 Found 2749 hits with Last Name = 'schmid' and Initial = 'b'
Found 2749 hits with Last Name = 'schmid' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50381654
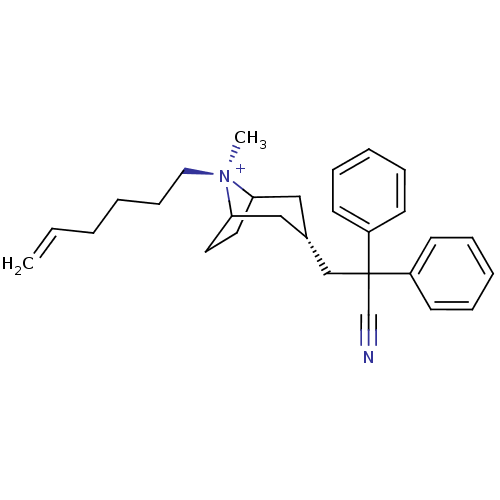
(CHEMBL2023764)Show SMILES C[N@+]1(CCCCC=C)C2CCC1C[C@@H](CC(C#N)(c1ccccc1)c1ccccc1)C2 |r,wU:1.1,wD:13.14,1.0,TLB:0:1:13.12.30:10.9,THB:2:1:13.12.30:10.9,14:13:1:10.9,(20.22,-1.4,;21.72,-1.81,;22.48,-.47,;21.7,.86,;22.46,2.2,;21.68,3.53,;22.44,4.87,;21.66,6.2,;22.77,-2.64,;22.17,-3.98,;20.99,-4.69,;21.98,-3.34,;23.82,-3.34,;24.8,-4.15,;25.57,-5.48,;27.11,-5.48,;27.5,-3.98,;27.89,-2.48,;27.88,-6.81,;27.11,-8.14,;27.89,-9.47,;29.43,-9.47,;30.19,-8.12,;29.42,-6.8,;28.59,-5.07,;29.68,-6.15,;31.16,-5.75,;31.55,-4.26,;30.45,-3.18,;28.97,-3.59,;24.53,-2.61,)| Show InChI InChI=1S/C29H37N2/c1-3-4-5-12-19-31(2)27-17-18-28(31)21-24(20-27)22-29(23-30,25-13-8-6-9-14-25)26-15-10-7-11-16-26/h3,6-11,13-16,24,27-28H,1,4-5,12,17-22H2,2H3/q+1/t24-,27?,28?,31- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-methyl scopolamine from muscarinic acetylcholine M2 receptor expressed in CHO cell membrane |
Bioorg Med Chem Lett 22: 3366-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.015
BindingDB Entry DOI: 10.7270/Q2C82B9Q |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50381654

(CHEMBL2023764)Show SMILES C[N@+]1(CCCCC=C)C2CCC1C[C@@H](CC(C#N)(c1ccccc1)c1ccccc1)C2 |r,wU:1.1,wD:13.14,1.0,TLB:0:1:13.12.30:10.9,THB:2:1:13.12.30:10.9,14:13:1:10.9,(20.22,-1.4,;21.72,-1.81,;22.48,-.47,;21.7,.86,;22.46,2.2,;21.68,3.53,;22.44,4.87,;21.66,6.2,;22.77,-2.64,;22.17,-3.98,;20.99,-4.69,;21.98,-3.34,;23.82,-3.34,;24.8,-4.15,;25.57,-5.48,;27.11,-5.48,;27.5,-3.98,;27.89,-2.48,;27.88,-6.81,;27.11,-8.14,;27.89,-9.47,;29.43,-9.47,;30.19,-8.12,;29.42,-6.8,;28.59,-5.07,;29.68,-6.15,;31.16,-5.75,;31.55,-4.26,;30.45,-3.18,;28.97,-3.59,;24.53,-2.61,)| Show InChI InChI=1S/C29H37N2/c1-3-4-5-12-19-31(2)27-17-18-28(31)21-24(20-27)22-29(23-30,25-13-8-6-9-14-25)26-15-10-7-11-16-26/h3,6-11,13-16,24,27-28H,1,4-5,12,17-22H2,2H3/q+1/t24-,27?,28?,31- | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-methyl scopolamine from muscarinic acetylcholine M1 receptor expressed in CHO cell membrane |
Bioorg Med Chem Lett 22: 3366-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.015
BindingDB Entry DOI: 10.7270/Q2C82B9Q |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50381654

