 Found 539 hits with Last Name = 'scheufler' and Initial = 'c'
Found 539 hits with Last Name = 'scheufler' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50235302

(CHEMBL4099771)Show SMILES CN(CCCNC(=O)CNC(=O)Nc1ccc2sc(Cl)c(-c3cccnc3C)c2c1)[C@@H]1CCCN(C1)c1ncnc2[nH]ccc12 |r,wU:30.32,(40.26,-16.4,;40.26,-17.94,;41.59,-18.71,;42.93,-17.94,;44.26,-18.71,;45.59,-17.94,;46.93,-18.71,;46.93,-20.25,;48.26,-17.94,;49.59,-18.71,;50.93,-17.94,;50.93,-16.4,;52.26,-18.71,;53.6,-17.95,;53.59,-16.41,;54.92,-15.64,;56.26,-16.41,;57.73,-15.93,;58.64,-17.18,;60.18,-17.18,;57.73,-18.43,;58.21,-19.89,;57.17,-21.03,;57.65,-22.5,;59.16,-22.82,;60.19,-21.66,;59.71,-20.2,;60.74,-19.06,;56.26,-17.95,;54.93,-18.72,;38.93,-18.71,;37.59,-17.93,;36.26,-18.71,;36.26,-20.25,;37.59,-21.01,;38.93,-20.24,;37.59,-22.55,;38.93,-23.31,;38.93,-24.86,;37.6,-25.63,;36.26,-24.86,;34.8,-25.34,;33.89,-24.1,;34.79,-22.85,;36.26,-23.32,)| Show InChI InChI=1S/C32H36ClN9O2S/c1-20-23(7-3-11-34-20)28-25-16-21(8-9-26(25)45-29(28)33)40-32(44)37-17-27(43)35-12-5-14-41(2)22-6-4-15-42(18-22)31-24-10-13-36-30(24)38-19-39-31/h3,7-11,13,16,19,22H,4-6,12,14-15,17-18H2,1-2H3,(H,35,43)(H,36,38,39)(H2,37,40,44)/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Competitive inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by... |
ACS Med Chem Lett 8: 338-343 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00519
BindingDB Entry DOI: 10.7270/Q2WW7KZW |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50075098

(CHEMBL3414626 | US10143704, Compound A2 | US944606...)Show SMILES CC(C)N(C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@@H]1C[C@H](CCc2nc3cc(ccc3[nH]2)C(C)(C)C)C1 |r,wU:24.27,22.24,10.11,8.8,wD:5.4,7.12,(-.55,4.43,;-1.02,5.57,;-2.24,5.73,;-.08,6.8,;1.45,6.59,;2.04,5.17,;1.23,3.86,;2.24,2.7,;3.65,3.27,;4.7,2.61,;3.54,4.8,;4.48,5.6,;1.76,1.24,;2.66,.02,;1.76,-1.24,;.3,-.77,;-1.03,-1.56,;-1.03,-2.79,;-2.38,-.77,;-2.38,.77,;-1.03,1.56,;.3,.77,;-.67,8.22,;-.05,9.59,;-1.48,10.19,;-2.06,11.61,;-1.11,12.83,;-1.7,14.26,;-.89,15.53,;-1.87,16.71,;-1.63,18.24,;-2.86,19.2,;-4.29,18.63,;-4.52,17.09,;-3.29,16.14,;-3.19,14.61,;-2.65,20.73,;-1.51,21.19,;-3.62,21.48,;-2.48,21.95,;-2.07,8.77,)| Show InChI InChI=1S/C30H42N8O3/c1-16(2)37(13-22-25(39)26(40)29(41-22)38-15-34-24-27(31)32-14-33-28(24)38)19-10-17(11-19)6-9-23-35-20-8-7-18(30(3,4)5)12-21(20)36-23/h7-8,12,14-17,19,22,25-26,29,39-40H,6,9-11,13H2,1-5H3,(H,35,36)(H2,31,32,33)/t17-,19+,22-,25-,26-,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Competitive inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by... |
ACS Med Chem Lett 8: 338-343 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00519
BindingDB Entry DOI: 10.7270/Q2WW7KZW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50536826

(CHEMBL4590355)Show SMILES CNc1ccnc(Nc2ccc3cc(C)n(-c4ccccc4Oc4cnc5n(C)cnc5c4)c3c2)n1 |(29.26,-10.25,;27.93,-11.02,;27.94,-12.56,;29.28,-13.33,;29.28,-14.87,;27.95,-15.64,;26.62,-14.88,;25.29,-15.64,;23.96,-14.88,;23.95,-13.33,;22.62,-12.56,;21.29,-13.34,;19.82,-12.85,;18.9,-14.1,;17.36,-14.08,;19.8,-15.36,;19.31,-16.81,;20.33,-17.95,;19.85,-19.4,;18.34,-19.72,;17.32,-18.56,;17.81,-17.11,;16.79,-15.96,;15.29,-16.26,;14.81,-17.71,;13.31,-18.01,;12.29,-16.86,;10.74,-16.84,;9.83,-18.07,;10.28,-15.37,;11.54,-14.47,;12.78,-15.39,;14.28,-15.1,;21.28,-14.89,;22.62,-15.65,;26.61,-13.34,)| Show InChI InChI=1S/C27H24N8O/c1-17-12-18-8-9-19(32-27-29-11-10-25(28-2)33-27)13-23(18)35(17)22-6-4-5-7-24(22)36-20-14-21-26(30-15-20)34(3)16-31-21/h4-16H,1-3H3,(H2,28,29,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... |
ACS Med Chem Lett 7: 735-40 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00167
BindingDB Entry DOI: 10.7270/Q2V69P3G |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50536819
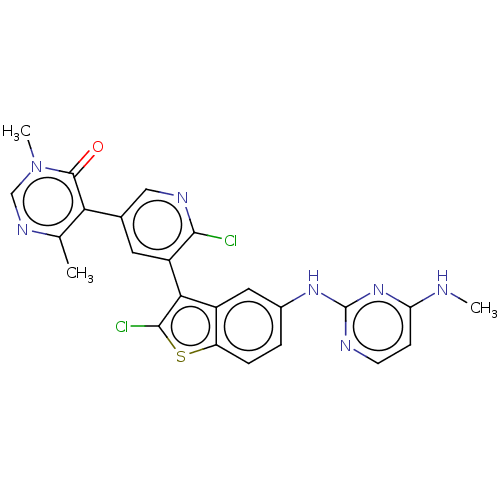
(CHEMBL4534250)Show SMILES CNc1ccnc(Nc2ccc3sc(Cl)c(-c4cc(cnc4Cl)-c4c(C)ncn(C)c4=O)c3c2)n1 |(34.66,-43.04,;33.33,-43.82,;33.34,-45.35,;34.68,-46.12,;34.68,-47.66,;33.35,-48.43,;32.02,-47.66,;30.69,-48.43,;29.36,-47.67,;29.35,-46.12,;28.02,-45.36,;26.69,-46.13,;25.23,-45.65,;24.32,-46.89,;22.78,-46.88,;25.22,-48.14,;24.74,-49.6,;23.23,-49.91,;22.75,-51.37,;23.77,-52.52,;25.29,-52.2,;25.76,-50.74,;27.27,-50.42,;21.24,-51.68,;20.76,-53.14,;21.78,-54.29,;19.25,-53.46,;18.22,-52.31,;18.71,-50.84,;17.68,-49.69,;20.22,-50.53,;20.7,-49.07,;26.69,-47.67,;28.02,-48.44,;32.01,-46.13,)| Show InChI InChI=1S/C24H19Cl2N7OS/c1-12-19(23(34)33(3)11-30-12)13-8-16(21(25)29-10-13)20-15-9-14(4-5-17(15)35-22(20)26)31-24-28-7-6-18(27-2)32-24/h4-11H,1-3H3,(H2,27,28,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... |
ACS Med Chem Lett 7: 735-40 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00167
BindingDB Entry DOI: 10.7270/Q2V69P3G |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50075098

