

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029464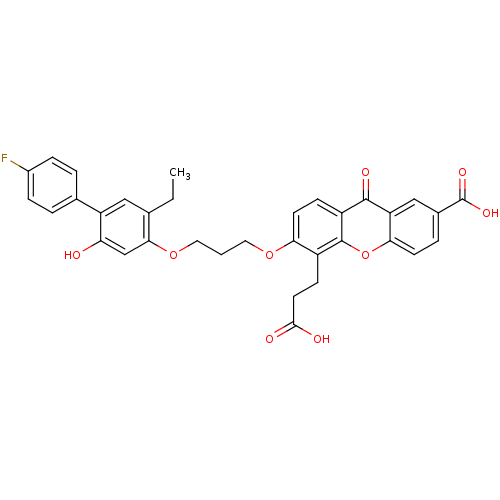 (5-(2-Carboxy-ethyl)-6-[3-(5-ethyl-4'-fluoro-2-hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description LTB4 receptor antagonist activity was determined by inhibition of specific binding of [3H]-LTB4 in guinea pig lung membranes | Bioorg Med Chem Lett 4: 2077-2082 (1994) Article DOI: 10.1016/S0960-894X(01)80105-0 BindingDB Entry DOI: 10.7270/Q27D2V2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029464 (5-(2-Carboxy-ethyl)-6-[3-(5-ethyl-4'-fluoro-2-hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]LTB4 to guinea pig lung membranes expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50283055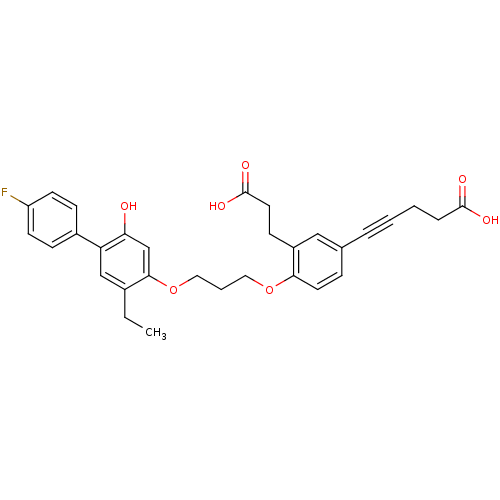 (5-{3-(2-Carboxy-ethyl)-4-[3-(5-ethyl-4'-fluoro-2-h...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description LTB4 receptor antagonist activity was determined by inhibition of specific binding of [3H]-LTB4 in human neutrophil | Bioorg Med Chem Lett 4: 2077-2082 (1994) Article DOI: 10.1016/S0960-894X(01)80105-0 BindingDB Entry DOI: 10.7270/Q27D2V2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50283055 (5-{3-(2-Carboxy-ethyl)-4-[3-(5-ethyl-4'-fluoro-2-h...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description LTB4 receptor antagonist activity was determined by inhibition of specific binding of [3H]-LTB4 in guinea pig lung membranes | Bioorg Med Chem Lett 4: 2077-2082 (1994) Article DOI: 10.1016/S0960-894X(01)80105-0 BindingDB Entry DOI: 10.7270/Q27D2V2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029477 (5-(2-Carboxy-ethyl)-6-[3-(5-ethyl-2-hydroxy-biphen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]-LTB4 to guinea pig lung membranes expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50013889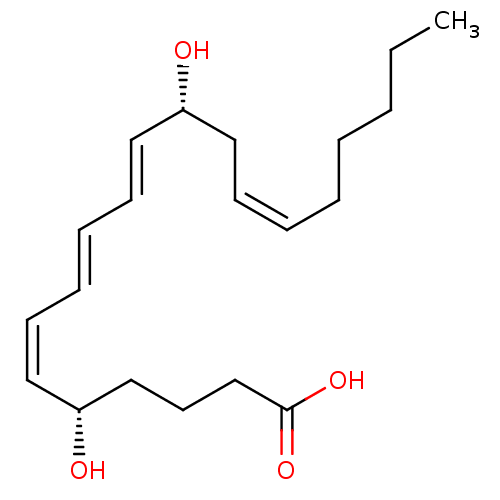 ((5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxyicosa-6,8,10,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]LTB4 to guinea pig lung membranes expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50013889 ((5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxyicosa-6,8,10,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description LTB4 receptor antagonist activity was determined by inhibition of specific binding of [3H]-LTB4 in guinea pig lung membranes | Bioorg Med Chem Lett 4: 2077-2082 (1994) Article DOI: 10.1016/S0960-894X(01)80105-0 BindingDB Entry DOI: 10.7270/Q27D2V2X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50013889 ((5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxyicosa-6,8,10,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of binding of [3H]LTB4 to LTB4 receptor in guinea-pig lung membranes | Bioorg Med Chem Lett 3: 1981-1984 (1993) Article DOI: 10.1016/S0960-894X(01)80999-9 BindingDB Entry DOI: 10.7270/Q2VH5P9R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50283054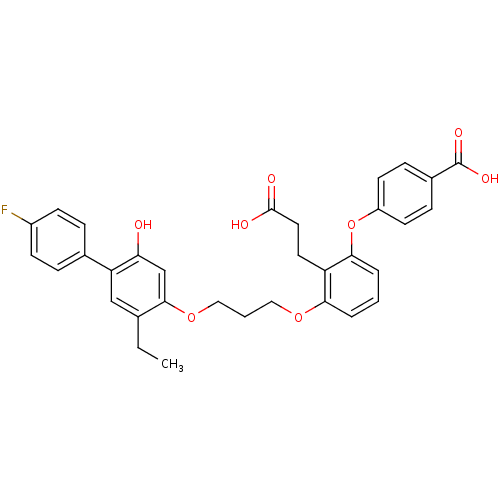 (4-{2-(2-Carboxy-ethyl)-3-[3-(5-ethyl-4'-fluoro-2-h...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description LTB4 receptor antagonist activity was determined by inhibition of specific binding of [3H]-LTB4 in human neutrophil | Bioorg Med Chem Lett 4: 2077-2082 (1994) Article DOI: 10.1016/S0960-894X(01)80105-0 BindingDB Entry DOI: 10.7270/Q27D2V2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029464 (5-(2-Carboxy-ethyl)-6-[3-(5-ethyl-4'-fluoro-2-hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description LTB4 receptor antagonist activity was determined by inhibition of specific binding of [3H]-LTB4 in human neutrophil | Bioorg Med Chem Lett 4: 2077-2082 (1994) Article DOI: 