 Found 240 hits with Last Name = 'schiffler' and Initial = 'ma'
Found 240 hits with Last Name = 'schiffler' and Initial = 'ma' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50140256

(CHEMBL3740223)Show SMILES Cc1ccc(C(O)=O)c(C)c1NC(=O)c1cc(cc2ccccc12)-c1cccc(CO)c1 Show InChI InChI=1S/C27H23NO4/c1-16-10-11-22(27(31)32)17(2)25(16)28-26(30)24-14-21(13-20-7-3-4-9-23(20)24)19-8-5-6-18(12-19)15-29/h3-14,29H,15H2,1-2H3,(H,28,30)(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 receptor |
Bioorg Med Chem Lett 26: 105-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.023
BindingDB Entry DOI: 10.7270/Q2G73HRK |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50140258
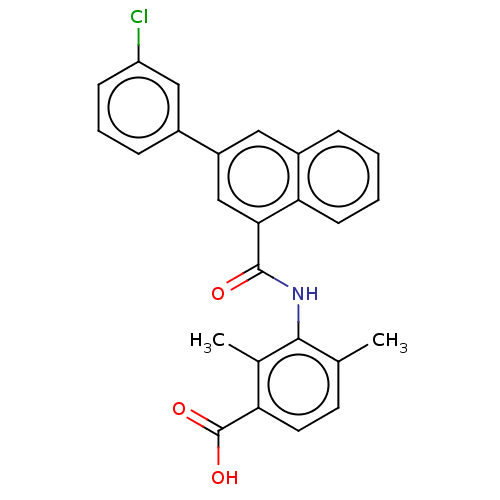
(CHEMBL3740325)Show SMILES Cc1ccc(C(O)=O)c(C)c1NC(=O)c1cc(cc2ccccc12)-c1cccc(Cl)c1 Show InChI InChI=1S/C26H20ClNO3/c1-15-10-11-21(26(30)31)16(2)24(15)28-25(29)23-14-19(17-7-5-8-20(27)13-17)12-18-6-3-4-9-22(18)23/h3-14H,1-2H3,(H,28,29)(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 receptor |
Bioorg Med Chem Lett 26: 105-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.023
BindingDB Entry DOI: 10.7270/Q2G73HRK |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50499955

(CHEMBL3741430)Show SMILES Cc1cc(cc(C)c1NC(=O)c1cc(cc2ccccc12)-c1cccc(CO)c1)C(O)=O Show InChI InChI=1S/C27H23NO4/c1-16-10-22(27(31)32)11-17(2)25(16)28-26(30)24-14-21(13-20-7-3-4-9-23(20)24)19-8-5-6-18(12-19)15-29/h3-14,29H,15H2,1-2H3,(H,28,30)(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 receptor |
Bioorg Med Chem Lett 26: 105-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.023
BindingDB Entry DOI: 10.7270/Q2G73HRK |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50499950
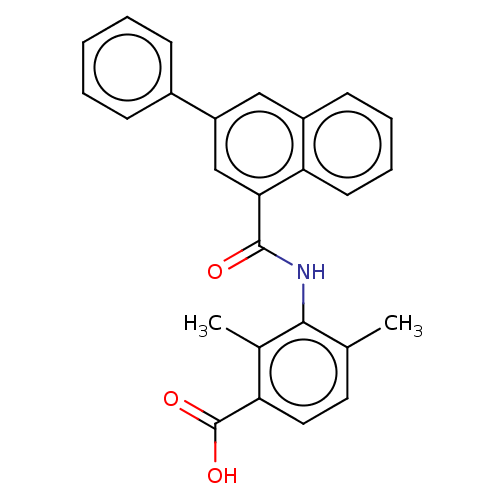
(CHEMBL3739435)Show SMILES Cc1ccc(C(O)=O)c(C)c1NC(=O)c1cc(cc2ccccc12)-c1ccccc1 Show InChI InChI=1S/C26H21NO3/c1-16-12-13-21(26(29)30)17(2)24(16)27-25(28)23-15-20(18-8-4-3-5-9-18)14-19-10-6-7-11-22(19)23/h3-15H,1-2H3,(H,27,28)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 receptor |
Bioorg Med Chem Lett 26: 105-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.023
BindingDB Entry DOI: 10.7270/Q2G73HRK |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50499949

(CHEMBL3742015)Show SMILES Cc1cc(cc(C)c1NC(=O)c1cc(cc2ccccc12)-c1ccccc1)C(O)=O Show InChI InChI=1S/C26H21NO3/c1-16-12-21(26(29)30)13-17(2)24(16)27-25(28)23-15-20(18-8-4-3-5-9-18)14-19-10-6-7-11-22(19)23/h3-15H,1-2H3,(H,27,28)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 receptor |
Bioorg Med Chem Lett 26: 105-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.023
BindingDB Entry DOI: 10.7270/Q2G73HRK |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50499954