(CHEMBL2023764)Show SMILES C[N@+]1(CCCCC=C)C2CCC1C[C@@H](CC(C#N)(c1ccccc1)c1ccccc1)C2 |r,wU:1.1,wD:13.14,1.0,TLB:0:1:13.12.30:10.9,THB:2:1:13.12.30:10.9,14:13:1:10.9,(20.22,-1.4,;21.72,-1.81,;22.48,-.47,;21.7,.86,;22.46,2.2,;21.68,3.53,;22.44,4.87,;21.66,6.2,;22.77,-2.64,;22.17,-3.98,;20.99,-4.69,;21.98,-3.34,;23.82,-3.34,;24.8,-4.15,;25.57,-5.48,;27.11,-5.48,;27.5,-3.98,;27.89,-2.48,;27.88,-6.81,;27.11,-8.14,;27.89,-9.47,;29.43,-9.47,;30.19,-8.12,;29.42,-6.8,;28.59,-5.07,;29.68,-6.15,;31.16,-5.75,;31.55,-4.26,;30.45,-3.18,;28.97,-3.59,;24.53,-2.61,)| Show InChI InChI=1S/C29H37N2/c1-3-4-5-12-19-31(2)27-17-18-28(31)21-24(20-27)22-29(23-30,25-13-8-6-9-14-25)26-15-10-7-11-16-26/h3,6-11,13-16,24,27-28H,1,4-5,12,17-22H2,2H3/q+1/t24-,27?,28?,31- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-methyl scopolamine from muscarinic acetylcholine M3 receptor expressed in CHO cell membrane |
Bioorg Med Chem Lett 22: 3366-9 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.015
BindingDB Entry DOI: 10.7270/Q2C82B9Q |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50071112
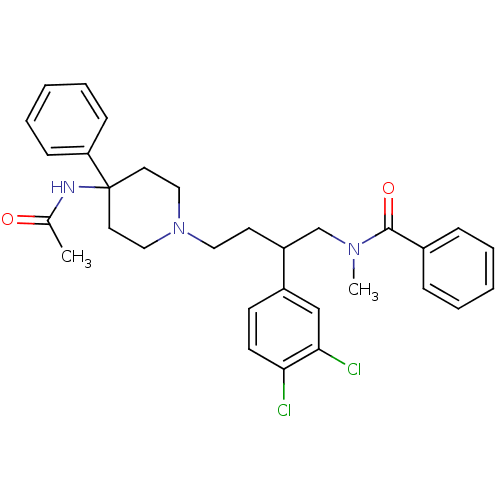
(CHEMBL56835 | N-[4-(4-Acetylamino-4-phenyl-piperid...)Show SMILES CN(CC(CCN1CCC(CC1)(NC(C)=O)c1ccccc1)c1ccc(Cl)c(Cl)c1)C(=O)c1ccccc1 Show InChI InChI=1S/C31H35Cl2N3O2/c1-23(37)34-31(27-11-7-4-8-12-27)16-19-36(20-17-31)18-15-26(25-13-14-28(32)29(33)21-25)22-35(2)30(38)24-9-5-3-6-10-24/h3-14,21,26H,15-20,22H2,1-2H3,(H,34,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 281: 1303-11 (1997)
BindingDB Entry DOI: 10.7270/Q2K35S61 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM85083
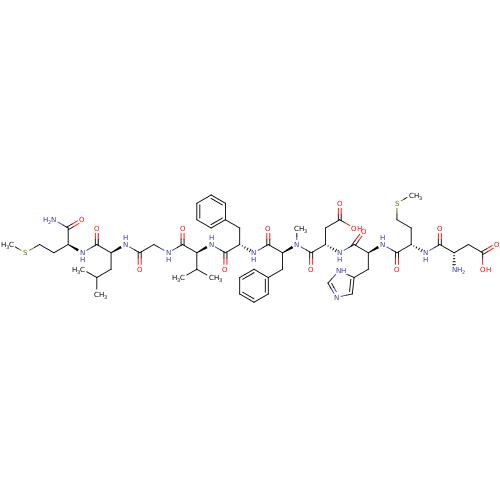
(NKB [MePhe7])Show SMILES CSCC[C@H](NC(=O)[C@H](CC(C)C)NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)N(C)C(=O)[C@H](CC(O)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](CCSC)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(N)=O Show InChI InChI=1S/C56H81N13O14S2/c1-31(2)22-39(51(78)63-37(48(58)75)18-20-84-6)62-44(70)29-60-55(82)47(32(3)4)68-53(80)40(23-33-14-10-8-11-15-33)66-54(81)43(24-34-16-12-9-13-17-34)69(5)56(83)42(27-46(73)74)67-52(79)41(25-35-28-59-30-61-35)65-50(77)38(19-21-85-7)64-49(76)36(57)26-45(71)72/h8-17,28,30-32,36-43,47H,18-27,29,57H2,1-7H3,(H2,58,75)(H,59,61)(H,60,82)(H,62,70)(H,63,78)(H,64,76)(H,65,77)(H,66,81)(H,67,79)(H,68,80)(H,71,72)(H,73,74)/t36-,37-,38-,39-,40-,41-,42-,43-,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 281: 1303-11 (1997)