(CHEMBL3414626 | US10143704, Compound A2 | US944606...)Show SMILES CC(C)N(C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)[C@@H]1C[C@H](CCc2nc3cc(ccc3[nH]2)C(C)(C)C)C1 |r,wU:24.27,22.24,10.11,8.8,wD:5.4,7.12,(-.55,4.43,;-1.02,5.57,;-2.24,5.73,;-.08,6.8,;1.45,6.59,;2.04,5.17,;1.23,3.86,;2.24,2.7,;3.65,3.27,;4.7,2.61,;3.54,4.8,;4.48,5.6,;1.76,1.24,;2.66,.02,;1.76,-1.24,;.3,-.77,;-1.03,-1.56,;-1.03,-2.79,;-2.38,-.77,;-2.38,.77,;-1.03,1.56,;.3,.77,;-.67,8.22,;-.05,9.59,;-1.48,10.19,;-2.06,11.61,;-1.11,12.83,;-1.7,14.26,;-.89,15.53,;-1.87,16.71,;-1.63,18.24,;-2.86,19.2,;-4.29,18.63,;-4.52,17.09,;-3.29,16.14,;-3.19,14.61,;-2.65,20.73,;-1.51,21.19,;-3.62,21.48,;-2.48,21.95,;-2.07,8.77,)| Show InChI InChI=1S/C30H42N8O3/c1-16(2)37(13-22-25(39)26(40)29(41-22)38-15-34-24-27(31)32-14-33-28(24)38)19-10-17(11-19)6-9-23-35-20-8-7-18(30(3,4)5)12-21(20)36-23/h7-8,12,14-17,19,22,25-26,29,39-40H,6,9-11,13H2,1-5H3,(H,35,36)(H2,31,32,33)/t17-,19+,22-,25-,26-,29-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... |
ACS Med Chem Lett 8: 338-343 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00519
BindingDB Entry DOI: 10.7270/Q2WW7KZW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50235302

(CHEMBL4099771)Show SMILES CN(CCCNC(=O)CNC(=O)Nc1ccc2sc(Cl)c(-c3cccnc3C)c2c1)[C@@H]1CCCN(C1)c1ncnc2[nH]ccc12 |r,wU:30.32,(40.26,-16.4,;40.26,-17.94,;41.59,-18.71,;42.93,-17.94,;44.26,-18.71,;45.59,-17.94,;46.93,-18.71,;46.93,-20.25,;48.26,-17.94,;49.59,-18.71,;50.93,-17.94,;50.93,-16.4,;52.26,-18.71,;53.6,-17.95,;53.59,-16.41,;54.92,-15.64,;56.26,-16.41,;57.73,-15.93,;58.64,-17.18,;60.18,-17.18,;57.73,-18.43,;58.21,-19.89,;57.17,-21.03,;57.65,-22.5,;59.16,-22.82,;60.19,-21.66,;59.71,-20.2,;60.74,-19.06,;56.26,-17.95,;54.93,-18.72,;38.93,-18.71,;37.59,-17.93,;36.26,-18.71,;36.26,-20.25,;37.59,-21.01,;38.93,-20.24,;37.59,-22.55,;38.93,-23.31,;38.93,-24.86,;37.6,-25.63,;36.26,-24.86,;34.8,-25.34,;33.89,-24.1,;34.79,-22.85,;36.26,-23.32,)| Show InChI InChI=1S/C32H36ClN9O2S/c1-20-23(7-3-11-34-20)28-25-16-21(8-9-26(25)45-29(28)33)40-32(44)37-17-27(43)35-12-5-14-41(2)22-6-4-15-42(18-22)31-24-10-13-36-30(24)38-19-39-31/h3,7-11,13,16,19,22H,4-6,12,14-15,17-18H2,1-2H3,(H,35,43)(H,36,38,39)(H2,37,40,44)/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... |
ACS Med Chem Lett 8: 338-343 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00519
BindingDB Entry DOI: 10.7270/Q2WW7KZW |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50529550
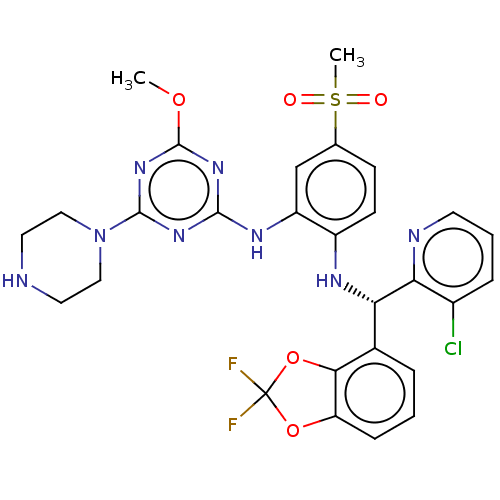
(CHEMBL4446126)Show SMILES COc1nc(Nc2cc(ccc2N[C@@H](c2cccc3OC(F)(F)Oc23)c2ncccc2Cl)S(C)(=O)=O)nc(n1)N1CCNCC1 |r| Show InChI InChI=1S/C28H27ClF2N8O5S/c1-42-27-37-25(36-26(38-27)39-13-11-32-12-14-39)35-20-15-16(45(2,40)41)8-9-19(20)34-22(23-18(29)6-4-10-33-23)17-5-3-7-21-24(17)44-28(30,31)43-21/h3-10,15,22,32,34H,11-14H2,1-2H3,(H,35,36,37,38)/t22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of 0.05 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for... |
ACS Med Chem Lett 10: 1655-1660 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00452
BindingDB Entry DOI: 10.7270/Q2RV0S5T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50235301
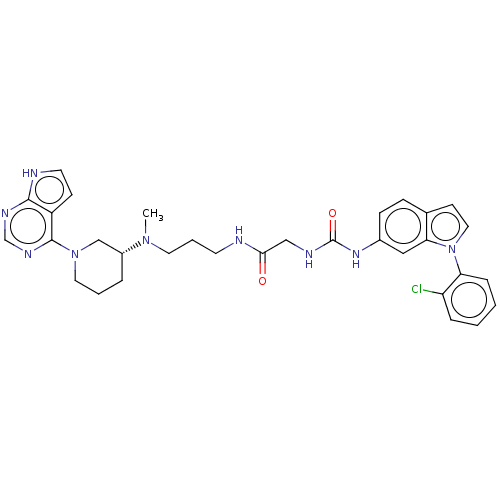
(CHEMBL4081752)Show SMILES CN(CCCNC(=O)CNC(=O)Nc1ccc2ccn(-c3ccccc3Cl)c2c1)[C@@H]1CCCN(C1)c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C32H36ClN9O2/c1-40(24-6-4-16-41(20-24)31-25-11-14-35-30(25)37-21-38-31)15-5-13-34-29(43)19-36-32(44)39-23-10-9-22-12-17-42(28(22)18-23)27-8-3-2-7-26(27)33/h2-3,7-12,14,17-18,21,24H,4-6,13,15-16,19-20H2,1H3,(H,34,43)(H,35,37,38)(H2,36,39,44)/t24-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... |
ACS Med Chem Lett 8: 338-343 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00519
BindingDB Entry DOI: 10.7270/Q2WW7KZW |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50529551
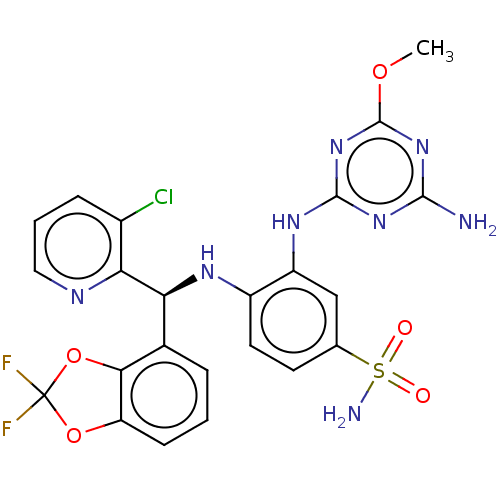
(CHEMBL4435508)Show SMILES COc1nc(N)nc(Nc2cc(ccc2N[C@@H](c2cccc3OC(F)(F)Oc23)c2ncccc2Cl)S(N)(=O)=O)n1 |r| Show InChI InChI=1S/C23H19ClF2N8O5S/c1-37-22-33-20(27)32-21(34-22)31-15-10-11(40(28,35)36)7-8-14(15)30-17(18-13(24)5-3-9-29-18)12-4-2-6-16-19(12)39-23(25,26)38-16/h2-10,17,30H,1H3,(H2,28,35,36)(H3,27,31,32,33,34)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of 0.05 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for... |
ACS Med Chem Lett 10: 1655-1660 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00452
BindingDB Entry DOI: 10.7270/Q2RV0S5T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50529554
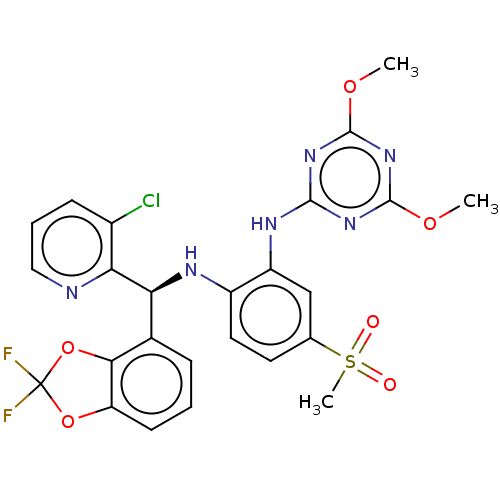
(CHEMBL4567485)Show SMILES COc1nc(Nc2cc(ccc2N[C@@H](c2cccc3OC(F)(F)Oc23)c2ncccc2Cl)S(C)(=O)=O)nc(OC)n1 |r| Show InChI InChI=1S/C25H21ClF2N6O6S/c1-37-23-32-22(33-24(34-23)38-2)31-17-12-13(41(3,35)36)9-10-16(17)30-19(20-15(26)7-5-11-29-20)14-6-4-8-18-21(14)40-25(27,28)39-18/h4-12,19,30H,1-3H3,(H,31,32,33,34)/t19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of 0.05 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for... |
ACS Med Chem Lett 10: 1655-1660 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00452
BindingDB Entry DOI: 10.7270/Q2RV0S5T |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50452149
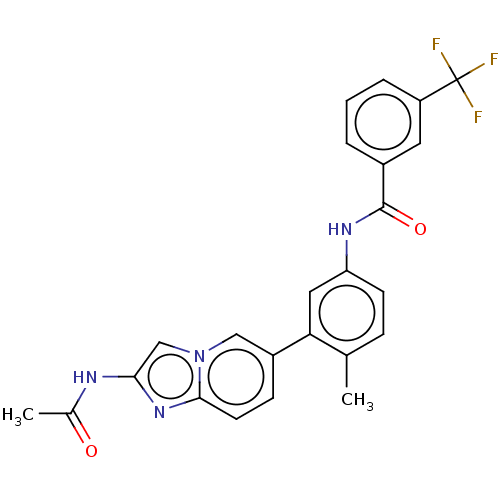
(CHEMBL4216073)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cccc(c2)C(F)(F)F)ccc1C Show InChI InChI=1S/C24H19F3N4O2/c1-14-6-8-19(29-23(33)16-4-3-5-18(10-16)24(25,26)27)11-20(14)17-7-9-22-30-21(28-15(2)32)13-31(22)12-17/h3-13H,1-2H3,(H,28,32)(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal his6-tagged B-Raf (437 to 765 residues) V600E mutant (unknown origin) catalytic domain expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50452150
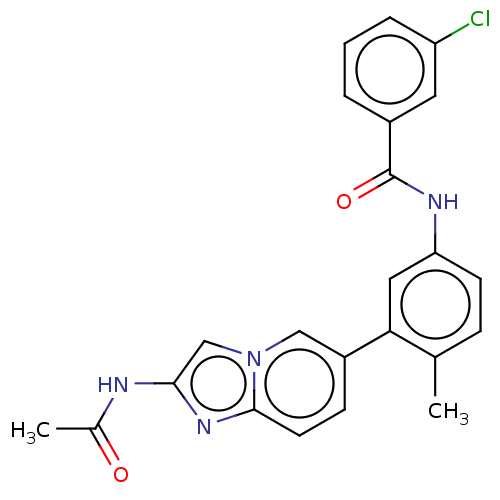
(CHEMBL4216386)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cccc(Cl)c2)ccc1C Show InChI InChI=1S/C23H19ClN4O2/c1-14-6-8-19(26-23(30)16-4-3-5-18(24)10-16)11-20(14)17-7-9-22-27-21(25-15(2)29)13-28(22)12-17/h3-13H,1-2H3,(H,25,29)(H,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal his6-tagged B-Raf (437 to 765 residues) V600E mutant (unknown origin) catalytic domain expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50452152
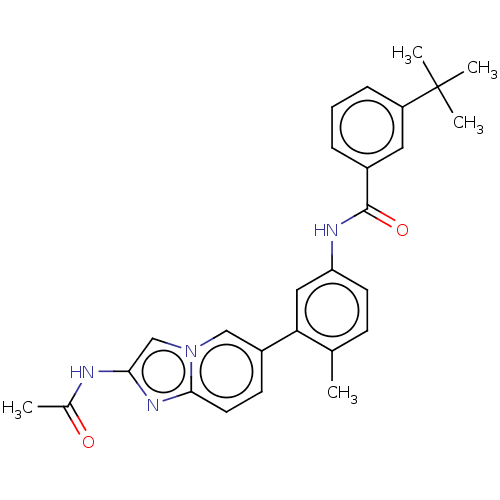
(CHEMBL4217462)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cccc(c2)C(C)(C)C)ccc1C Show InChI InChI=1S/C27H28N4O2/c1-17-9-11-22(29-26(33)19-7-6-8-21(13-19)27(3,4)5)14-23(17)20-10-12-25-30-24(28-18(2)32)16-31(25)15-20/h6-16H,1-5H3,(H,28,32)(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal his6-tagged B-Raf (437 to 765 residues) V600E mutant (unknown origin) catalytic domain expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50536826