(CHEMBL3741902)Show SMILES Cc1ccc(C(O)=O)c(C)c1NC(=O)c1cc(nc2ccccc12)-c1cccc(CO)c1 Show InChI InChI=1S/C26H22N2O4/c1-15-10-11-19(26(31)32)16(2)24(15)28-25(30)21-13-23(18-7-5-6-17(12-18)14-29)27-22-9-4-3-8-20(21)22/h3-13,29H,14H2,1-2H3,(H,28,30)(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 receptor |
Bioorg Med Chem Lett 26: 105-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.023
BindingDB Entry DOI: 10.7270/Q2G73HRK |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50499952
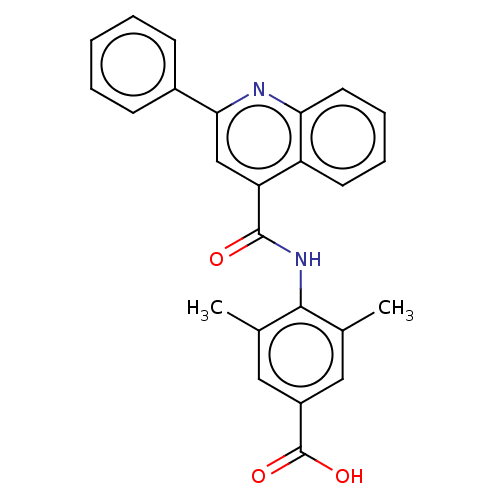
(CHEMBL3741642)Show SMILES Cc1cc(cc(C)c1NC(=O)c1cc(nc2ccccc12)-c1ccccc1)C(O)=O Show InChI InChI=1S/C25H20N2O3/c1-15-12-18(25(29)30)13-16(2)23(15)27-24(28)20-14-22(17-8-4-3-5-9-17)26-21-11-7-6-10-19(20)21/h3-14H,1-2H3,(H,27,28)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 receptor |
Bioorg Med Chem Lett 26: 105-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.023
BindingDB Entry DOI: 10.7270/Q2G73HRK |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107278
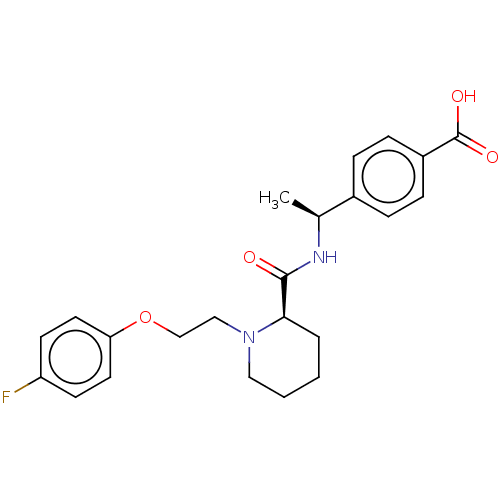
(CHEMBL3600884)Show SMILES Cl.C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccc(F)cc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H27FN2O4/c1-16(17-5-7-18(8-6-17)23(28)29)25-22(27)21-4-2-3-13-26(21)14-15-30-20-11-9-19(24)10-12-20/h5-12,16,21H,2-4,13-15H2,1H3,(H,25,27)(H,28,29)/t16-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from recombinant human EP4 receptor expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting analy... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50446847

(CHEMBL3115074)Show SMILES C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H28N2O4/c1-17(18-10-12-19(13-11-18)23(27)28)24-22(26)21-9-5-6-14-25(21)15-16-29-20-7-3-2-4-8-20/h2-4,7-8,10-13,17,21H,5-6,9,14-16H2,1H3,(H,24,26)(H,27,28)/t17-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from recombinant human EP4 receptor expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting analy... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107280
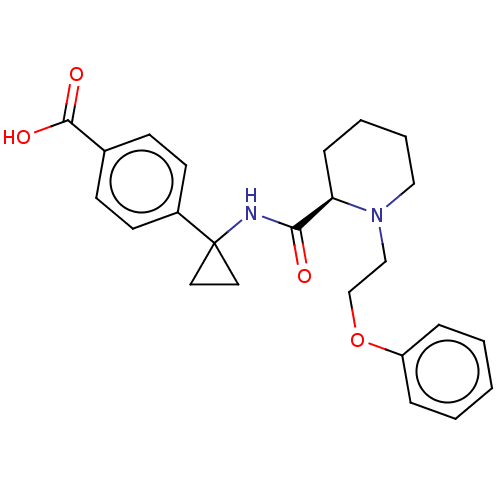
(CHEMBL3600788)Show SMILES Cl.OC(=O)c1ccc(cc1)C1(CC1)NC(=O)[C@H]1CCCCN1CCOc1ccccc1 |r| Show InChI InChI=1S/C24H28N2O4/c27-22(25-24(13-14-24)19-11-9-18(10-12-19)23(28)29)21-8-4-5-15-26(21)16-17-30-20-6-2-1-3-7-20/h1-3,6-7,9-12,21H,4-5,8,13-17H2,(H,25,27)(H,28,29)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from recombinant human EP4 receptor expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting analy... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50499953

(CHEMBL3739779)Show SMILES Cc1cc(cc(C)c1NC(=O)c1cc(nc2ccccc12)-c1cccc(CO)c1)C(O)=O Show InChI InChI=1S/C26H22N2O4/c1-15-10-19(26(31)32)11-16(2)24(15)28-25(30)21-13-23(18-7-5-6-17(12-18)14-29)27-22-9-4-3-8-20(21)22/h3-13,29H,14H2,1-2H3,(H,28,30)(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 receptor |
Bioorg Med Chem Lett 26: 105-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.023
BindingDB Entry DOI: 10.7270/Q2G73HRK |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50446847

