78,;17.5,-19.5,;21.47,-18.86,;22.76,-19.65,;21.51,-17.33,;12.33,-26.12,;13.44,-27.2,;6.73,-26.74,;7.7,-25.59,;6.94,-20.7,;5.58,-21.43,;5.54,-22.97,;4.27,-20.62,;4.31,-19.08,;2.99,-18.27,;3.03,-16.73,;1.72,-15.92,;.36,-16.66,;.32,-18.21,;1.64,-19.01,)| Show InChI InChI=1S/C44H51N9O6.3C2HF3O2/c45-41(46)34-12-6-32(7-13-34)28-47-42(56)37-26-30-8-14-35(15-9-30)48-39(54)18-20-52-22-24-53(25-23-52)21-19-40(55)49-36-16-10-31(11-17-36)27-38(43(57)50-37)51-44(58)59-29-33-4-2-1-3-5-33;3*3-2(4,5)1(6)7/h1-17,37-38H,18-29H2,(H3,45,46)(H,47,56)(H,48,54)(H,49,55)(H,50,57)(H,51,58);3*(H,6,7)/t37-,38+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532401

(CHEMBL4515955)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@@]([H])(NC(=O)OC)C(=O)N1)cc3)cc2)C(=O)NCc1ccc(cc1)C(N)=N |r,wU:22.18,wD:49.47,(22.95,-24.3,;23.75,-25.64,;23,-27,;25.32,-25.61,;26.74,-25.59,;26.37,-26.85,;26.33,-24.33,;27.58,-20.32,;28.38,-21.66,;27.63,-23.02,;29.95,-21.62,;31.37,-21.6,;31,-22.87,;30.96,-20.34,;22.33,-19.99,;23.13,-21.33,;22.37,-22.69,;24.7,-21.29,;26.11,-21.27,;25.75,-22.54,;25.7,-20.01,;14.55,-14.2,;14.5,-15.74,;14.46,-17.69,;15.01,-19.55,;16.51,-19.12,;17.64,-20.2,;17.26,-21.71,;19.07,-23.21,;18.3,-24.57,;19.04,-25.94,;16.74,-24.52,;15.93,-25.85,;14.37,-25.8,;13.57,-27.11,;12.04,-27.06,;11.31,-25.72,;12.11,-24.41,;13.65,-24.45,;9.75,-25.68,;9.01,-24.31,;7.45,-24.27,;6.63,-25.6,;6.7,-22.9,;7.46,-21.41,;6.94,-19.96,;7.94,-18.8,;9.45,-19.09,;10.45,-17.92,;10.5,-16.39,;10.54,-14.86,;9.19,-15.58,;7.82,-16.32,;7.78,-17.88,;6.49,-15.51,;6.53,-13.95,;11.85,-15.66,;11.89,-14.13,;13.15,-16.46,;9.96,-20.52,;8.97,-21.69,;15.76,-22.15,;14.63,-21.06,;15.8,-16.54,;15.76,-18.08,;17.15,-15.82,;18.46,-16.62,;19.81,-15.9,;21.11,-16.71,;22.46,-15.98,;22.51,-14.45,;21.2,-13.65,;19.85,-14.38,;23.86,-13.72,;25.17,-14.52,;23.9,-12.18,)| Show InChI InChI=1S/C38H47N9O6.3C2HF3O2/c1-53-38(52)45-32-23-26-6-12-30(13-7-26)43-34(49)15-17-47-20-18-46(19-21-47)16-14-33(48)42-29-10-4-25(5-11-29)22-31(44-37(32)51)36(50)41-24-27-2-8-28(9-3-27)35(39)40;3*3-2(4,5)1(6)7/h2-13,31-32H,14-24H2,1H3,(H3,39,40)(H,41,50)(H,42,48)(H,43,49)(H,44,51)(H,45,52);3*(H,6,7)/t31-,32+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532401