(CHEMBL4590355)Show SMILES CNc1ccnc(Nc2ccc3cc(C)n(-c4ccccc4Oc4cnc5n(C)cnc5c4)c3c2)n1 |(29.26,-10.25,;27.93,-11.02,;27.94,-12.56,;29.28,-13.33,;29.28,-14.87,;27.95,-15.64,;26.62,-14.88,;25.29,-15.64,;23.96,-14.88,;23.95,-13.33,;22.62,-12.56,;21.29,-13.34,;19.82,-12.85,;18.9,-14.1,;17.36,-14.08,;19.8,-15.36,;19.31,-16.81,;20.33,-17.95,;19.85,-19.4,;18.34,-19.72,;17.32,-18.56,;17.81,-17.11,;16.79,-15.96,;15.29,-16.26,;14.81,-17.71,;13.31,-18.01,;12.29,-16.86,;10.74,-16.84,;9.83,-18.07,;10.28,-15.37,;11.54,-14.47,;12.78,-15.39,;14.28,-15.1,;21.28,-14.89,;22.62,-15.65,;26.61,-13.34,)| Show InChI InChI=1S/C27H24N8O/c1-17-12-18-8-9-19(32-27-29-11-10-25(28-2)33-27)13-23(18)35(17)22-6-4-5-7-24(22)36-20-14-21-26(30-15-20)34(3)16-31-21/h4-16H,1-3H3,(H2,28,29,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... |
ACS Med Chem Lett 7: 735-40 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00167
BindingDB Entry DOI: 10.7270/Q2V69P3G |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50529544
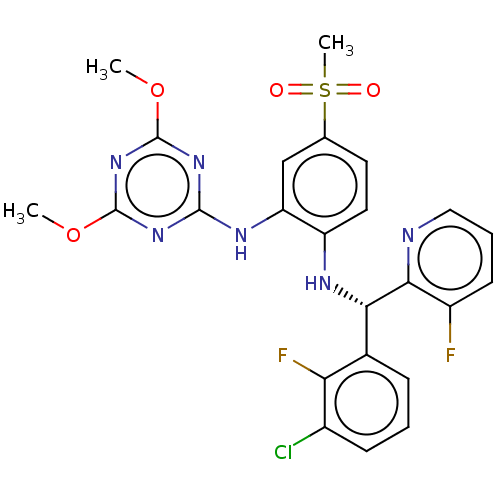
(CHEMBL4557484)Show SMILES COc1nc(Nc2cc(ccc2N[C@@H](c2cccc(Cl)c2F)c2ncccc2F)S(C)(=O)=O)nc(OC)n1 |r| Show InChI InChI=1S/C24H21ClF2N6O4S/c1-36-23-31-22(32-24(33-23)37-2)30-18-12-13(38(3,34)35)9-10-17(18)29-20(21-16(26)8-5-11-28-21)14-6-4-7-15(25)19(14)27/h4-12,20,29H,1-3H3,(H,30,31,32,33)/t20-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of 0.05 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for... |
ACS Med Chem Lett 10: 1655-1660 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00452
BindingDB Entry DOI: 10.7270/Q2RV0S5T |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM180790
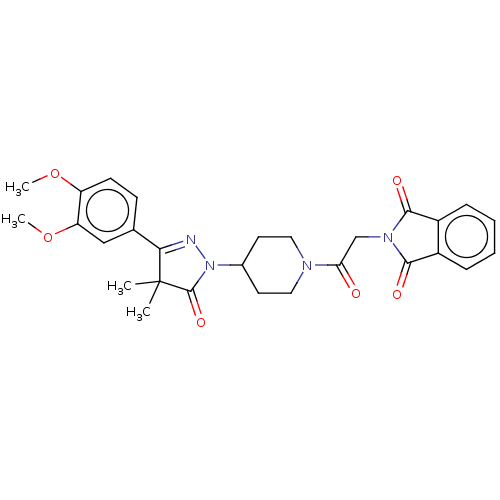
(US8865745, 14)Show SMILES COc1ccc(cc1OC)C1=NN(C2CCN(CC2)C(=O)CN2C(=O)c3ccccc3C2=O)C(=O)C1(C)C |t:11| Show InChI InChI=1S/C28H30N4O6/c1-28(2)24(17-9-10-21(37-3)22(15-17)38-4)29-32(27(28)36)18-11-13-30(14-12-18)23(33)16-31-25(34)19-7-5-6-8-20(19)26(31)35/h5-10,15,18H,11-14,16H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Takeda GmbH
US Patent
| Assay Description
PDE4B1 activity was inhibited by the compounds according to the invention in a modified SPA (scintillation proximity assay) test, supplied by Amersha... |
US Patent US8865745 (2014)
BindingDB Entry DOI: 10.7270/Q2XK8DBB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50452147
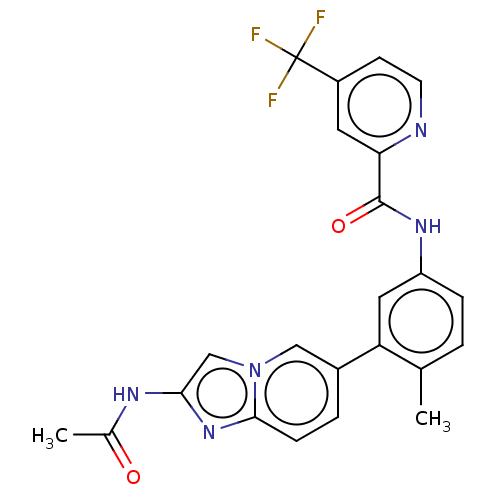
(CHEMBL4204192)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cc(ccn2)C(F)(F)F)ccc1C Show InChI InChI=1S/C23H18F3N5O2/c1-13-3-5-17(29-22(33)19-9-16(7-8-27-19)23(24,25)26)10-18(13)15-4-6-21-30-20(28-14(2)32)12-31(21)11-15/h3-12H,1-2H3,(H,28,32)(H,29,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal his6-tagged B-Raf (437 to 765 residues) V600E mutant (unknown origin) catalytic domain expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259415
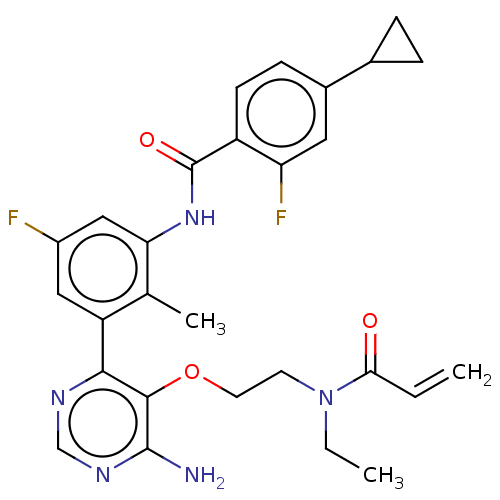
(US10457647, Example 14 | US11180460, Example 14 | ...)Show SMILES CCN(CCOc1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2F)C2CC2)c1C)C(=O)C=C Show InChI InChI=1S/C28H29F2N5O3/c1-4-24(36)35(5-2)10-11-38-26-25(32-15-33-27(26)31)21-13-19(29)14-23(16(21)3)34-28(37)20-9-8-18(12-22(20)30)17-6-7-17/h4,8-9,12-15,17H,1,5-7,10-11H2,2-3H3,(H,34,37)(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50514642