10.1016/S0960-894X(01)80105-0 BindingDB Entry DOI: 10.7270/Q27D2V2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029464 (5-(2-Carboxy-ethyl)-6-[3-(5-ethyl-4'-fluoro-2-hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]-LTB4 to human neutrophil expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029477 (5-(2-Carboxy-ethyl)-6-[3-(5-ethyl-2-hydroxy-biphen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]-LTB4 to human neutrophil expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50283054 (4-{2-(2-Carboxy-ethyl)-3-[3-(5-ethyl-4'-fluoro-2-h...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description LTB4 receptor antagonist activity was determined by inhibition of specific binding of [3H]-LTB4 in human neutrophil | Bioorg Med Chem Lett 4: 2077-2082 (1994) Article DOI: 10.1016/S0960-894X(01)80105-0 BindingDB Entry DOI: 10.7270/Q27D2V2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029482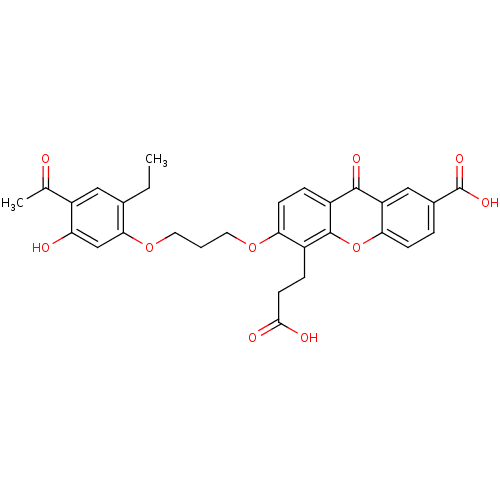 (6-[3-(4-Acetyl-2-ethyl-5-hydroxy-phenoxy)-propoxy]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]-LTB4 to guinea pig lung membranes expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029482 (6-[3-(4-Acetyl-2-ethyl-5-hydroxy-phenoxy)-propoxy]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]-LTB4 to guinea pig lung membranes expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50280884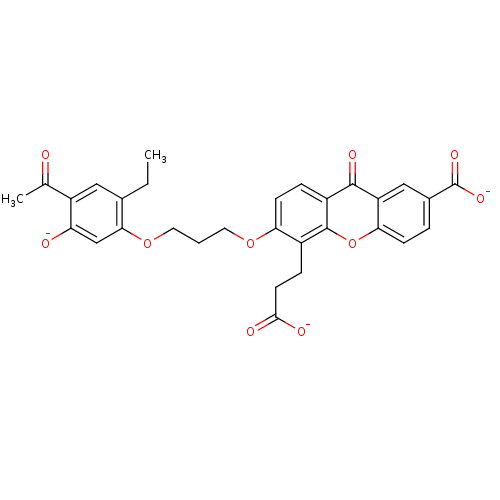 (3-{3-[3-(4-acetyl-2-ethyl-5-olatophenoxy)propoxy]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of binding of [3H]LTB4 to LTB4 receptor in guinea-pig lung membranes | Bioorg Med Chem Lett 3: 1981-1984 (1993) Article DOI: 10.1016/S0960-894X(01)80999-9 BindingDB Entry DOI: 10.7270/Q2VH5P9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50013889 ((5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxyicosa-6,8,10,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description LTB4 receptor antagonist activity was determined by inhibition of specific binding of [3H]-LTB4 in guinea pig lung membranes | Bioorg Med Chem Lett 4: 2077-2082 (1994) Article DOI: 10.1016/S0960-894X(01)80105-0 BindingDB Entry DOI: 10.7270/Q27D2V2X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50013889 ((5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxyicosa-6,8,10,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]-LTB4 to human neutrophil expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM60917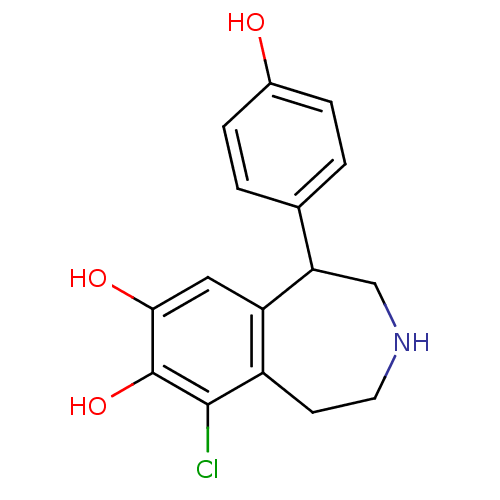 (9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50045092 (5-(2-Carboxy-ethyl)-6-[(E)-6-(4-methoxy-phenyl)-he...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition constant against binding of [3H]-LTB4 to human neutrophils | J Med Chem 36: 1726-34 (1993) BindingDB Entry DOI: 10.7270/Q2JQ1023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029462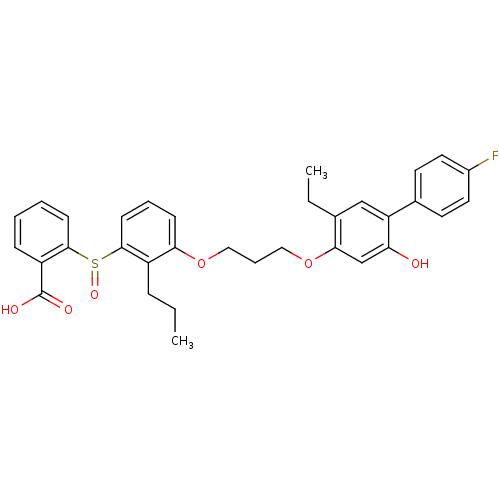 (2-{3-[3-(5-Ethyl-4'-fluoro-2-hydroxy-biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards LTB4 receptor guinea pig spleen cells using [3H]-LTB4 as radioligand | J Med Chem 38: 4411-32 (1995) BindingDB Entry DOI: 10.7270/Q20V8BSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50280886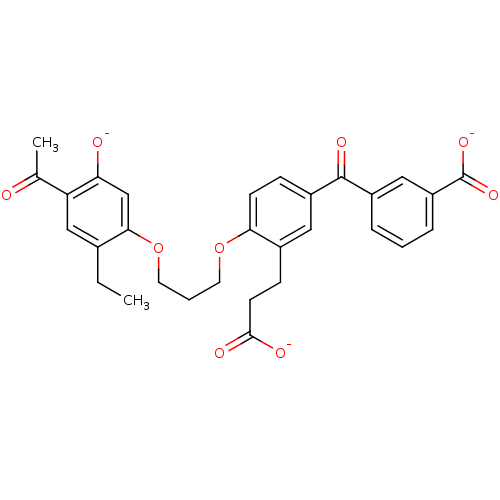 (3-[2-[3-(4-acetyl-2-ethyl-5-olatophenoxy)propoxy]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of binding of [3H]LTB4 to LTB4 receptor in guinea-pig lung membranes | Bioorg Med Chem Lett 3: 1981-1984 (1993) Article DOI: 10.1016/S0960-894X(01)80999-9 BindingDB Entry DOI: 10.7270/Q2VH5P9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001611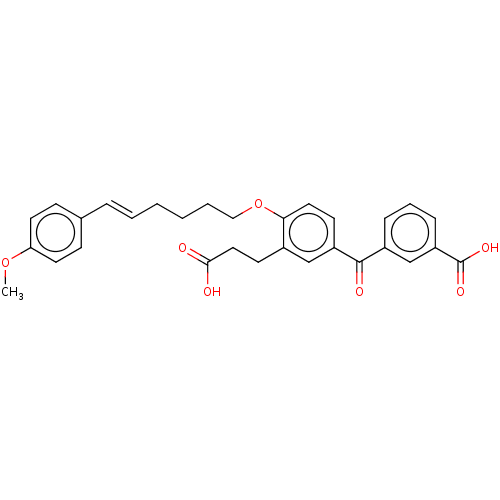 ((E)-3-{3-(2-Carboxy-ethyl)-4-[6-(4-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of