BindingDB Entry DOI: 10.7270/Q2K35S61 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Rattus norvegicus (Rat)) | BDBM50291261
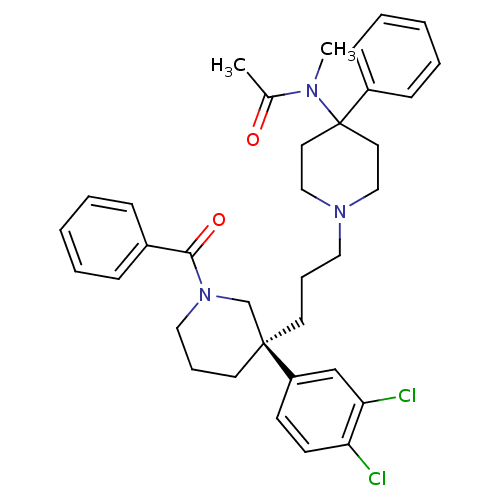
((S)-(+)-N-((3-[1-Benzoyl-3-(3,4-dichlorophenyl)pip...)Show SMILES CN(C(C)=O)C1(CCN(CCC[C@@]2(CCCN(C2)C(=O)c2ccccc2)c2ccc(Cl)c(Cl)c2)CC1)c1ccccc1 |r| Show InChI InChI=1S/C35H41Cl2N3O2/c1-27(41)38(2)35(29-13-7-4-8-14-29)19-23-39(24-20-35)21-9-17-34(30-15-16-31(36)32(37)25-30)18-10-22-40(26-34)33(42)28-11-5-3-6-12-28/h3-8,11-16,25H,9-10,17-24,26H2,1-2H3/t34-/m0/s1 | PDB
Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A.
Curated by ChEMBL
| Assay Description
Ability to displace [125I]NKA from Tachykinin receptor 2 in rat deodenum membrane |
J Med Chem 39: 2281-4 (1996)
Article DOI: 10.1021/jm9602423
BindingDB Entry DOI: 10.7270/Q22J6CJR |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM81891
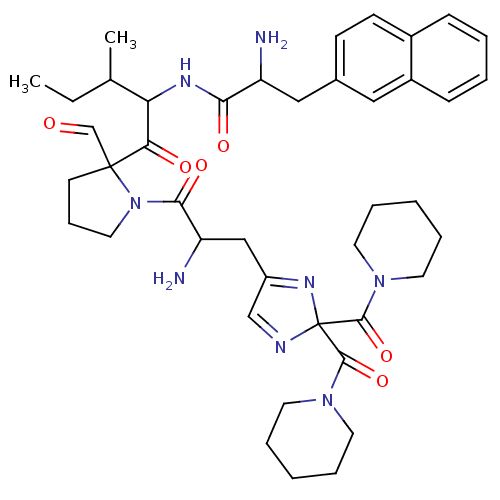
(CAS_188397 | L-366,948 | NSC_188397)Show SMILES CCC(C)C(NC(=O)C(N)Cc1ccc2ccccc2c1)C(=O)C1(CCCN1C(=O)C(N)CC1=NC(N=C1)(C(=O)N1CCCCC1)C(=O)N1CCCCC1)C=O |c:39,t:36| Show InChI InChI=1S/C42H56N8O6/c1-3-28(2)35(46-37(53)33(43)24-29-15-16-30-13-6-7-14-31(30)23-29)36(52)41(27-51)17-12-22-50(41)38(54)34(44)25-32-26-45-42(47-32,39(55)48-18-8-4-9-19-48)40(56)49-20-10-5-11-21-49/h6-7,13-16,23,26-28,33-35H,3-5,8-12,17-22,24-25,43-44H2,1-2H3,(H,46,53) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM81942
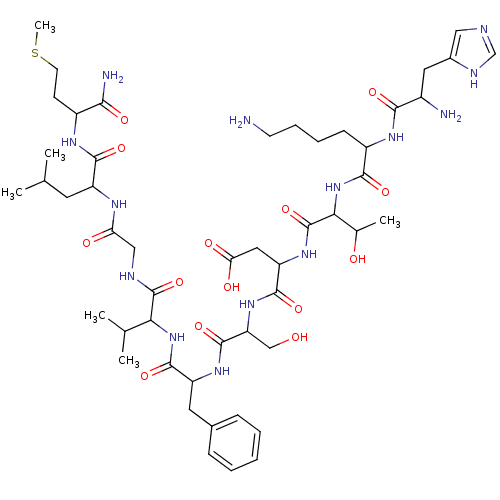
(CAS_55582 | NKA | NSC_55582 | Neurokinin alpha | S...)Show SMILES CSCCC(NC(=O)C(CC(C)C)NC(=O)CNC(=O)C(NC(=O)C(Cc1ccccc1)NC(=O)C(CO)NC(=O)C(CC(O)=O)NC(=O)C(NC(=O)C(CCCCN)NC(=O)C(N)Cc1cnc[nH]1)C(C)O)C(C)C)C(N)=O Show InChI InChI=1S/C50H80N14O14S/c1-26(2)18-34(45(73)58-32(42(53)70)15-17-79-6)57-38(67)23-55-49(77)40(27(3)4)63-47(75)35(19-29-12-8-7-9-13-29)60-48(76)37(24-65)62-46(74)36(21-39(68)69)61-50(78)41(28(5)66)64-44(72)33(14-10-11-16-51)59-43(71)31(52)20-30-22-54-25-56-30/h7-9,12-13,22,25-28,31-37,40-41,65-66H,10-11,14-21,23-24,51-52H2,1-6H3,(H2,53,70)(H,54,56)(H,55,77)(H,57,67)(H,58,73)(H,59,71)(H,60,76)(H,61,78)(H,62,74)(H,63,75)(H,64,72)(H,68,69) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 281: 1303-11 (1997)