(CHEMBL4515955)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@@]([H])(NC(=O)OC)C(=O)N1)cc3)cc2)C(=O)NCc1ccc(cc1)C(N)=N |r,wU:22.18,wD:49.47,(22.95,-24.3,;23.75,-25.64,;23,-27,;25.32,-25.61,;26.74,-25.59,;26.37,-26.85,;26.33,-24.33,;27.58,-20.32,;28.38,-21.66,;27.63,-23.02,;29.95,-21.62,;31.37,-21.6,;31,-22.87,;30.96,-20.34,;22.33,-19.99,;23.13,-21.33,;22.37,-22.69,;24.7,-21.29,;26.11,-21.27,;25.75,-22.54,;25.7,-20.01,;14.55,-14.2,;14.5,-15.74,;14.46,-17.69,;15.01,-19.55,;16.51,-19.12,;17.64,-20.2,;17.26,-21.71,;19.07,-23.21,;18.3,-24.57,;19.04,-25.94,;16.74,-24.52,;15.93,-25.85,;14.37,-25.8,;13.57,-27.11,;12.04,-27.06,;11.31,-25.72,;12.11,-24.41,;13.65,-24.45,;9.75,-25.68,;9.01,-24.31,;7.45,-24.27,;6.63,-25.6,;6.7,-22.9,;7.46,-21.41,;6.94,-19.96,;7.94,-18.8,;9.45,-19.09,;10.45,-17.92,;10.5,-16.39,;10.54,-14.86,;9.19,-15.58,;7.82,-16.32,;7.78,-17.88,;6.49,-15.51,;6.53,-13.95,;11.85,-15.66,;11.89,-14.13,;13.15,-16.46,;9.96,-20.52,;8.97,-21.69,;15.76,-22.15,;14.63,-21.06,;15.8,-16.54,;15.76,-18.08,;17.15,-15.82,;18.46,-16.62,;19.81,-15.9,;21.11,-16.71,;22.46,-15.98,;22.51,-14.45,;21.2,-13.65,;19.85,-14.38,;23.86,-13.72,;25.17,-14.52,;23.9,-12.18,)| Show InChI InChI=1S/C38H47N9O6.3C2HF3O2/c1-53-38(52)45-32-23-26-6-12-30(13-7-26)43-34(49)15-17-47-20-18-46(19-21-47)16-14-33(48)42-29-10-4-25(5-11-29)22-31(44-37(32)51)36(50)41-24-27-2-8-28(9-3-27)35(39)40;3*3-2(4,5)1(6)7/h2-13,31-32H,14-24H2,1H3,(H3,39,40)(H,41,50)(H,42,48)(H,43,49)(H,44,51)(H,45,52);3*(H,6,7)/t31-,32+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of human plasmin using Mes-DSer(Bzl)-Phe-Arg-AMC as substrate by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50380617

(CHEMBL2016873)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2Cc3cn(Cc4cccc(Cn5cc(C[C@@H](NS(=O)(=O)Cc6ccccc6)C(=O)N2)nn5)c4)nn3)cc1 |r| Show InChI InChI=1S/C33H35N11O4S/c34-31(35)26-11-9-22(10-12-26)16-36-32(45)29-14-27-19-43(41-38-27)17-24-7-4-8-25(13-24)18-44-20-28(39-42-44)15-30(33(46)37-29)40-49(47,48)21-23-5-2-1-3-6-23/h1-13,19-20,29-30,40H,14-18,21H2,(H3,34,35)(H,36,45)(H,37,46)/t29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
The Medicines Company (Leipzig) GmbH
US Patent
| Assay Description
The inhibition constants for human plasmin (h plasmin), human plasma kallikrein (h PK), thrombin and factor Xa were determined in analogy to a previo... |
US Patent US8921319 (2014)
BindingDB Entry DOI: 10.7270/Q2RX99S6 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50380619

(CHEMBL2016865)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-c1cccc(-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-[#6]-c2ccccc2)-[#7]S(=O)(=O)[#6]-c2cccc(c2)-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-c2ccc(cc2)-[#6](-[#7])=[#7])c1 |r| Show InChI InChI=1S/C38H44N8O6S/c39-34(40)30-17-15-26(16-18-30)22-43-35(47)33(21-27-10-4-11-28(19-27)23-44-38(41)42)45-36(48)32(14-6-9-25-7-2-1-3-8-25)46-53(51,52)24-29-12-5-13-31(20-29)37(49)50/h1-5,7-8,10-13,15-20,32-33,46H,6,9,14,21-24H2,(H3,39,40)(H,43,47)(H,45,48)(H,49,50)(H4,41,42,44)/t32-,33+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of plasmin by dixon plot method |
J Med Chem 55: 1171-80 (2012)
Article DOI: 10.1021/jm2011996
BindingDB Entry DOI: 10.7270/Q26T0NN4 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532401