(CHEMBL4593663)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc2[nH]cc(C3=CCN(CC3)C(=O)C=C)c12 |t:31| Show InChI InChI=1S/C31H28FN5O2/c1-3-27(38)37-12-10-21(11-13-37)25-16-33-30-28(25)29(34-17-35-30)24-14-23(32)15-26(18(24)2)36-31(39)22-8-6-20(7-9-22)19-4-5-19/h3,6-10,14-17,19H,1,4-5,11-13H2,2H3,(H,36,39)(H,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348545

(CHEMBL1801384)Show SMILES O=C1NCCc2[nH]c3c(ccc4cnc(\C=C\c5ccc(CN6CCOCC6)cc5)cc34)c12 Show InChI InChI=1S/C27H26N4O2/c32-27-25-22-8-6-20-16-29-21(15-23(20)26(22)30-24(25)9-10-28-27)7-5-18-1-3-19(4-2-18)17-31-11-13-33-14-12-31/h1-8,15-16,30H,9-14,17H2,(H,28,32)/b7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using hsp27 peptide biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET assay |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50348537

(CHEMBL1801376)Show SMILES Fc1cccc(Nc2cc3c4[nH]c5CNC(=O)c5c4ccc3cn2)c1 Show InChI InChI=1S/C19H13FN4O/c20-11-2-1-3-12(6-11)23-16-7-14-10(8-21-16)4-5-13-17-15(24-18(13)14)9-22-19(17)25/h1-8,24H,9H2,(H,21,23)(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259407
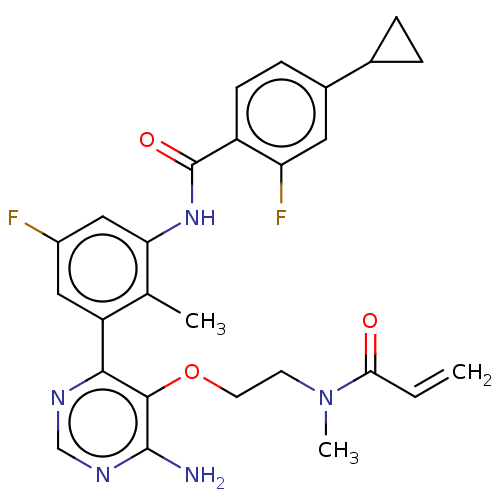
(US10457647, Example 6 | US11180460, Example 6 | US...)Show SMILES CN(CCOc1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2F)C2CC2)c1C)C(=O)C=C Show InChI InChI=1S/C27H27F2N5O3/c1-4-23(35)34(3)9-10-37-25-24(31-14-32-26(25)30)20-12-18(28)13-22(15(20)2)33-27(36)19-8-7-17(11-21(19)29)16-5-6-16/h4,7-8,11-14,16H,1,5-6,9-10H2,2-3H3,(H,33,36)(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50536819