binding of [3H]LTB4 to LTB4 receptor in guinea-pig lung membranes | Bioorg Med Chem Lett 3: 1981-1984 (1993) Article DOI: 10.1016/S0960-894X(01)80999-9 BindingDB Entry DOI: 10.7270/Q2VH5P9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029448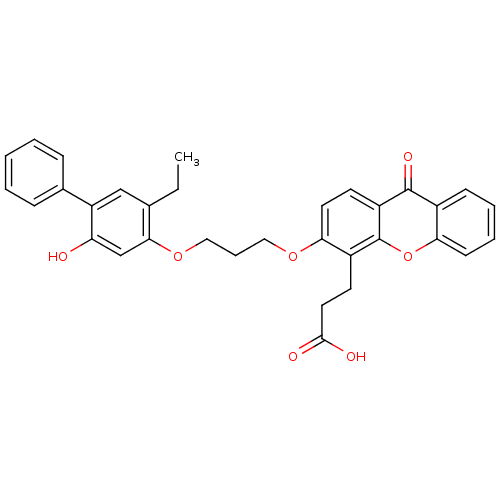 (3-{3-[3-(5-Ethyl-2-hydroxy-biphenyl-4-yloxy)-propo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]-LTB4 to guinea pig lung membranes expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50283056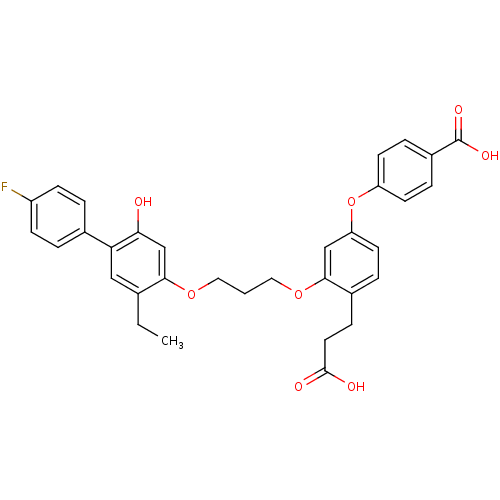 (4-{4-(2-Carboxy-ethyl)-3-[3-(5-ethyl-4'-fluoro-2-h...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description LTB4 receptor antagonist activity was determined by inhibition of specific binding of [3H]-LTB4 in guinea pig lung membranes | Bioorg Med Chem Lett 4: 2077-2082 (1994) Article DOI: 10.1016/S0960-894X(01)80105-0 BindingDB Entry DOI: 10.7270/Q27D2V2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50283056 (4-{4-(2-Carboxy-ethyl)-3-[3-(5-ethyl-4'-fluoro-2-h...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description LTB4 receptor antagonist activity was determined by inhibition of specific binding of [3H]-LTB4 in human neutrophil | Bioorg Med Chem Lett 4: 2077-2082 (1994) Article DOI: 10.1016/S0960-894X(01)80105-0 BindingDB Entry DOI: 10.7270/Q27D2V2X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029448 (3-{3-[3-(5-Ethyl-2-hydroxy-biphenyl-4-yloxy)-propo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]-LTB4 to guinea pig lung membranes expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029452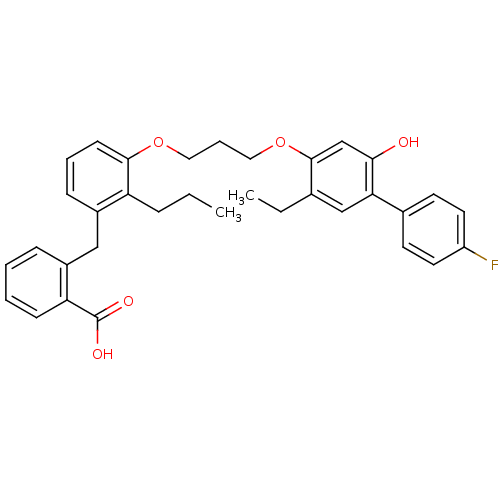 (2-{3-[3-(5-Ethyl-4'-fluoro-2-hydroxy-biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards LTB4 receptor guinea pig spleen cells using [3H]-LTB4 as radioligand | J Med Chem 38: 4411-32 (1995) BindingDB Entry DOI: 10.7270/Q20V8BSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029468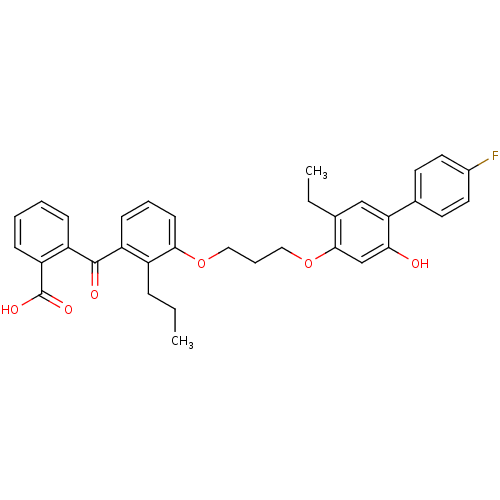 (2-{3-[3-(5-Ethyl-4'-fluoro-2-hydroxy-biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards LTB4 receptor guinea pig spleen cells using [3H]-LTB4 as radioligand | J Med Chem 38: 4411-32 (1995) BindingDB Entry DOI: 10.7270/Q20V8BSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029460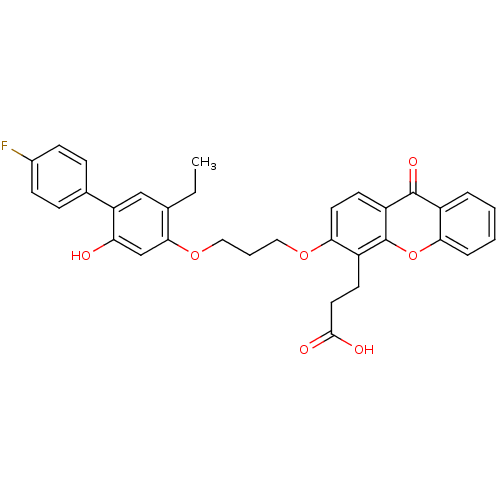 (3-{3-[3-(5-Ethyl-4'-fluoro-2-hydroxy-biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]-LTB4 to human neutrophil expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029460 (3-{3-[3-(5-Ethyl-4'-fluoro-2-hydroxy-biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]-LTB4 to guinea pig lung membranes expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029467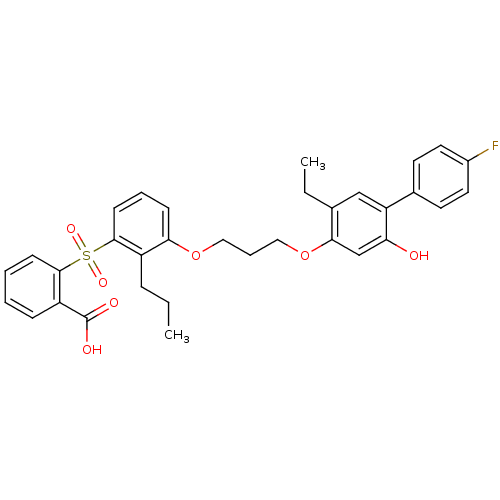 (2-{3-[3-(5-Ethyl-4'-fluoro-2-hydroxy-biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards LTB4 receptor guinea pig spleen cells using [3H]-LTB4 as radioligand | J Med Chem 38: 4411-32 (1995) BindingDB Entry DOI: 10.7270/Q20V8BSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029484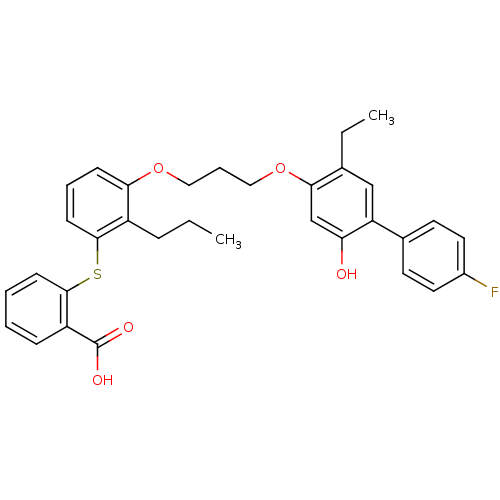 (2-{3-[3-(5-Ethyl-4'-fluoro-2-hydroxy-biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards LTB4 receptor guinea pig spleen cells using [3H]-LTB4 as radioligand | J Med Chem 38: 4411-32 (1995) BindingDB Entry DOI: 10.7270/Q20V8BSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025202 (9-Aminomethyl-9H-fluorene-2,5,6-triol | CHEMBL5767...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50045092 (5-(2-Carboxy-ethyl)-6-[(E)-6-(4-methoxy-phenyl)-he...