BindingDB Entry DOI: 10.7270/Q2K35S61 |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(BOVINE) | BDBM50021195
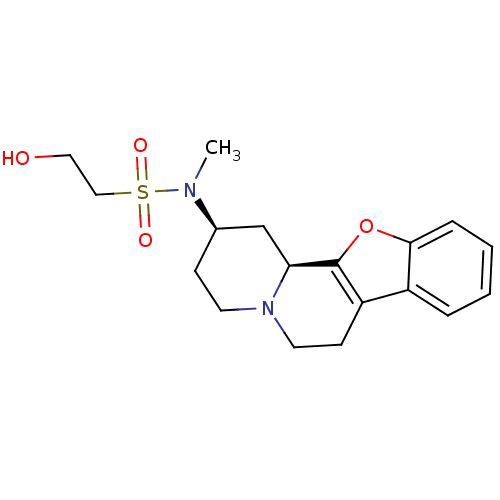
(2-Hydroxy-ethanesulfonic acid (1,3,4,5,6,11b-hexah...)Show SMILES CN([C@@H]1CCN2CCc3c(oc4ccccc34)[C@@H]2C1)S(=O)(=O)CCO Show InChI InChI=1S/C18H24N2O4S/c1-19(25(22,23)11-10-21)13-6-8-20-9-7-15-14-4-2-3-5-17(14)24-18(15)16(20)12-13/h2-5,13,16,21H,6-12H2,1H3/t13-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for its ability to displace [3H]-clonidine from alpha-2 adrenergic receptor of calf cerebral cortex |
J Med Chem 28: 1756-9 (1986)
BindingDB Entry DOI: 10.7270/Q2JW8CWS |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50052027

((E)-3-[6-(2,6-Dichloro-phenylsulfanylmethyl)-3-phe...)Show SMILES OC(=O)\C=C\c1nc(CSc2c(Cl)cccc2Cl)ccc1OCCc1ccccc1 Show InChI InChI=1S/C23H19Cl2NO3S/c24-18-7-4-8-19(25)23(18)30-15-17-9-11-21(20(26-17)10-12-22(27)28)29-14-13-16-5-2-1-3-6-16/h1-12H,13-15H2,(H,27,28)/b12-10+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-LTB4 binding to human neutrophils |
J Med Chem 39: 3837-41 (1996)
Article DOI: 10.1021/jm960248s
BindingDB Entry DOI: 10.7270/Q2VX0FKP |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM81891

(CAS_188397 | L-366,948 | NSC_188397)Show SMILES CCC(C)C(NC(=O)C(N)Cc1ccc2ccccc2c1)C(=O)C1(CCCN1C(=O)C(N)CC1=NC(N=C1)(C(=O)N1CCCCC1)C(=O)N1CCCCC1)C=O |c:39,t:36| Show InChI InChI=1S/C42H56N8O6/c1-3-28(2)35(46-37(53)33(43)24-29-15-16-30-13-6-7-14-31(30)23-29)36(52)41(27-51)17-12-22-50(41)38(54)34(44)25-32-26-45-42(47-32,39(55)48-18-8-4-9-19-48)40(56)49-20-10-5-11-21-49/h6-7,13-16,23,26-28,33-35H,3-5,8-12,17-22,24-25,43-44H2,1-2H3,(H,46,53) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM81944
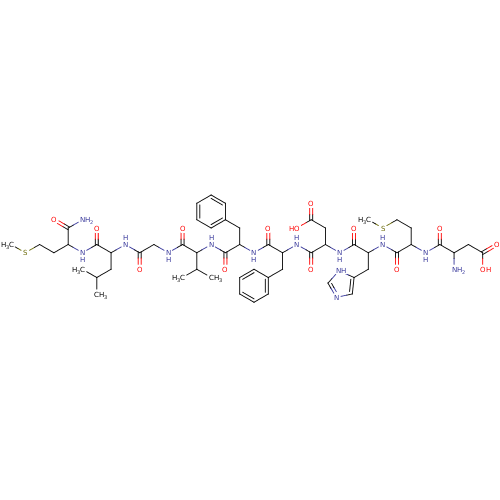
(CAS_86933-75-7 | NKB | NSC_5311312 | neuromedin K)Show SMILES CSCCC(NC(=O)C(CC(C)C)NC(=O)CNC(=O)C(NC(=O)C(Cc1ccccc1)NC(=O)C(Cc1ccccc1)NC(=O)C(CC(O)=O)NC(=O)C(Cc1cnc[nH]1)NC(=O)C(CCSC)NC(=O)C(N)CC(O)=O)C(C)C)C(N)=O Show InChI InChI=1S/C55H79N13O14S2/c1-30(2)21-38(50(77)62-36(47(57)74)17-19-83-5)61-43(69)28-59-55(82)46(31(3)4)68-54(81)40(23-33-15-11-8-12-16-33)65-51(78)39(22-32-13-9-7-10-14-32)64-53(80)42(26-45(72)73)67-52(79)41(24-34-27-58-29-60-34)66-49(76)37(18-20-84-6)63-48(75)35(56)25-44(70)71/h7-16,27,29-31,35-42,46H,17-26,28,56H2,1-6H3,(H2,57,74)(H,58,60)(H,59,82)(H,61,69)(H,62,77)(H,63,75)(H,64,80)(H,65,78)(H,66,76)(H,67,79)(H,68,81)(H,70,71)(H,72,73) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 281: 1303-11 (1997)