(CHEMBL3115074)Show SMILES C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H28N2O4/c1-17(18-10-12-19(13-11-18)23(27)28)24-22(26)21-9-5-6-14-25(21)15-16-29-20-7-3-2-4-8-20/h2-4,7-8,10-13,17,21H,5-6,9,14-16H2,1H3,(H,24,26)(H,27,28)/t17-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| US Patent
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Eli Lilly and Company
US Patent
| Assay Description
hEP1 and hEP4 membranes are prepared from recombinant HEK293 cells stably expressing the human EP1 (Genbank accession number AY275470) or EP4 (Genban... |
US Patent US8962659 (2015)
BindingDB Entry DOI: 10.7270/Q2028Q75 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107282
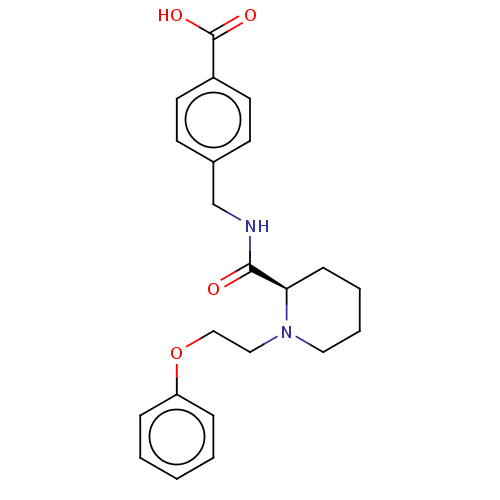
(CHEMBL3600786)Show SMILES Cl.OC(=O)c1ccc(CNC(=O)[C@H]2CCCCN2CCOc2ccccc2)cc1 |r| Show InChI InChI=1S/C22H26N2O4/c25-21(23-16-17-9-11-18(12-10-17)22(26)27)20-8-4-5-13-24(20)14-15-28-19-6-2-1-3-7-19/h1-3,6-7,9-12,20H,4-5,8,13-16H2,(H,23,25)(H,26,27)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from recombinant human EP4 receptor expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting analy... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107281
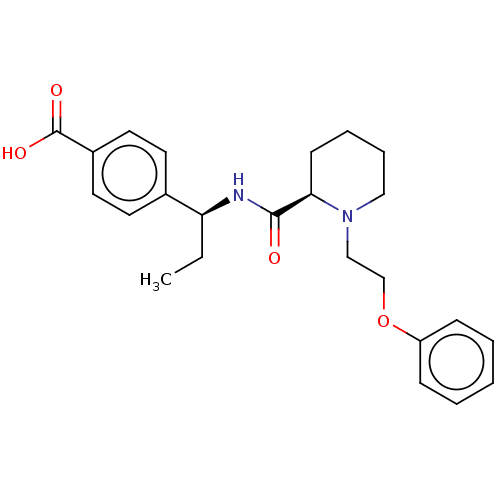
(CHEMBL3600787)Show SMILES Cl.CC[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C24H30N2O4/c1-2-21(18-11-13-19(14-12-18)24(28)29)25-23(27)22-10-6-7-15-26(22)16-17-30-20-8-4-3-5-9-20/h3-5,8-9,11-14,21-22H,2,6-7,10,15-17H2,1H3,(H,25,27)(H,28,29)/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from recombinant human EP4 receptor expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting analy... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107279

(CHEMBL3600883)Show SMILES Cl.C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccc(cc1)C#N)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C24H27N3O4/c1-17(19-7-9-20(10-8-19)24(29)30)26-23(28)22-4-2-3-13-27(22)14-15-31-21-11-5-18(16-25)6-12-21/h5-12,17,22H,2-4,13-15H2,1H3,(H,26,28)(H,29,30)/t17-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from recombinant human EP4 receptor expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting analy... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107283
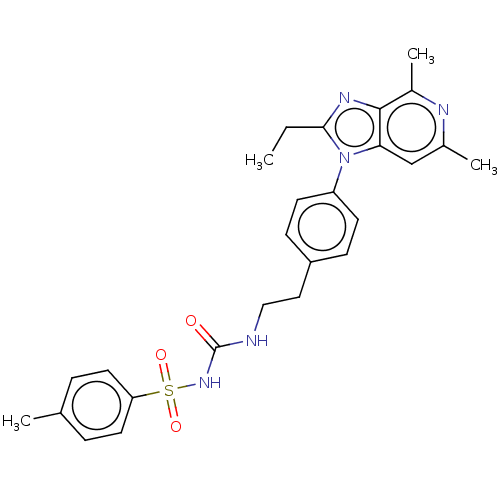
(AAT-007 | CJ-023 | Grapiprant | MR-10A7 | RQ-00000...)Show SMILES CCc1nc2c(C)nc(C)cc2n1-c1ccc(CCNC(=O)NS(=O)(=O)c2ccc(C)cc2)cc1 Show InChI InChI=1S/C26H29N5O3S/c1-5-24-29-25-19(4)28-18(3)16-23(25)31(24)21-10-8-20(9-11-21)14-15-27-26(32)30-35(33,34)22-12-6-17(2)7-13-22/h6-13,16H,5,14-15H2,1-4H3,(H2,27,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 448 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 receptor |
Bioorg Med Chem Lett 26: 105-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.023
BindingDB Entry DOI: 10.7270/Q2G73HRK |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107283

(AAT-007 | CJ-023 | Grapiprant | MR-10A7 | RQ-00000...)Show SMILES CCc1nc2c(C)nc(C)cc2n1-c1ccc(CCNC(=O)NS(=O)(=O)c2ccc(C)cc2)cc1 Show InChI InChI=1S/C26H29N5O3S/c1-5-24-29-25-19(4)28-18(3)16-23(25)31(24)21-10-8-20(9-11-21)14-15-27-26(32)30-35(33,34)22-12-6-17(2)7-13-22/h6-13,16H,5,14-15H2,1-4H3,(H2,27,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 449 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from recombinant human EP4 receptor expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting analy... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50499956

(CHEMBL3741710)Show SMILES Cc1cccc(C)c1NC(=O)c1cc(nc2ccccc12)-c1ccccc1 Show InChI InChI=1S/C24H20N2O/c1-16-9-8-10-17(2)23(16)26-24(27)20-15-22(18-11-4-3-5-12-18)25-21-14-7-6-13-19(20)21/h3-15H,1-2H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 512 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 receptor |
Bioorg Med Chem Lett 26: 105-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.023
BindingDB Entry DOI: 10.7270/Q2G73HRK |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50446847