(CHEMBL4534250)Show SMILES CNc1ccnc(Nc2ccc3sc(Cl)c(-c4cc(cnc4Cl)-c4c(C)ncn(C)c4=O)c3c2)n1 |(34.66,-43.04,;33.33,-43.82,;33.34,-45.35,;34.68,-46.12,;34.68,-47.66,;33.35,-48.43,;32.02,-47.66,;30.69,-48.43,;29.36,-47.67,;29.35,-46.12,;28.02,-45.36,;26.69,-46.13,;25.23,-45.65,;24.32,-46.89,;22.78,-46.88,;25.22,-48.14,;24.74,-49.6,;23.23,-49.91,;22.75,-51.37,;23.77,-52.52,;25.29,-52.2,;25.76,-50.74,;27.27,-50.42,;21.24,-51.68,;20.76,-53.14,;21.78,-54.29,;19.25,-53.46,;18.22,-52.31,;18.71,-50.84,;17.68,-49.69,;20.22,-50.53,;20.7,-49.07,;26.69,-47.67,;28.02,-48.44,;32.01,-46.13,)| Show InChI InChI=1S/C24H19Cl2N7OS/c1-12-19(23(34)33(3)11-30-12)13-8-16(21(25)29-10-13)20-15-9-14(4-5-17(15)35-22(20)26)31-24-28-7-6-18(27-2)32-24/h4-11H,1-3H3,(H2,27,28,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... |
ACS Med Chem Lett 7: 735-40 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00167
BindingDB Entry DOI: 10.7270/Q2V69P3G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259423
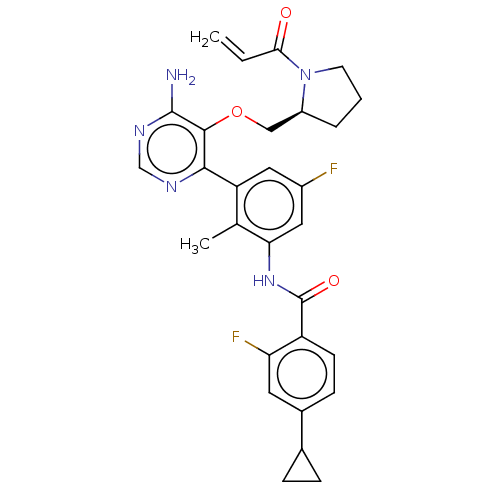
(US10457647, Example 22 | US11180460, Example 22 | ...)Show SMILES Cc1c(NC(=O)c2ccc(cc2F)C2CC2)cc(F)cc1-c1ncnc(N)c1OC[C@@H]1CCCN1C(=O)C=C |r| Show InChI InChI=1S/C29H29F2N5O3/c1-3-25(37)36-10-4-5-20(36)14-39-27-26(33-15-34-28(27)32)22-12-19(30)13-24(16(22)2)35-29(38)21-9-8-18(11-23(21)31)17-6-7-17/h3,8-9,11-13,15,17,20H,1,4-7,10,14H2,2H3,(H,35,38)(H2,32,33,34)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50514634

(CHEMBL4450082)Show SMILES CN(CC\C=C\c1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2)C2CC2)c1C)C(=O)C=C Show InChI InChI=1S/C29H30FN5O2/c1-4-26(36)35(3)14-6-5-7-23-27(32-17-33-28(23)31)24-15-22(30)16-25(18(24)2)34-29(37)21-12-10-20(11-13-21)19-8-9-19/h4-5,7,10-13,15-17,19H,1,6,8-9,14H2,2-3H3,(H,34,37)(H2,31,32,33)/b7-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259409
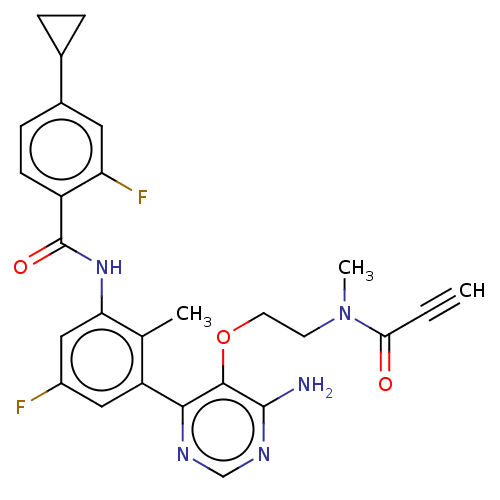
(US10457647, Example 8 | US11180460, Example 8 | US...)Show SMILES CN(CCOc1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2F)C2CC2)c1C)C(=O)C#C Show InChI InChI=1S/C27H25F2N5O3/c1-4-23(35)34(3)9-10-37-25-24(31-14-32-26(25)30)20-12-18(28)13-22(15(20)2)33-27(36)19-8-7-17(11-21(19)29)16-5-6-16/h1,7-8,11-14,16H,5-6,9-10H2,2-3H3,(H,33,36)(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50514645
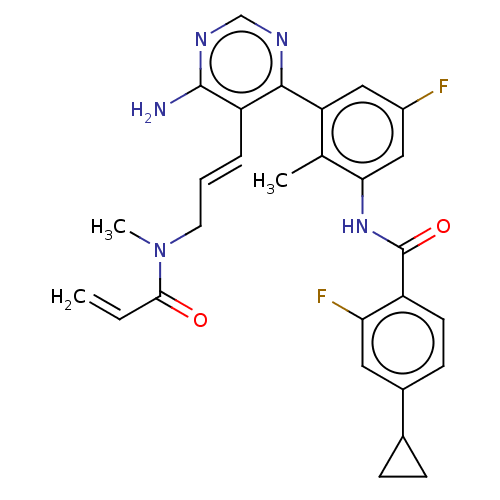
(CHEMBL4546324)Show SMILES CN(C\C=C\c1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2F)C2CC2)c1C)C(=O)C=C Show InChI InChI=1S/C28H27F2N5O2/c1-4-25(36)35(3)11-5-6-21-26(32-15-33-27(21)31)22-13-19(29)14-24(16(22)2)34-28(37)20-10-9-18(12-23(20)30)17-7-8-17/h4-6,9-10,12-15,17H,1,7-8,11H2,2-3H3,(H,34,37)(H2,31,32,33)/b6-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50452152

(CHEMBL4217462)Show SMILES CC(=O)Nc1cn2cc(ccc2n1)-c1cc(NC(=O)c2cccc(c2)C(C)(C)C)ccc1C Show InChI InChI=1S/C27H28N4O2/c1-17-9-11-22(29-26(33)19-7-6-8-21(13-19)27(3,4)5)14-23(17)20-10-12-25-30-24(28-18(2)32)16-31(25)15-20/h6-16H,1-5H3,(H,28,32)(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P38 (unknown origin) |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50348534
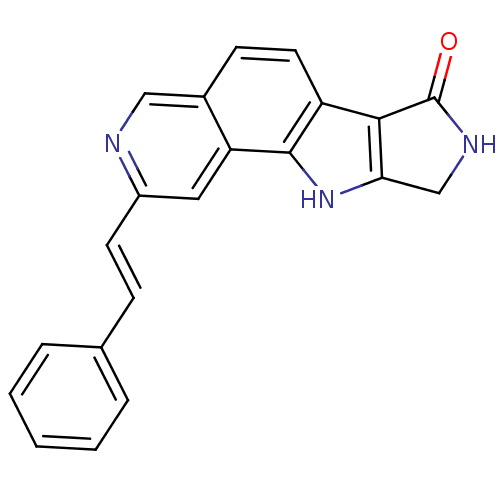
(CHEMBL1801373)Show SMILES O=C1NCc2[nH]c3c(ccc4cnc(\C=C\c5ccccc5)cc34)c12 Show InChI InChI=1S/C21H15N3O/c25-21-19-16-9-7-14-11-22-15(8-6-13-4-2-1-3-5-13)10-17(14)20(16)24-18(19)12-23-21/h1-11,24H,12H2,(H,23,25)/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
Bioorg Med Chem Lett 20: 4715-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.04.024
BindingDB Entry DOI: 10.7270/Q2765FNN |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50452151
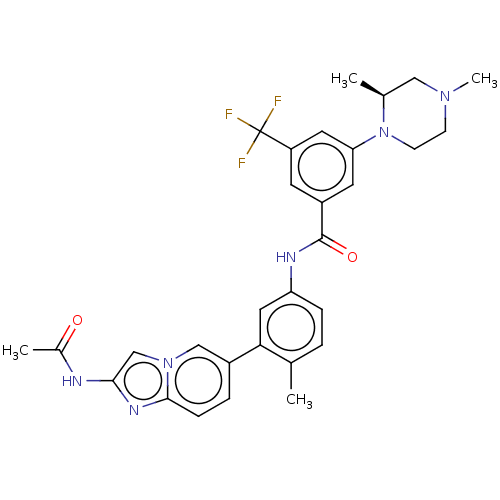
(CHEMBL4208527)Show SMILES C[C@H]1CN(C)CCN1c1cc(cc(c1)C(F)(F)F)C(=O)Nc1ccc(C)c(c1)-c1ccc2nc(NC(C)=O)cn2c1 |r| Show InChI InChI=1S/C30H31F3N6O2/c1-18-5-7-24(14-26(18)21-6-8-28-36-27(34-20(3)40)17-38(28)16-21)35-29(41)22-11-23(30(31,32)33)13-25(12-22)39-10-9-37(4)15-19(39)2/h5-8,11-14,16-17,19H,9-10,15H2,1-4H3,(H,34,40)(H,35,41)/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P38 (unknown origin) |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50529549