BindingDB Entry DOI: 10.7270/Q2K35S61 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50205990

(CHEMBL395429 | OXYTOCIN)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](N)CSSC[C@H](NC(=O)[C@H](CC(N)=O)NC(=O)[C@H](CCC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCC(N)=O |r| Show InChI InChI=1S/C43H66N12O12S2/c1-5-22(4)35-42(66)49-26(12-13-32(45)57)38(62)51-29(17-33(46)58)39(63)53-30(20-69-68-19-25(44)36(60)50-28(40(64)54-35)16-23-8-10-24(56)11-9-23)43(67)55-14-6-7-31(55)41(65)52-27(15-21(2)3)37(61)48-18-34(47)59/h8-11,21-22,25-31,35,56H,5-7,12-20,44H2,1-4H3,(H2,45,57)(H2,46,58)(H2,47,59)(H,48,61)(H,49,66)(H,50,60)(H,51,62)(H,52,65)(H,53,63)(H,54,64)/t22-,25-,26-,27-,28-,29-,30-,31-,35-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Binding affinity against oxytocin receptor in rat uterus |
J Med Chem 33: 2321-3 (1990)
BindingDB Entry DOI: 10.7270/Q2F76D60 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50042180
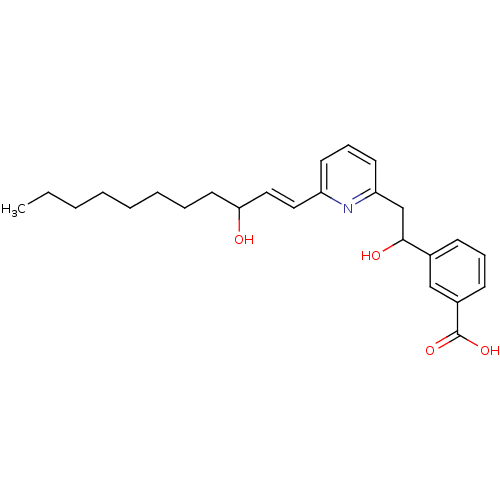
(3-{1-Hydroxy-2-[6-(3-hydroxy-undec-1-enyl)-pyridin...)Show SMILES CCCCCCCCC(O)\C=C\c1cccc(CC(O)c2cccc(c2)C(O)=O)n1 Show InChI InChI=1S/C25H33NO4/c1-2-3-4-5-6-7-14-23(27)16-15-21-12-9-13-22(26-21)18-24(28)19-10-8-11-20(17-19)25(29)30/h8-13,15-17,23-24,27-28H,2-7,14,18H2,1H3,(H,29,30)/b16-15+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity of [3H]-LTB4 to receptors on intact human polymorphonuclear leukocytes |
J Med Chem 36: 3321-32 (1993)
BindingDB Entry DOI: 10.7270/Q2G73CRX |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50051293
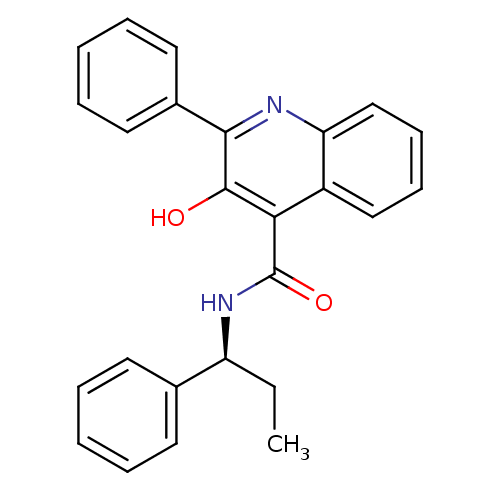
((S)-(-)-N-(R-ethylbenzyl)-3-hydroxy-2-phenylquinol...)Show SMILES CC[C@H](NC(=O)c1c(O)c(nc2ccccc12)-c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C25H22N2O2/c1-2-20(17-11-5-3-6-12-17)27-25(29)22-19-15-9-10-16-21(19)26-23(24(22)28)18-13-7-4-8-14-18/h3-16,20,28H,2H2,1H3,(H,27,29)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 281: 1303-11 (1997)
BindingDB Entry DOI: 10.7270/Q2K35S61 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50042149
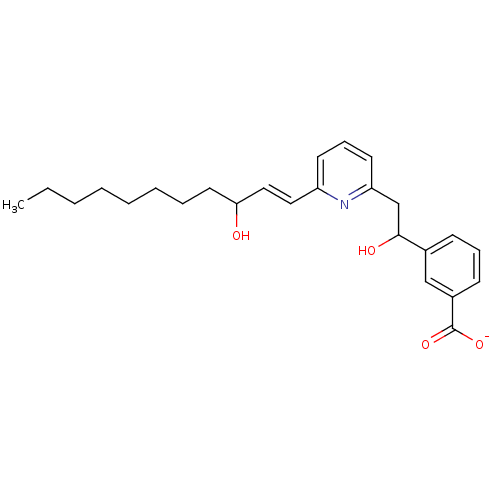