(CHEMBL4515955)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@@]([H])(NC(=O)OC)C(=O)N1)cc3)cc2)C(=O)NCc1ccc(cc1)C(N)=N |r,wU:22.18,wD:49.47,(22.95,-24.3,;23.75,-25.64,;23,-27,;25.32,-25.61,;26.74,-25.59,;26.37,-26.85,;26.33,-24.33,;27.58,-20.32,;28.38,-21.66,;27.63,-23.02,;29.95,-21.62,;31.37,-21.6,;31,-22.87,;30.96,-20.34,;22.33,-19.99,;23.13,-21.33,;22.37,-22.69,;24.7,-21.29,;26.11,-21.27,;25.75,-22.54,;25.7,-20.01,;14.55,-14.2,;14.5,-15.74,;14.46,-17.69,;15.01,-19.55,;16.51,-19.12,;17.64,-20.2,;17.26,-21.71,;19.07,-23.21,;18.3,-24.57,;19.04,-25.94,;16.74,-24.52,;15.93,-25.85,;14.37,-25.8,;13.57,-27.11,;12.04,-27.06,;11.31,-25.72,;12.11,-24.41,;13.65,-24.45,;9.75,-25.68,;9.01,-24.31,;7.45,-24.27,;6.63,-25.6,;6.7,-22.9,;7.46,-21.41,;6.94,-19.96,;7.94,-18.8,;9.45,-19.09,;10.45,-17.92,;10.5,-16.39,;10.54,-14.86,;9.19,-15.58,;7.82,-16.32,;7.78,-17.88,;6.49,-15.51,;6.53,-13.95,;11.85,-15.66,;11.89,-14.13,;13.15,-16.46,;9.96,-20.52,;8.97,-21.69,;15.76,-22.15,;14.63,-21.06,;15.8,-16.54,;15.76,-18.08,;17.15,-15.82,;18.46,-16.62,;19.81,-15.9,;21.11,-16.71,;22.46,-15.98,;22.51,-14.45,;21.2,-13.65,;19.85,-14.38,;23.86,-13.72,;25.17,-14.52,;23.9,-12.18,)| Show InChI InChI=1S/C38H47N9O6.3C2HF3O2/c1-53-38(52)45-32-23-26-6-12-30(13-7-26)43-34(49)15-17-47-20-18-46(19-21-47)16-14-33(48)42-29-10-4-25(5-11-29)22-31(44-37(32)51)36(50)41-24-27-2-8-28(9-3-27)35(39)40;3*3-2(4,5)1(6)7/h2-13,31-32H,14-24H2,1H3,(H3,39,40)(H,41,50)(H,42,48)(H,43,49)(H,44,51)(H,45,52);3*(H,6,7)/t31-,32+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Binding affinity to human plasmin assessed as slow binding constant in presence of Mes-DArg-Phe-Arg-AMC after 20 mins by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50532401