(CHEMBL4448208)Show SMILES CS(=O)(=O)c1ccc(N[C@H](c2ccccn2)c2cccc(Cl)c2F)c(Nc2nccc(n2)C(N)=O)c1 |r| Show InChI InChI=1S/C24H20ClFN6O3S/c1-36(34,35)14-8-9-17(20(13-14)32-24-29-12-10-19(31-24)23(27)33)30-22(18-7-2-3-11-28-18)15-5-4-6-16(25)21(15)26/h2-13,22,30H,1H3,(H2,27,33)(H,29,31,32)/t22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of 0.05 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for... |
ACS Med Chem Lett 10: 1655-1660 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00452
BindingDB Entry DOI: 10.7270/Q2RV0S5T |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50514637

(CHEMBL4463185)Show SMILES Cc1c(NC(=O)c2ccc(c(F)c2F)C(C)(C)O)cc(F)cc1-c1ncnc(N)c1OC1CN(C1)C(=O)C=C Show InChI InChI=1S/C27H26F3N5O4/c1-5-20(36)35-10-15(11-35)39-24-23(32-12-33-25(24)31)17-8-14(28)9-19(13(17)2)34-26(37)16-6-7-18(27(3,4)38)22(30)21(16)29/h5-9,12,15,38H,1,10-11H2,2-4H3,(H,34,37)(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50357312

(IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...)Show SMILES Nc1ncnc2n(nc(-c3ccc(Oc4ccccc4)cc3)c12)[C@@H]1CCCN(C1)C(=O)C=C Show InChI InChI=1S/C25H24N6O2/c1-2-21(32)30-14-6-7-18(15-30)31-25-22(24(26)27-16-28-25)23(29-31)17-10-12-20(13-11-17)33-19-8-4-3-5-9-19/h2-5,8-13,16,18H,1,6-7,14-15H2,(H2,26,27,28)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259407

(US10457647, Example 6 | US11180460, Example 6 | US...)Show SMILES CN(CCOc1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2F)C2CC2)c1C)C(=O)C=C Show InChI InChI=1S/C27H27F2N5O3/c1-4-23(35)34(3)9-10-37-25-24(31-14-32-26(25)30)20-12-18(28)13-22(15(20)2)33-27(36)19-8-7-17(11-21(19)29)16-5-6-16/h4,7-8,11-14,16H,1,5-6,9-10H2,2-3H3,(H,33,36)(H2,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of BTK in vitamin D3 differentiated human THP1 cells assessed as inhibition of FCgammaR-induced IL8 production measured after 24 hrs by HT... |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM60633

(US8865745, 8)Show SMILES CCCC1(C)C(=O)N(N=C1c1ccc(OC)c(OC)c1)C1CCN(CC1)C(=O)CCl |c:8| Show InChI InChI=1S/C22H30ClN3O4/c1-5-10-22(2)20(15-6-7-17(29-3)18(13-15)30-4)24-26(21(22)28)16-8-11-25(12-9-16)19(27)14-23/h6-7,13,16H,5,8-12,14H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.95 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Takeda GmbH
US Patent
| Assay Description
PDE4B1 activity was inhibited by the compounds according to the invention in a modified SPA (scintillation proximity assay) test, supplied by Amersha... |
US Patent US8865745 (2014)
BindingDB Entry DOI: 10.7270/Q2XK8DBB |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50235302

(CHEMBL4099771)Show SMILES CN(CCCNC(=O)CNC(=O)Nc1ccc2sc(Cl)c(-c3cccnc3C)c2c1)[C@@H]1CCCN(C1)c1ncnc2[nH]ccc12 |r,wU:30.32,(40.26,-16.4,;40.26,-17.94,;41.59,-18.71,;42.93,-17.94,;44.26,-18.71,;45.59,-17.94,;46.93,-18.71,;46.93,-20.25,;48.26,-17.94,;49.59,-18.71,;50.93,-17.94,;50.93,-16.4,;52.26,-18.71,;53.6,-17.95,;53.59,-16.41,;54.92,-15.64,;56.26,-16.41,;57.73,-15.93,;58.64,-17.18,;60.18,-17.18,;57.73,-18.43,;58.21,-19.89,;57.17,-21.03,;57.65,-22.5,;59.16,-22.82,;60.19,-21.66,;59.71,-20.2,;60.74,-19.06,;56.26,-17.95,;54.93,-18.72,;38.93,-18.71,;37.59,-17.93,;36.26,-18.71,;36.26,-20.25,;37.59,-21.01,;38.93,-20.24,;37.59,-22.55,;38.93,-23.31,;38.93,-24.86,;37.6,-25.63,;36.26,-24.86,;34.8,-25.34,;33.89,-24.1,;34.79,-22.85,;36.26,-23.32,)| Show InChI InChI=1S/C32H36ClN9O2S/c1-20-23(7-3-11-34-20)28-25-16-21(8-9-26(25)45-29(28)33)40-32(44)37-17-27(43)35-12-5-14-41(2)22-6-4-15-42(18-22)31-24-10-13-36-30(24)38-19-39-31/h3,7-11,13,16,19,22H,4-6,12,14-15,17-18H2,1-2H3,(H,35,43)(H,36,38,39)(H2,37,40,44)/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of DOT1L in human HeLa cells assessed as reduction in H3K79me2 level after 72 hrs by ELISA |
ACS Med Chem Lett 8: 338-343 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00519
BindingDB Entry DOI: 10.7270/Q2WW7KZW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348515
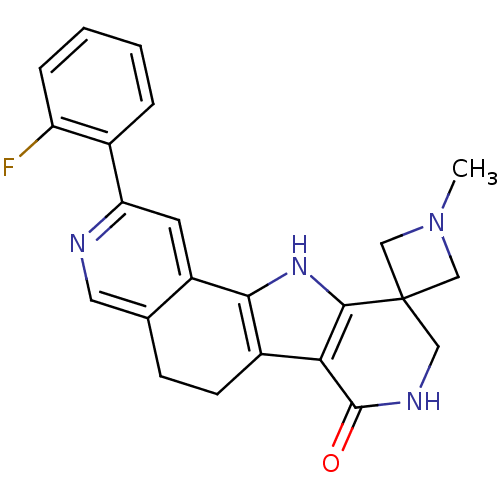
(CHEMBL1233942)Show SMILES CN1CC2(C1)CNC(=O)c1c3CCc4cnc(cc4-c3[nH]c21)-c1ccccc1F Show InChI InChI=1S/C23H21FN4O/c1-28-11-23(12-28)10-26-22(29)19-15-7-6-13-9-25-18(14-4-2-3-5-17(14)24)8-16(13)20(15)27-21(19)23/h2-5,8-9,27H,6-7,10-12H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of MK2 mediated anisomycin-stimulated hsp27 phosphorylation in human THP-1 cells by fluorometric analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50452151