(CHEMBL3115074)Show SMILES C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H28N2O4/c1-17(18-10-12-19(13-11-18)23(27)28)24-22(26)21-9-5-6-14-25(21)15-16-29-20-7-3-2-4-8-20/h2-4,7-8,10-13,17,21H,5-6,9,14-16H2,1H3,(H,24,26)(H,27,28)/t17-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from human EP2 receptor by liquid scintillation counting analysis |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50446847

(CHEMBL3115074)Show SMILES C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H28N2O4/c1-17(18-10-12-19(13-11-18)23(27)28)24-22(26)21-9-5-6-14-25(21)15-16-29-20-7-3-2-4-8-20/h2-4,7-8,10-13,17,21H,5-6,9,14-16H2,1H3,(H,24,26)(H,27,28)/t17-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| US Patent
| 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Eli Lilly and Company
US Patent
| Assay Description
hEP1 and hEP4 membranes are prepared from recombinant HEK293 cells stably expressing the human EP1 (Genbank accession number AY275470) or EP4 (Genban... |
US Patent US8962659 (2015)
BindingDB Entry DOI: 10.7270/Q2028Q75 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50140258

(CHEMBL3740325)Show SMILES Cc1ccc(C(O)=O)c(C)c1NC(=O)c1cc(cc2ccccc12)-c1cccc(Cl)c1 Show InChI InChI=1S/C26H20ClNO3/c1-15-10-11-21(26(30)31)16(2)24(15)28-25(29)23-14-19(17-7-5-8-20(27)13-17)12-18-6-3-4-9-22(18)23/h3-14H,1-2H3,(H,28,29)(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP2 receptor |
Bioorg Med Chem Lett 26: 105-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.023
BindingDB Entry DOI: 10.7270/Q2G73HRK |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50446847

(CHEMBL3115074)Show SMILES C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H28N2O4/c1-17(18-10-12-19(13-11-18)23(27)28)24-22(26)21-9-5-6-14-25(21)15-16-29-20-7-3-2-4-8-20/h2-4,7-8,10-13,17,21H,5-6,9,14-16H2,1H3,(H,24,26)(H,27,28)/t17-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from human EP3 receptor by liquid scintillation counting analysis |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50446847

(CHEMBL3115074)Show SMILES C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H28N2O4/c1-17(18-10-12-19(13-11-18)23(27)28)24-22(26)21-9-5-6-14-25(21)15-16-29-20-7-3-2-4-8-20/h2-4,7-8,10-13,17,21H,5-6,9,14-16H2,1H3,(H,24,26)(H,27,28)/t17-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| US Patent
| >1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Eli Lilly and Company
US Patent
| Assay Description
hEP1 and hEP4 membranes are prepared from recombinant HEK293 cells stably expressing the human EP1 (Genbank accession number AY275470) or EP4 (Genban... |
US Patent US8962659 (2015)
BindingDB Entry DOI: 10.7270/Q2028Q75 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50140258

(CHEMBL3740325)Show SMILES Cc1ccc(C(O)=O)c(C)c1NC(=O)c1cc(cc2ccccc12)-c1cccc(Cl)c1 Show InChI InChI=1S/C26H20ClNO3/c1-15-10-11-21(26(30)31)16(2)24(15)28-25(29)23-14-19(17-7-5-8-20(27)13-17)12-18-6-3-4-9-22(18)23/h3-14H,1-2H3,(H,28,29)(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor |
Bioorg Med Chem Lett 26: 105-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.023
BindingDB Entry DOI: 10.7270/Q2G73HRK |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50446847

(CHEMBL3115074)Show SMILES C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H28N2O4/c1-17(18-10-12-19(13-11-18)23(27)28)24-22(26)21-9-5-6-14-25(21)15-16-29-20-7-3-2-4-8-20/h2-4,7-8,10-13,17,21H,5-6,9,14-16H2,1H3,(H,24,26)(H,27,28)/t17-,21+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| US Patent
| >1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Eli Lilly and Company
US Patent
| Assay Description
hEP1 and hEP4 membranes are prepared from recombinant HEK293 cells stably expressing the human EP1 (Genbank accession number AY275470) or EP4 (Genban... |
US Patent US8962659 (2015)
BindingDB Entry DOI: 10.7270/Q2028Q75 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50446847

(CHEMBL3115074)Show SMILES C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccccc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H28N2O4/c1-17(18-10-12-19(13-11-18)23(27)28)24-22(26)21-9-5-6-14-25(21)15-16-29-20-7-3-2-4-8-20/h2-4,7-8,10-13,17,21H,5-6,9,14-16H2,1H3,(H,24,26)(H,27,28)/t17-,21+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-PGE2 from human EP1 receptor by liquid scintillation counting analysis |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50499951
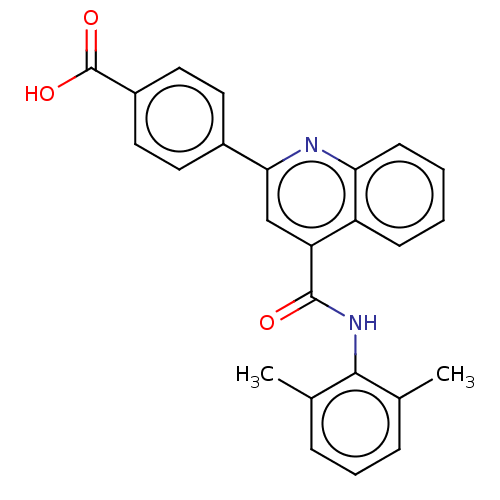
(CHEMBL3739886)Show SMILES Cc1cccc(C)c1NC(=O)c1cc(nc2ccccc12)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H20N2O3/c1-15-6-5-7-16(2)23(15)27-24(28)20-14-22(26-21-9-4-3-8-19(20)21)17-10-12-18(13-11-17)25(29)30/h3-14H,1-2H3,(H,27,28)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 receptor |
Bioorg Med Chem Lett 26: 105-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.023
BindingDB Entry DOI: 10.7270/Q2G73HRK |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50140258