(CHEMBL112520 | Lithium; 3-{1-hydroxy-2-[6-(3-hydro...)Show SMILES CCCCCCCCC(O)\C=C\c1cccc(CC(O)c2cccc(c2)C([O-])=O)n1 Show InChI InChI=1S/C25H33NO4/c1-2-3-4-5-6-7-14-23(27)16-15-21-12-9-13-22(26-21)18-24(28)19-10-8-11-20(17-19)25(29)30/h8-13,15-17,23-24,27-28H,2-7,14,18H2,1H3,(H,29,30)/p-1/b16-15+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- LTB4 binding on human whole cells |
J Med Chem 36: 3308-20 (1993)
BindingDB Entry DOI: 10.7270/Q21R6R4P |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50051293

((S)-(-)-N-(R-ethylbenzyl)-3-hydroxy-2-phenylquinol...)Show SMILES CC[C@H](NC(=O)c1c(O)c(nc2ccccc12)-c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C25H22N2O2/c1-2-20(17-11-5-3-6-12-17)27-25(29)22-19-15-9-10-16-21(19)26-23(24(22)28)18-13-7-4-8-14-18/h3-16,20,28H,2H2,1H3,(H,27,29)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A.
Curated by ChEMBL
| Assay Description
Binding Affinity of [125I]-MePhe7-NKB towards Tachykinin receptor 3-CHO cell membranes(n=3-8) |
J Med Chem 39: 2281-4 (1996)
Article DOI: 10.1021/jm9602423
BindingDB Entry DOI: 10.7270/Q22J6CJR |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50021195

(2-Hydroxy-ethanesulfonic acid (1,3,4,5,6,11b-hexah...)Show SMILES CN([C@@H]1CCN2CCc3c(oc4ccccc34)[C@@H]2C1)S(=O)(=O)CCO Show InChI InChI=1S/C18H24N2O4S/c1-19(25(22,23)11-10-21)13-6-8-20-9-7-15-14-4-2-3-5-17(14)24-18(15)16(20)12-13/h2-5,13,16,21H,6-12H2,1H3/t13-,16+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of human plasma renin |
J Med Chem 28: 1756-9 (1986)
BindingDB Entry DOI: 10.7270/Q2JW8CWS |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM50368134
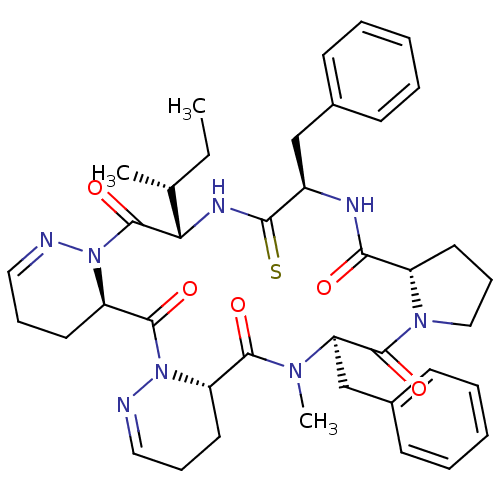
(CHEMBL1790544)Show SMILES CC[C@@H](C)[C@@H]1NC(=S)[C@@H](Cc2ccccc2)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](Cc2ccccc2)N(C)C(=O)[C@@H]2CCC=NN2C(=O)[C@H]2CCC=NN2C1=O |c:44,53| Show InChI InChI=1S/C40H50N8O5S/c1-4-26(2)34-40(53)48-32(19-12-22-42-48)39(52)47-31(18-11-21-41-47)37(50)45(3)33(25-28-16-9-6-10-17-28)38(51)46-23-13-20-30(46)35(49)43-29(36(54)44-34)24-27-14-7-5-8-15-27/h5-10,14-17,21-22,26,29-34H,4,11-13,18-20,23-25H2,1-3H3,(H,43,49)(H,44,54)/t26-,29-,30+,31+,32-,33-,34+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck
Curated by ChEMBL
| Assay Description
Binding affinity against oxytocin receptor in rat uterus |
J Med Chem 33: 2321-3 (1990)
BindingDB Entry DOI: 10.7270/Q2F76D60 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50074789