(CHEMBL4208527)Show SMILES C[C@H]1CN(C)CCN1c1cc(cc(c1)C(F)(F)F)C(=O)Nc1ccc(C)c(c1)-c1ccc2nc(NC(C)=O)cn2c1 |r| Show InChI InChI=1S/C30H31F3N6O2/c1-18-5-7-24(14-26(18)21-6-8-28-36-27(34-20(3)40)17-38(28)16-21)35-29(41)22-11-23(30(31,32)33)13-25(12-22)39-10-9-37(4)15-19(39)2/h5-8,11-14,16-17,19H,9-10,15H2,1-4H3,(H,34,40)(H,35,41)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal his6-tagged B-Raf (437 to 765 residues) V600E mutant (unknown origin) catalytic domain expressed in baculovirus ... |
Bioorg Med Chem Lett 27: 5221-5224 (2017)
Article DOI: 10.1016/j.bmcl.2017.10.047
BindingDB Entry DOI: 10.7270/Q2SB4892 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259434
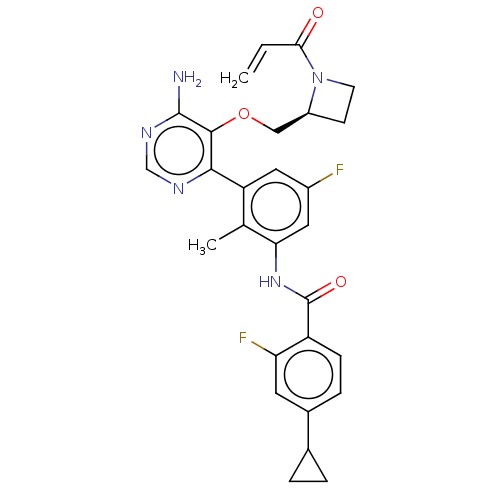
(US10457647, Example 33 | US11180460, Example 33 | ...)Show SMILES Cc1c(NC(=O)c2ccc(cc2F)C2CC2)cc(F)cc1-c1ncnc(N)c1OC[C@@H]1CCN1C(=O)C=C |r| Show InChI InChI=1S/C28H27F2N5O3/c1-3-24(36)35-9-8-19(35)13-38-26-25(32-14-33-27(26)31)21-11-18(29)12-23(15(21)2)34-28(37)20-7-6-17(10-22(20)30)16-4-5-16/h3,6-7,10-12,14,16,19H,1,4-5,8-9,13H2,2H3,(H,34,37)(H2,31,32,33)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50514644

(CHEMBL4463650)Show SMILES Cc1c(NC(=O)c2ccc(cc2)C2CC2)cc(F)cc1-c1ncnc(N)c1OC1CN(C1)C(=O)C=C Show InChI InChI=1S/C27H26FN5O3/c1-3-23(34)33-12-20(13-33)36-25-24(30-14-31-26(25)29)21-10-19(28)11-22(15(21)2)32-27(35)18-8-6-17(7-9-18)16-4-5-16/h3,6-11,14,16,20H,1,4-5,12-13H2,2H3,(H,32,35)(H2,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259422
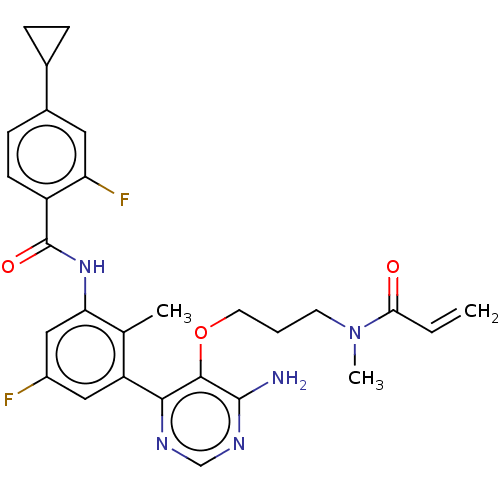
(US10457647, Example 21 | US11180460, Example 21 | ...)Show SMILES CN(CCCOc1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2F)C2CC2)c1C)C(=O)C=C Show InChI InChI=1S/C28H29F2N5O3/c1-4-24(36)35(3)10-5-11-38-26-25(32-15-33-27(26)31)21-13-19(29)14-23(16(21)2)34-28(37)20-9-8-18(12-22(20)30)17-6-7-17/h4,8-9,12-15,17H,1,5-7,10-11H2,2-3H3,(H,34,37)(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
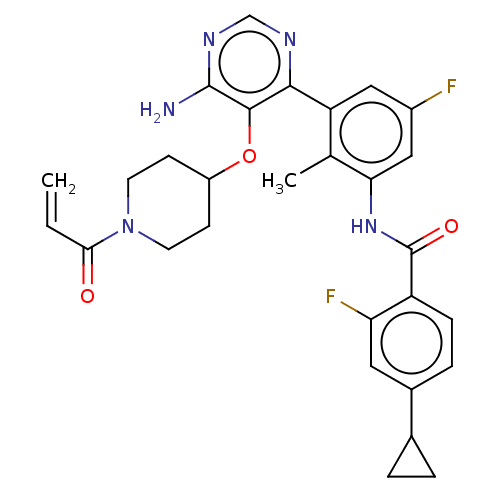
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM259406
(US10457647, Example 5 | US11180460, Example 5 | US...)Show SMILES Cc1c(NC(=O)c2ccc(cc2F)C2CC2)cc(F)cc1-c1ncnc(N)c1OC1CCN(CC1)C(=O)C=C Show InChI InChI=1S/C29H29F2N5O3/c1-3-25(37)36-10-8-20(9-11-36)39-27-26(33-15-34-28(27)32)22-13-19(30)14-24(16(22)2)35-29(38)21-7-6-18(12-23(21)31)17-4-5-17/h3,6-7,12-15,17,20H,1,4-5,8-11H2,2H3,(H,35,38)(H2,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
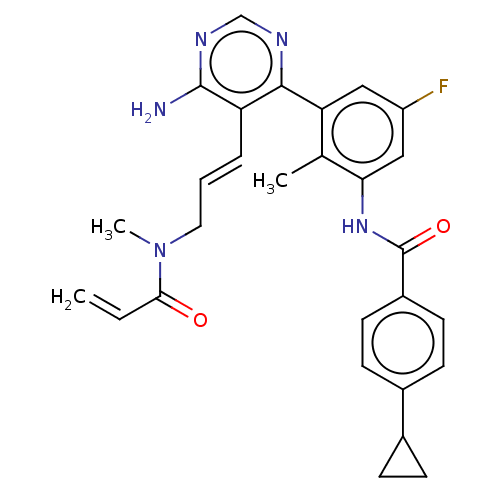
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50514635
(CHEMBL4559123)Show SMILES CN(C\C=C\c1c(N)ncnc1-c1cc(F)cc(NC(=O)c2ccc(cc2)C2CC2)c1C)C(=O)C=C Show InChI InChI=1S/C28H28FN5O2/c1-4-25(35)34(3)13-5-6-22-26(31-16-32-27(22)30)23-14-21(29)15-24(17(23)2)33-28(36)20-11-9-19(10-12-20)18-7-8-18/h4-6,9-12,14-16,18H,1,7-8,13H2,2-3H3,(H,33,36)(H2,30,31,32)/b6-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay |
J Med Chem 63: 5102-5118 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01916
BindingDB Entry DOI: 10.7270/Q2GB27FW |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM180793
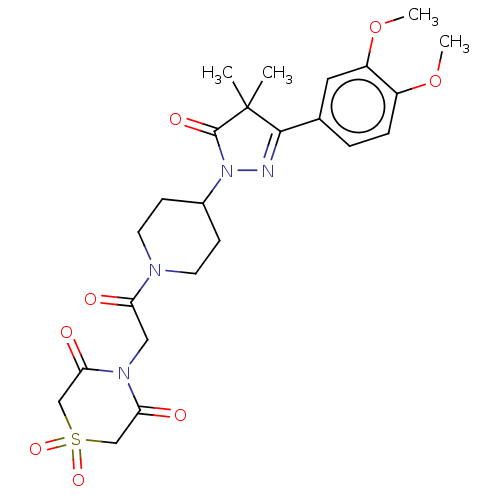
(US8865745, 18)Show SMILES COc1ccc(cc1OC)C1=NN(C2CCN(CC2)C(=O)CN2C(=O)CS(=O)(=O)CC2=O)C(=O)C1(C)C |t:11| Show InChI InChI=1S/C24H30N4O8S/c1-24(2)22(15-5-6-17(35-3)18(11-15)36-4)25-28(23(24)32)16-7-9-26(10-8-16)19(29)12-27-20(30)13-37(33,34)14-21(27)31/h5-6,11,16H,7-10,12-14H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Takeda GmbH
US Patent
| Assay Description
PDE4B1 activity was inhibited by the compounds according to the invention in a modified SPA (scintillation proximity assay) test, supplied by Amersha... |
US Patent US8865745 (2014)
BindingDB Entry DOI: 10.7270/Q2XK8DBB |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50529549