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antagonistic activity towards LTB4 receptor was evaluated by inhibition of binding of [3H]LTB4 to human neutrophils | Bioorg Med Chem Lett 3: 1981-1984 (1993) Article DOI: 10.1016/S0960-894X(01)80999-9 BindingDB Entry DOI: 10.7270/Q2VH5P9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50001955 ((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014968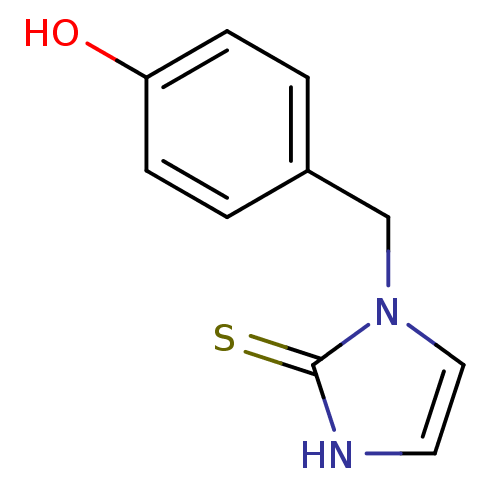 (1-(4-Hydroxy-benzyl)-1,3-dihydro-imidazole-2-thion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | 4.5 | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Dopamine beta hydroxylase at pH 4.5 | J Med Chem 29: 2465-72 (1987) BindingDB Entry DOI: 10.7270/Q2BR8R5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025206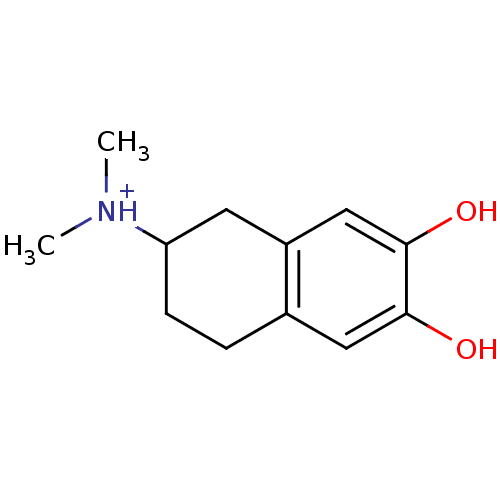 ((6,7-Dihydroxy-1,2,3,4-tetrahydro-naphthalen-2-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM81519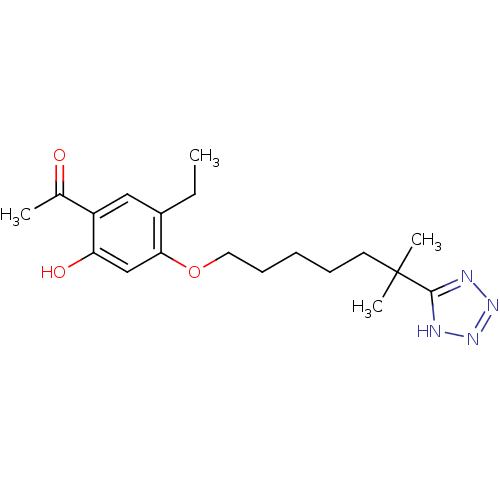 (CAS_117690-79-6 | CGS 23356 | CHEMBL15766 | LY 255...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of binding of [3H]LTB4 to LTB4 receptor in guinea-pig lung membranes | Bioorg Med Chem Lett 3: 1981-1984 (1993) Article DOI: 10.1016/S0960-894X(01)80999-9 BindingDB Entry DOI: 10.7270/Q2VH5P9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50000439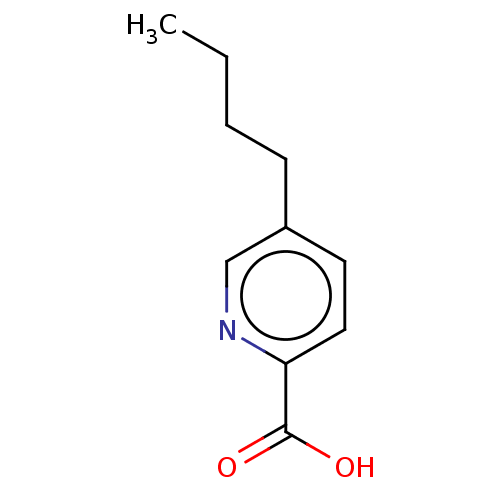 (5-Butyl-pyridine-2-carboxylic acid | 5-Butyl-pyrid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents | PubMed | 149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Dopamine beta hydroxylase at pH 4.5 | J Med Chem 29: 2465-72 (1987) BindingDB Entry DOI: 10.7270/Q2BR8R5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50001955 ((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank PC cid PC sid PDB UniChem Patents Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50025206 ((6,7-Dihydroxy-1,2,3,4-tetrahydro-naphthalen-2-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 