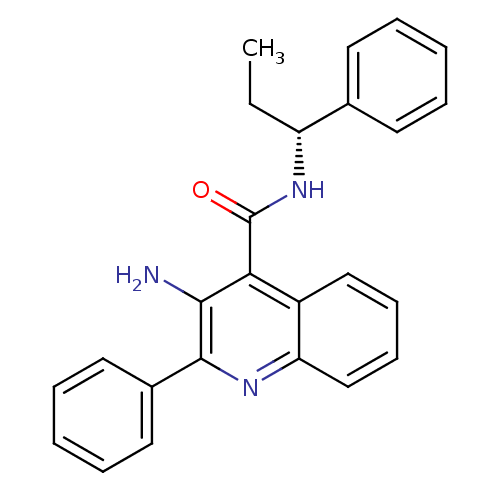
(3-Amino-2-phenyl-quinoline-4-carboxylic acid ((R)-...)Show SMILES CC[C@@H](NC(=O)c1c(N)c(nc2ccccc12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C25H23N3O/c1-2-20(17-11-5-3-6-12-17)28-25(29)22-19-15-9-10-16-21(19)27-24(23(22)26)18-13-7-4-8-14-18/h3-16,20H,2,26H2,1H3,(H,28,29)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A.
Curated by ChEMBL
| Assay Description
Binding affinity towards cloned human Tachykinin receptor 3 (hNK-3) expressed in CHO cells using [125I][MePhe7]-NKB |
J Med Chem 42: 1053-65 (1999)
Article DOI: 10.1021/jm980633c
BindingDB Entry DOI: 10.7270/Q2F47PTQ |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50051290

(CHEMBL299377 | N-(1-{3-[1-Benzoyl-3-(3,4-dichloro-...)Show SMILES CN(C(C)=O)C1(CCN(CCCC2(CCCN(C2)C(=O)c2ccccc2)c2ccc(Cl)c(Cl)c2)CC1)c1ccccc1 Show InChI InChI=1S/C35H41Cl2N3O2/c1-27(41)38(2)35(29-13-7-4-8-14-29)19-23-39(24-20-35)21-9-17-34(30-15-16-31(36)32(37)25-30)18-10-22-40(26-34)33(42)28-11-5-3-6-12-28/h3-8,11-16,25H,9-10,17-24,26H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 281: 1303-11 (1997)
BindingDB Entry DOI: 10.7270/Q2K35S61 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50291261

((S)-(+)-N-((3-[1-Benzoyl-3-(3,4-dichlorophenyl)pip...)Show SMILES CN(C(C)=O)C1(CCN(CCC[C@@]2(CCCN(C2)C(=O)c2ccccc2)c2ccc(Cl)c(Cl)c2)CC1)c1ccccc1 |r| Show InChI InChI=1S/C35H41Cl2N3O2/c1-27(41)38(2)35(29-13-7-4-8-14-29)19-23-39(24-20-35)21-9-17-34(30-15-16-31(36)32(37)25-30)18-10-22-40(26-34)33(42)28-11-5-3-6-12-28/h3-8,11-16,25H,9-10,17-24,26H2,1-2H3/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A.
Curated by ChEMBL
| Assay Description
Binding affinity towards cloned human Tachykinin receptor 3 (hNK-3) expressed in CHO cells using [125I][MePhe7]-NKB |
J Med Chem 42: 1053-65 (1999)
Article DOI: 10.1021/jm980633c
BindingDB Entry DOI: 10.7270/Q2F47PTQ |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50291261

((S)-(+)-N-((3-[1-Benzoyl-3-(3,4-dichlorophenyl)pip...)Show SMILES CN(C(C)=O)C1(CCN(CCC[C@@]2(CCCN(C2)C(=O)c2ccccc2)c2ccc(Cl)c(Cl)c2)CC1)c1ccccc1 |r| Show InChI InChI=1S/C35H41Cl2N3O2/c1-27(41)38(2)35(29-13-7-4-8-14-29)19-23-39(24-20-35)21-9-17-34(30-15-16-31(36)32(37)25-30)18-10-22-40(26-34)33(42)28-11-5-3-6-12-28/h3-8,11-16,25H,9-10,17-24,26H2,1-2H3/t34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A.