(CHEMBL3740325)Show SMILES Cc1ccc(C(O)=O)c(C)c1NC(=O)c1cc(cc2ccccc12)-c1cccc(Cl)c1 Show InChI InChI=1S/C26H20ClNO3/c1-15-10-11-21(26(30)31)16(2)24(15)28-25(29)23-14-19(17-7-5-8-20(27)13-17)12-18-6-3-4-9-22(18)23/h3-14H,1-2H3,(H,28,29)(H,30,31) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP1 receptor |
Bioorg Med Chem Lett 26: 105-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.023
BindingDB Entry DOI: 10.7270/Q2G73HRK |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50028854
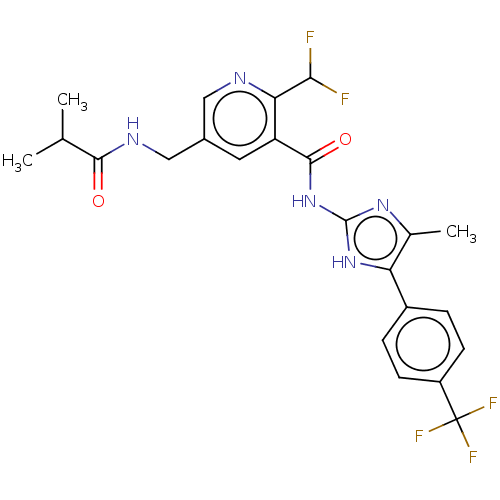
(CHEMBL3342693)Show SMILES CC(C)C(=O)NCc1cnc(C(F)F)c(c1)C(=O)Nc1nc(C)c([nH]1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C23H22F5N5O2/c1-11(2)20(34)30-10-13-8-16(18(19(24)25)29-9-13)21(35)33-22-31-12(3)17(32-22)14-4-6-15(7-5-14)23(26,27)28/h4-9,11,19H,10H2,1-3H3,(H,30,34)(H2,31,32,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 59: 194-205 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01249
BindingDB Entry DOI: 10.7270/Q2474CQR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50028854

(CHEMBL3342693)Show SMILES CC(C)C(=O)NCc1cnc(C(F)F)c(c1)C(=O)Nc1nc(C)c([nH]1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C23H22F5N5O2/c1-11(2)20(34)30-10-13-8-16(18(19(24)25)29-9-13)21(35)33-22-31-12(3)17(32-22)14-4-6-15(7-5-14)23(26,27)28/h4-9,11,19H,10H2,1-3H3,(H,30,34)(H2,31,32,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human microsomal PGES1 expressed in 293E cells by LC/MS/MS analysis |
J Med Chem 59: 194-205 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01249
BindingDB Entry DOI: 10.7270/Q2474CQR |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233281

(CHEMBL4084995)Show SMILES OC(=O)c1ccccc1CC[C@@H]1CCC[C@@H]1NC(=O)C1CCN(CC1)c1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C27H31F3N2O4/c28-27(29,30)36-22-12-10-21(11-13-22)32-16-14-20(15-17-32)25(33)31-24-7-3-5-19(24)9-8-18-4-1-2-6-23(18)26(34)35/h1-2,4,6,10-13,19-20,24H,3,5,7-9,14-17H2,(H,31,33)(H,34,35)/t19-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50194141

(CHEMBL3938686)Show SMILES Cc1cccc2ccc(nc12)N1CC[C@H](C(=O)N[C@@H]2CC[C@H](CO)CC2)C(C)(C)C1 |r,wU:14.16,18.19,21.23,(40.97,-19.87,;39.64,-20.64,;39.65,-22.18,;38.31,-22.96,;36.97,-22.19,;36.97,-20.65,;35.63,-19.88,;35.63,-18.35,;36.97,-17.58,;38.3,-18.34,;38.3,-19.88,;36.97,-16.05,;38.3,-15.28,;38.3,-13.74,;36.97,-12.97,;36.97,-11.43,;38.3,-10.66,;35.63,-10.66,;35.63,-9.12,;36.97,-8.34,;36.98,-6.81,;35.65,-6.03,;35.65,-4.49,;36.99,-3.72,;34.31,-6.8,;34.3,-8.34,;35.64,-13.74,;34.14,-14.14,;34.55,-12.65,;35.64,-15.28,)| Show InChI InChI=1S/C25H35N3O2/c1-17-5-4-6-19-9-12-22(27-23(17)19)28-14-13-21(25(2,3)16-28)24(30)26-20-10-7-18(15-29)8-11-20/h4-6,9,12,18,20-21,29H,7-8,10-11,13-16H2,1-3H3,(H,26,30)/t18-,20+,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES-1 expressed in 293E cells assessed as reduction in conversion of PGH2 to PGE2 after 1.5 min by LC/MS analysis |
Bioorg Med Chem Lett 26: 4824-4828 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.023
BindingDB Entry DOI: 10.7270/Q2125VMX |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233283