(CHEMBL4515955)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.[H][C@]1(Cc2ccc(NC(=O)CCN3CCN(CC3)CCC(=O)Nc3ccc(C[C@@]([H])(NC(=O)OC)C(=O)N1)cc3)cc2)C(=O)NCc1ccc(cc1)C(N)=N |r,wU:22.18,wD:49.47,(22.95,-24.3,;23.75,-25.64,;23,-27,;25.32,-25.61,;26.74,-25.59,;26.37,-26.85,;26.33,-24.33,;27.58,-20.32,;28.38,-21.66,;27.63,-23.02,;29.95,-21.62,;31.37,-21.6,;31,-22.87,;30.96,-20.34,;22.33,-19.99,;23.13,-21.33,;22.37,-22.69,;24.7,-21.29,;26.11,-21.27,;25.75,-22.54,;25.7,-20.01,;14.55,-14.2,;14.5,-15.74,;14.46,-17.69,;15.01,-19.55,;16.51,-19.12,;17.64,-20.2,;17.26,-21.71,;19.07,-23.21,;18.3,-24.57,;19.04,-25.94,;16.74,-24.52,;15.93,-25.85,;14.37,-25.8,;13.57,-27.11,;12.04,-27.06,;11.31,-25.72,;12.11,-24.41,;13.65,-24.45,;9.75,-25.68,;9.01,-24.31,;7.45,-24.27,;6.63,-25.6,;6.7,-22.9,;7.46,-21.41,;6.94,-19.96,;7.94,-18.8,;9.45,-19.09,;10.45,-17.92,;10.5,-16.39,;10.54,-14.86,;9.19,-15.58,;7.82,-16.32,;7.78,-17.88,;6.49,-15.51,;6.53,-13.95,;11.85,-15.66,;11.89,-14.13,;13.15,-16.46,;9.96,-20.52,;8.97,-21.69,;15.76,-22.15,;14.63,-21.06,;15.8,-16.54,;15.76,-18.08,;17.15,-15.82,;18.46,-16.62,;19.81,-15.9,;21.11,-16.71,;22.46,-15.98,;22.51,-14.45,;21.2,-13.65,;19.85,-14.38,;23.86,-13.72,;25.17,-14.52,;23.9,-12.18,)| Show InChI InChI=1S/C38H47N9O6.3C2HF3O2/c1-53-38(52)45-32-23-26-6-12-30(13-7-26)43-34(49)15-17-47-20-18-46(19-21-47)16-14-33(48)42-29-10-4-25(5-11-29)22-31(44-37(32)51)36(50)41-24-27-2-8-28(9-3-27)35(39)40;3*3-2(4,5)1(6)7/h2-13,31-32H,14-24H2,1H3,(H3,39,40)(H,41,50)(H,42,48)(H,43,49)(H,44,51)(H,45,52);3*(H,6,7)/t31-,32+;;;/m0.../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Binding affinity to human plasmin assessed as slow binding constant in presence of Mes-DArg-Phe-Arg-AMC after 20 mins by Dixon plot analysis |
J Med Chem 59: 6370-86 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00606
BindingDB Entry DOI: 10.7270/Q2377D5T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50349358
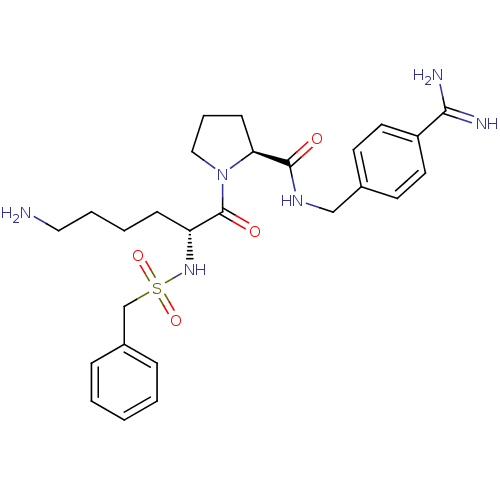
(CHEMBL1809216)Show SMILES NCCCC[C@@H](NS(=O)(=O)Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1ccc(cc1)C(N)=N |r| Show InChI InChI=1S/C26H36N6O4S/c27-15-5-4-9-22(31-37(35,36)18-20-7-2-1-3-8-20)26(34)32-16-6-10-23(32)25(33)30-17-19-11-13-21(14-12-19)24(28)29/h1-3,7-8,11-14,22-23,31H,4-6,9-10,15-18,27H2,(H3,28,29)(H,30,33)/t22-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps University Marburg
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 21: 4860-4 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.033
BindingDB Entry DOI: 10.7270/Q2ST7Q5M |
More data for this
Ligand-Target Pair | |
Suppressor of tumorigenicity 14 protein
(Homo sapiens (Human)) | BDBM104908

(CHEMBL468270 | US8569313, Inhibitor 16)Show SMILES NCCCCc1cccc(c1)S(=O)(=O)N[C@@H](Cc1cccc(c1)C(N)=N)C(=O)N1CCC(CCN)CC1 |r| Show InChI InChI=1S/C27H40N6O3S/c28-13-2-1-5-21-6-4-9-24(18-21)37(35,36)32-25(19-22-7-3-8-23(17-22)26(30)31)27(34)33-15-11-20(10-14-29)12-16-33/h3-4,6-9,17-18,20,25,32H,1-2,5,10-16,19,28-29H2,(H3,30,31)/t25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Curacyte Discovery GmbH
Curated by ChEMBL
| Assay Description
Inhibition of matriptase (unknown origin) |
Bioorg Med Chem Lett 19: 67-73 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.019
BindingDB Entry DOI: 10.7270/Q2HH6JXF |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50380623
(CHEMBL2016870)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2Cc3cccc(CNC(=O)Cc4cccc(CC(=O)NCc5cccc(C[C@@H](NS(=O)(=O)Cc6ccccc6)C(=O)N2)c5)c4)c3)cc1 |r| Show InChI InChI=1S/C45H47N7O6S/c46-43(47)38-17-15-30(16-18-38)26-50-44(55)39-22-32-9-5-13-36(20-32)27-48-41(53)24-34-11-4-12-35(19-34)25-42(54)49-28-37-14-6-10-33(21-37)23-40(45(56)51-39)52-59(57,58)29-31-7-2-1-3-8-31/h1-21,39-40,52H,22-29H2,(H3,46,47)(H,48,53)(H,49,54)(H,50,55)(H,51,56)/t39-,40+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
The Medicines Company (Leipzig) GmbH
US Patent
| Assay Description
The inhibition constants for human plasmin (h plasmin), human plasma kallikrein (h PK), thrombin and factor Xa were determined in analogy to a previo... |
US Patent US8921319 (2014)
BindingDB Entry DOI: 10.7270/Q2RX99S6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data