255 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dopamine beta-hydroxylase (Bos taurus) | BDBM50014968 (1-(4-Hydroxy-benzyl)-1,3-dihydro-imidazole-2-thion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 344 | n/a | n/a | n/a | n/a | n/a | n/a | 6.6 | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of Dopamine beta hydroxylase at pH 6.6 | J Med Chem 29: 2465-72 (1987) BindingDB Entry DOI: 10.7270/Q2BR8R5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025201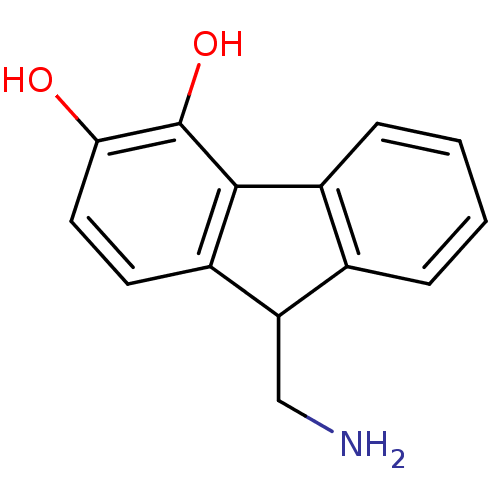 (9-Aminomethyl-9H-fluorene-3,4-diol | CHEMBL55693) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025208 (6-Chloro-9-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50280885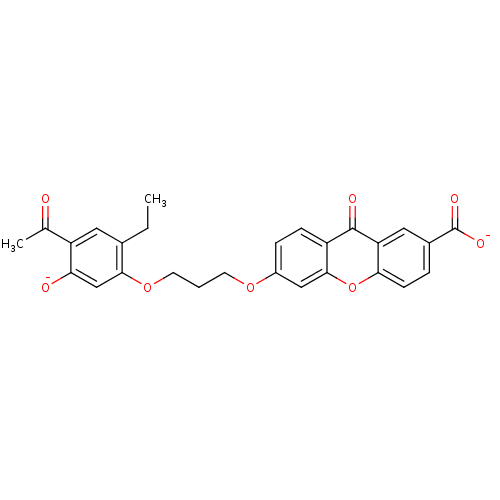 (CHEMBL58534 | disodium 6-[3-(4-acetyl-2-ethyl-5-ol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 751 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of binding of [3H]LTB4 to LTB4 receptor in guinea-pig lung membranes | Bioorg Med Chem Lett 3: 1981-1984 (1993) Article DOI: 10.1016/S0960-894X(01)80999-9 BindingDB Entry DOI: 10.7270/Q2VH5P9R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM60917 (9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50004821 (2,3,4,5-Tetrahydro-1H-benzo[d]azepine-7,8-diol | C...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for binding affinity against dDopamine receptor D1 using [3H]fenoldopam as a radioligand | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50025204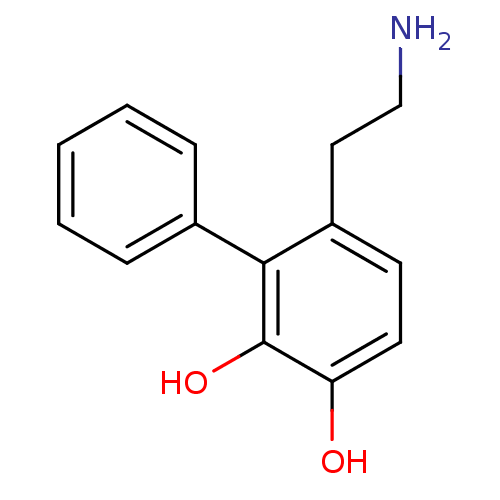 (6-(2-Amino-ethyl)-biphenyl-2,3-diol | CHEMBL299511) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes | J Med Chem 29: 1904-12 (1986) BindingDB Entry DOI: 10.7270/Q2TQ623R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 523 total ) | Next | Last >> |