(CHEMBL4102262)Show SMILES OC(=O)c1ccc(Cl)cc1CC[C@@H]1CCC[C@@H]1NC(=O)C1CCN(CC1)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C27H30ClF3N2O3/c28-21-8-11-23(26(35)36)19(16-21)5-4-17-2-1-3-24(17)32-25(34)18-12-14-33(15-13-18)22-9-6-20(7-10-22)27(29,30)31/h6-11,16-18,24H,1-5,12-15H2,(H,32,34)(H,35,36)/t17-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233285

(CHEMBL4101413)Show SMILES Cc1cccc2ccc(nc12)N1CCC(CC1)C(=O)N[C@H]1CCC[C@H]1CCc1ccccc1C(O)=O |r| Show InChI InChI=1S/C30H35N3O3/c1-20-6-4-9-23-14-15-27(32-28(20)23)33-18-16-24(17-19-33)29(34)31-26-11-5-8-22(26)13-12-21-7-2-3-10-25(21)30(35)36/h2-4,6-7,9-10,14-15,22,24,26H,5,8,11-13,16-19H2,1H3,(H,31,34)(H,35,36)/t22-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233283

(CHEMBL4102262)Show SMILES OC(=O)c1ccc(Cl)cc1CC[C@@H]1CCC[C@@H]1NC(=O)C1CCN(CC1)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C27H30ClF3N2O3/c28-21-8-11-23(26(35)36)19(16-21)5-4-17-2-1-3-24(17)32-25(34)18-12-14-33(15-13-18)22-9-6-20(7-10-22)27(29,30)31/h6-11,16-18,24H,1-5,12-15H2,(H,32,34)(H,35,36)/t17-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS addition meas... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50194190
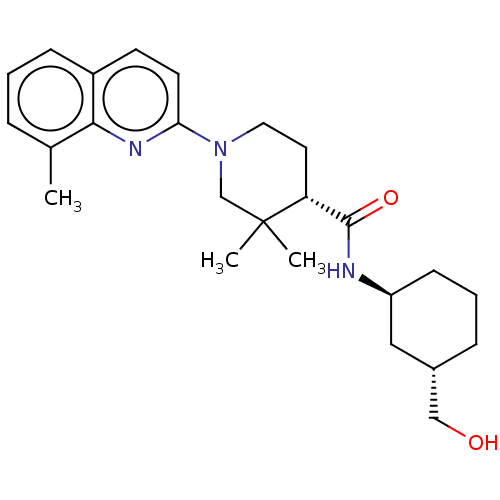
(CHEMBL3956184)Show SMILES Cc1cccc2ccc(nc12)N1CC[C@H](C(=O)N[C@H]2CCC[C@H](CO)C2)C(C)(C)C1 |r| Show InChI InChI=1S/C25H35N3O2/c1-17-6-4-8-19-10-11-22(27-23(17)19)28-13-12-21(25(2,3)16-28)24(30)26-20-9-5-7-18(14-20)15-29/h4,6,8,10-11,18,20-21,29H,5,7,9,12-16H2,1-3H3,(H,26,30)/t18-,20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS stimulation ... |
Bioorg Med Chem Lett 26: 4824-4828 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.023
BindingDB Entry DOI: 10.7270/Q2125VMX |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233278

(CHEMBL4064335)Show SMILES OC(=O)c1ccc(F)cc1CC[C@@H]1CCC[C@@H]1NC(=O)C1CCN(CC1)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C27H30F4N2O3/c28-21-8-11-23(26(35)36)19(16-21)5-4-17-2-1-3-24(17)32-25(34)18-12-14-33(15-13-18)22-9-6-20(7-10-22)27(29,30)31/h6-11,16-18,24H,1-5,12-15H2,(H,32,34)(H,35,36)/t17-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233277

(CHEMBL4085873)Show SMILES Cc1ccc(C(O)=O)c(CC[C@@H]2CCC[C@@H]2NC(=O)C2CCN(CC2)c2ccc(cc2)C(F)(F)F)c1 |r| Show InChI InChI=1S/C28H33F3N2O3/c1-18-5-12-24(27(35)36)21(17-18)7-6-19-3-2-4-25(19)32-26(34)20-13-15-33(16-14-20)23-10-8-22(9-11-23)28(29,30)31/h5,8-12,17,19-20,25H,2-4,6-7,13-16H2,1H3,(H,32,34)(H,35,36)/t19-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50194190

(CHEMBL3956184)Show SMILES Cc1cccc2ccc(nc12)N1CC[C@H](C(=O)N[C@H]2CCC[C@H](CO)C2)C(C)(C)C1 |r| Show InChI InChI=1S/C25H35N3O2/c1-17-6-4-8-19-10-11-22(27-23(17)19)28-13-12-21(25(2,3)16-28)24(30)26-20-9-5-7-18(14-20)15-29/h4,6,8,10-11,18,20-21,29H,5,7,9,12-16H2,1-3H3,(H,26,30)/t18-,20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES-1 expressed in 293E cells assessed as reduction in conversion of PGH2 to PGE2 after 1.5 min by LC/MS analysis |
Bioorg Med Chem Lett 26: 4824-4828 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.023
BindingDB Entry DOI: 10.7270/Q2125VMX |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50499955

(CHEMBL3741430)Show SMILES Cc1cc(cc(C)c1NC(=O)c1cc(cc2ccccc12)-c1cccc(CO)c1)C(O)=O Show InChI InChI=1S/C27H23NO4/c1-16-10-22(27(31)32)11-17(2)25(16)28-26(30)24-14-21(13-20-7-3-4-9-23(20)24)19-8-5-6-18(12-19)15-29/h3-14,29H,15H2,1-2H3,(H,28,30)(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 receptor in HEK293 cells assessed as inhibition of PGE2-induced cAMP accumulation |
Bioorg Med Chem Lett 26: 105-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.023
BindingDB Entry DOI: 10.7270/Q2G73HRK |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Mus musculus (Mouse)) | BDBM50030823