(CHEMBL4448208)Show SMILES CS(=O)(=O)c1ccc(N[C@H](c2ccccn2)c2cccc(Cl)c2F)c(Nc2nccc(n2)C(N)=O)c1 |r| Show InChI InChI=1S/C24H20ClFN6O3S/c1-36(34,35)14-8-9-17(20(13-14)32-24-29-12-10-19(31-24)23(27)33)30-22(18-7-2-3-11-28-18)15-5-4-6-16(25)21(15)26/h2-13,22,30H,1H3,(H2,27,33)(H,29,31,32)/t22-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of 0.5 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for ... |
ACS Med Chem Lett 10: 1655-1660 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00452
BindingDB Entry DOI: 10.7270/Q2RV0S5T |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50235299
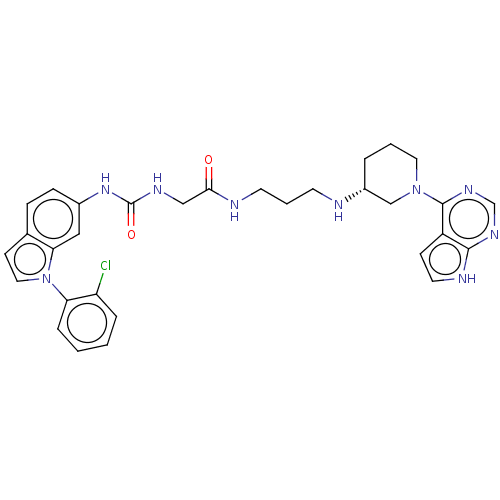
(CHEMBL4066397)Show SMILES Clc1ccccc1-n1ccc2ccc(NC(=O)NCC(=O)NCCCN[C@@H]3CCCN(C3)c3ncnc4[nH]ccc34)cc12 |r| Show InChI InChI=1S/C31H34ClN9O2/c32-25-6-1-2-7-26(25)41-16-11-21-8-9-22(17-27(21)41)39-31(43)36-18-28(42)34-13-4-12-33-23-5-3-15-40(19-23)30-24-10-14-35-29(24)37-20-38-30/h1-2,6-11,14,16-17,20,23,33H,3-5,12-13,15,18-19H2,(H,34,42)(H,35,37,38)(H2,36,39,43)/t23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... |
ACS Med Chem Lett 8: 338-343 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00519
BindingDB Entry DOI: 10.7270/Q2WW7KZW |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50235299

(CHEMBL4066397)Show SMILES Clc1ccccc1-n1ccc2ccc(NC(=O)NCC(=O)NCCCN[C@@H]3CCCN(C3)c3ncnc4[nH]ccc34)cc12 |r| Show InChI InChI=1S/C31H34ClN9O2/c32-25-6-1-2-7-26(25)41-16-11-21-8-9-22(17-27(21)41)39-31(43)36-18-28(42)34-13-4-12-33-23-5-3-15-40(19-23)30-24-10-14-35-29(24)37-20-38-30/h1-2,6-11,14,16-17,20,23,33H,3-5,12-13,15,18-19H2,(H,34,42)(H,35,37,38)(H2,36,39,43)/t23-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... |
ACS Med Chem Lett 8: 338-343 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00519
BindingDB Entry DOI: 10.7270/Q2WW7KZW |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 2
(Homo sapiens (Human)) | BDBM50348492
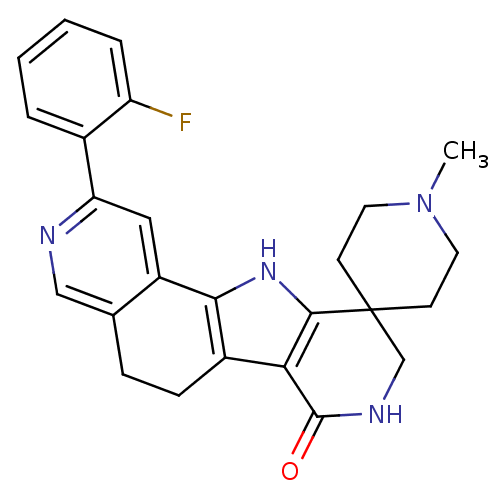
(CHEMBL1801296)Show SMILES CN1CCC2(CC1)CNC(=O)c1c3CCc4cnc(cc4-c3[nH]c21)-c1ccccc1F Show InChI InChI=1S/C25H25FN4O/c1-30-10-8-25(9-11-30)14-28-24(31)21-17-7-6-15-13-27-20(16-4-2-3-5-19(16)26)12-18(15)22(17)29-23(21)25/h2-5,12-13,29H,6-11,14H2,1H3,(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human MK2 using biotinyl-AYSRALSRQLSSGVSEIRCOOH as substrate after 45 mins by FRET analysis |
Bioorg Med Chem Lett 20: 4719-23 (2010)
Checked by Author
Article DOI: 10.1016/j.bmcl.2010.04.023
BindingDB Entry DOI: 10.7270/Q2BZ66C2 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50235300
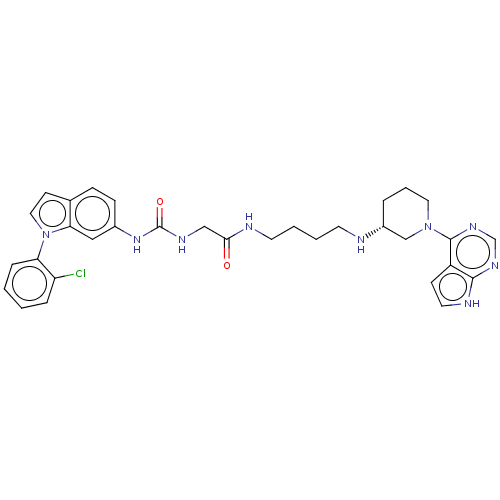
(CHEMBL4087730)Show SMILES Clc1ccccc1-n1ccc2ccc(NC(=O)NCC(=O)NCCCCN[C@@H]3CCCN(C3)c3ncnc4[nH]ccc34)cc12 |r| Show InChI InChI=1S/C32H36ClN9O2/c33-26-7-1-2-8-27(26)42-17-12-22-9-10-23(18-28(22)42)40-32(44)37-19-29(43)35-14-4-3-13-34-24-6-5-16-41(20-24)31-25-11-15-36-30(25)38-21-39-31/h1-2,7-12,15,17-18,21,24,34H,3-6,13-14,16,19-20H2,(H,35,43)(H,36,38,39)(H2,37,40,44)/t24-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... |
ACS Med Chem Lett 8: 338-343 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00519
BindingDB Entry DOI: 10.7270/Q2WW7KZW |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM50235300

(CHEMBL4087730)Show SMILES Clc1ccccc1-n1ccc2ccc(NC(=O)NCC(=O)NCCCCN[C@@H]3CCCN(C3)c3ncnc4[nH]ccc34)cc12 |r| Show InChI InChI=1S/C32H36ClN9O2/c33-26-7-1-2-8-27(26)42-17-12-22-9-10-23(18-28(22)42)40-32(44)37-19-29(43)35-14-4-3-13-34-24-6-5-16-41(20-24)31-25-11-15-36-30(25)38-21-39-31/h1-2,7-12,15,17-18,21,24,34H,3-6,13-14,16,19-20H2,(H,35,43)(H,36,38,39)(H2,37,40,44)/t24-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... |
ACS Med Chem Lett 8: 338-343 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00519
BindingDB Entry DOI: 10.7270/Q2WW7KZW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data