(CHEMBL3342553)Show SMILES CNC(=O)O[C@H]1COc2ccc(cc2[C@@H]1NC(=O)c1ccc(F)cc1)N1CCN(CC1)C1COC1 |r| Show InChI InChI=1S/C25H29FN4O5/c1-27-25(32)35-22-15-34-21-7-6-18(29-8-10-30(11-9-29)19-13-33-14-19)12-20(21)23(22)28-24(31)16-2-4-17(26)5-3-16/h2-7,12,19,22-23H,8-11,13-15H2,1H3,(H,27,32)(H,28,31)/t22-,23-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse cathepsin S using benzyloxycarbonyl-L-Leucyl-L-Arginine 4-Methyl-coumaryl-7-amide substrate by FRET assay |
ACS Med Chem Lett 5: 1138-42 (2014)
Article DOI: 10.1021/ml500283g
BindingDB Entry DOI: 10.7270/Q2765GXN |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233284
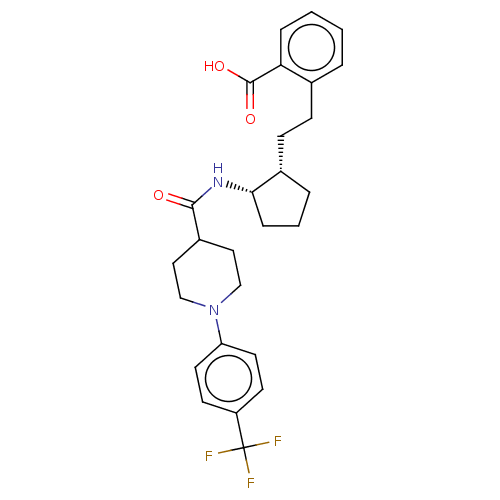
(CHEMBL4071976)Show SMILES OC(=O)c1ccccc1CC[C@@H]1CCC[C@@H]1NC(=O)C1CCN(CC1)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C27H31F3N2O3/c28-27(29,30)21-10-12-22(13-11-21)32-16-14-20(15-17-32)25(33)31-24-7-3-5-19(24)9-8-18-4-1-2-6-23(18)26(34)35/h1-2,4,6,10-13,19-20,24H,3,5,7-9,14-17H2,(H,31,33)(H,34,35)/t19-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50194141

(CHEMBL3938686)Show SMILES Cc1cccc2ccc(nc12)N1CC[C@H](C(=O)N[C@@H]2CC[C@H](CO)CC2)C(C)(C)C1 |r,wU:14.16,18.19,21.23,(40.97,-19.87,;39.64,-20.64,;39.65,-22.18,;38.31,-22.96,;36.97,-22.19,;36.97,-20.65,;35.63,-19.88,;35.63,-18.35,;36.97,-17.58,;38.3,-18.34,;38.3,-19.88,;36.97,-16.05,;38.3,-15.28,;38.3,-13.74,;36.97,-12.97,;36.97,-11.43,;38.3,-10.66,;35.63,-10.66,;35.63,-9.12,;36.97,-8.34,;36.98,-6.81,;35.65,-6.03,;35.65,-4.49,;36.99,-3.72,;34.31,-6.8,;34.3,-8.34,;35.64,-13.74,;34.14,-14.14,;34.55,-12.65,;35.64,-15.28,)| Show InChI InChI=1S/C25H35N3O2/c1-17-5-4-6-19-9-12-22(27-23(17)19)28-14-13-21(25(2,3)16-28)24(30)26-20-10-7-18(15-29)8-11-20/h4-6,9,12,18,20-21,29H,7-8,10-11,13-16H2,1-3H3,(H,26,30)/t18-,20+,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human A549 cells assessed as reduction in recombinant human interleukin-1 beta-induced PGE2 production preincubated for 30 m... |
Bioorg Med Chem Lett 26: 4824-4828 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.023
BindingDB Entry DOI: 10.7270/Q2125VMX |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50194138
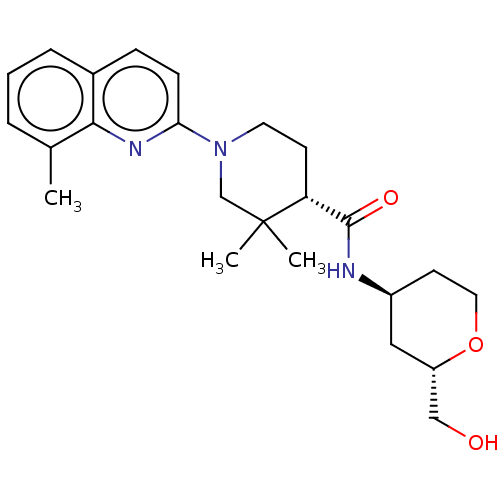
(CHEMBL3928608)Show SMILES Cc1cccc2ccc(nc12)N1CC[C@H](C(=O)N[C@H]2CCO[C@H](CO)C2)C(C)(C)C1 |r| Show InChI InChI=1S/C24H33N3O3/c1-16-5-4-6-17-7-8-21(26-22(16)17)27-11-9-20(24(2,3)15-27)23(29)25-18-10-12-30-19(13-18)14-28/h4-8,18-20,28H,9-15H2,1-3H3,(H,25,29)/t18-,19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES-1 expressed in 293E cells assessed as reduction in conversion of PGH2 to PGE2 after 1.5 min by LC/MS analysis |
Bioorg Med Chem Lett 26: 4824-4828 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.023
BindingDB Entry DOI: 10.7270/Q2125VMX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233280

(CHEMBL4063350)Show SMILES OC(=O)c1ccccc1CC[C@@H]1CCC[C@@H]1NC(=O)C1CCN(CC1)c1ccc(cn1)C(F)(F)F |r| Show InChI InChI=1S/C26H30F3N3O3/c27-26(28,29)20-10-11-23(30-16-20)32-14-12-19(13-15-32)24(33)31-22-7-3-5-18(22)9-8-17-4-1-2-6-21(17)25(34)35/h1-2,4,6,10-11,16,18-19,22H,3,5,7-9,12-15H2,(H,31,33)(H,34,35)/t18-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50194141

(CHEMBL3938686)Show SMILES Cc1cccc2ccc(nc12)N1CC[C@H](C(=O)N[C@@H]2CC[C@H](CO)CC2)C(C)(C)C1 |r,wU:14.16,18.19,21.23,(40.97,-19.87,;39.64,-20.64,;39.65,-22.18,;38.31,-22.96,;36.97,-22.19,;36.97,-20.65,;35.63,-19.88,;35.63,-18.35,;36.97,-17.58,;38.3,-18.34,;38.3,-19.88,;36.97,-16.05,;38.3,-15.28,;38.3,-13.74,;36.97,-12.97,;36.97,-11.43,;38.3,-10.66,;35.63,-10.66,;35.63,-9.12,;36.97,-8.34,;36.98,-6.81,;35.65,-6.03,;35.65,-4.49,;36.99,-3.72,;34.31,-6.8,;34.3,-8.34,;35.64,-13.74,;34.14,-14.14,;34.55,-12.65,;35.64,-15.28,)| Show InChI InChI=1S/C25H35N3O2/c1-17-5-4-6-19-9-12-22(27-23(17)19)28-14-13-21(25(2,3)16-28)24(30)26-20-10-7-18(15-29)8-11-20/h4-6,9,12,18,20-21,29H,7-8,10-11,13-16H2,1-3H3,(H,26,30)/t18-,20+,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mPGES-1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS stimulation ... |
Bioorg Med Chem Lett 26: 4824-4828 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.023
BindingDB Entry DOI: 10.7270/Q2125VMX |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233276

(CHEMBL4078000)Show SMILES OC(=O)c1ccc(Cl)cc1OC[C@@H]1CCC[C@@H]1NC(=O)C1CCN(CC1)c1ccc(cc1)C(F)(F)F |r| Show InChI InChI=1S/C26H28ClF3N2O4/c27-19-6-9-21(25(34)35)23(14-19)36-15-17-2-1-3-22(17)31-24(33)16-10-12-32(13-11-16)20-7-4-18(5-8-20)26(28,29)30/h4-9,14,16-17,22H,1-3,10-13,15H2,(H,31,33)(H,34,35)/t17-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM50233274
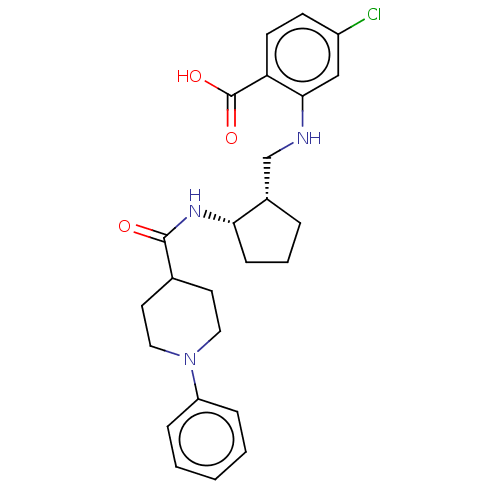
(CHEMBL4067045)Show SMILES OC(=O)c1ccc(Cl)cc1NC[C@@H]1CCC[C@@H]1NC(=O)C1CCN(CC1)c1ccccc1 |r| Show InChI InChI=1S/C25H30ClN3O3/c26-19-9-10-21(25(31)32)23(15-19)27-16-18-5-4-8-22(18)28-24(30)17-11-13-29(14-12-17)20-6-2-1-3-7-20/h1-3,6-7,9-10,15,17-18,22,27H,4-5,8,11-14,16H2,(H,28,30)(H,31,32)/t18-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... |
Bioorg Med Chem Lett 27: 1478-1483 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.011
BindingDB Entry DOI: 10.7270/Q28P62RZ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50140256

(CHEMBL3740223)Show SMILES Cc1ccc(C(O)=O)c(C)c1NC(=O)c1cc(cc2ccccc12)-c1cccc(CO)c1 Show InChI InChI=1S/C27H23NO4/c1-16-10-11-22(27(31)32)17(2)25(16)28-26(30)24-14-21(13-20-7-3-4-9-23(20)24)19-8-5-6-18(12-19)15-29/h3-14,29H,15H2,1-2H3,(H,28,30)(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 receptor in HEK293 cells assessed as inhibition of PGE2-induced cAMP accumulation |
Bioorg Med Chem Lett 26: 105-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.023
BindingDB Entry DOI: 10.7270/Q2G73HRK |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50107278

(CHEMBL3600884)Show SMILES Cl.C[C@H](NC(=O)[C@H]1CCCCN1CCOc1ccc(F)cc1)c1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C23H27FN2O4/c1-16(17-5-7-18(8-6-17)23(28)29)25-22(27)21-4-2-3-13-26(21)14-15-30-20-11-9-19(24)10-12-20/h5-12,16,21H,2-4,13-15H2,1H3,(H,25,27)(H,28,29)/t16-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at recombinant human EP4 receptor expressed in HEK293 cells assessed as inhibition of PGE2-stimulated cAMP accumulation by scinti... |
Bioorg Med Chem Lett 25: 3176-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.091
BindingDB Entry DOI: 10.7270/Q2FX7C79 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data