Curated by ChEMBL
| Assay Description
Binding Affinity of [125I]-MePhe7-NKB towards Tachykinin receptor 3-CHO cell membranes(n=3-8) |
J Med Chem 39: 2281-4 (1996)
Article DOI: 10.1021/jm9602423
BindingDB Entry DOI: 10.7270/Q22J6CJR |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50053354
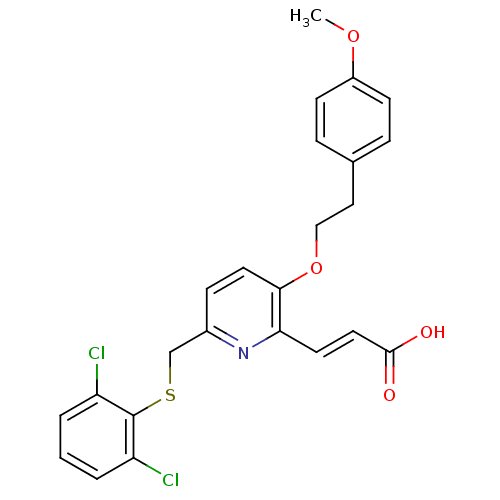
((E)-3-{6-(2,6-Dichloro-phenylsulfanylmethyl)-3-[2-...)Show SMILES COc1ccc(CCOc2ccc(CSc3c(Cl)cccc3Cl)nc2\C=C\C(O)=O)cc1 Show InChI InChI=1S/C24H21Cl2NO4S/c1-30-18-8-5-16(6-9-18)13-14-31-22-11-7-17(27-21(22)10-12-23(28)29)15-32-24-19(25)3-2-4-20(24)26/h2-12H,13-15H2,1H3,(H,28,29)/b12-10+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-LTB4 binding to human neutrophils |
J Med Chem 39: 3837-41 (1996)
Article DOI: 10.1021/jm960248s
BindingDB Entry DOI: 10.7270/Q2VX0FKP |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM81894
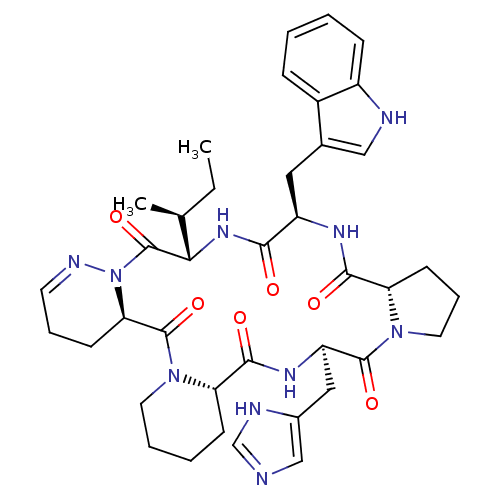
(Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@@H]2CCCCN2C(=O)[C@H]2CCC=NN2C1=O |c:55| Show InChI InChI=1S/C39H50N10O6/c1-3-23(2)33-39(55)49-32(13-8-15-43-49)38(54)48-16-7-6-12-30(48)36(52)45-29(19-25-21-40-22-42-25)37(53)47-17-9-14-31(47)35(51)44-28(34(50)46-33)18-24-20-41-27-11-5-4-10-26(24)27/h4-5,10-11,15,20-23,28-33,41H,3,6-9,12-14,16-19H2,1-2H3,(H,40,42)(H,44,51)(H,45,52)(H,46,50)/t23-,28+,29+,30-,31-,32+,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM81894

(Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@@H]2CCCCN2C(=O)[C@H]2CCC=NN2C1=O |c:55| Show InChI InChI=1S/C39H50N10O6/c1-3-23(2)33-39(55)49-32(13-8-15-43-49)38(54)48-16-7-6-12-30(48)36(52)45-29(19-25-21-40-22-42-25)37(53)47-17-9-14-31(47)35(51)44-28(34(50)46-33)18-24-20-41-27-11-5-4-10-26(24)27/h4-5,10-11,15,20-23,28-33,41H,3,6-9,12-14,16-19H2,1-2H3,(H,40,42)(H,44,51)(H,45,52)(H,46,50)/t23-,28+,29+,30-,31-,32+,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50053350
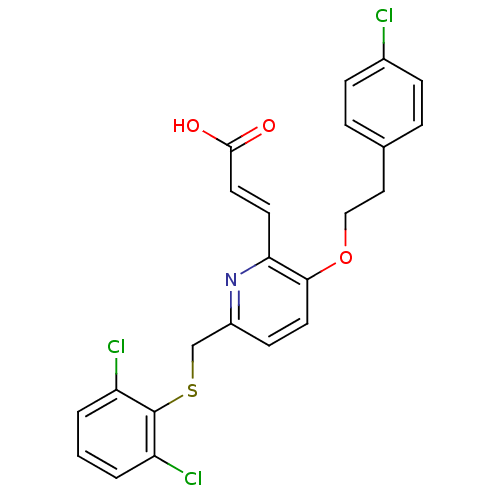
((E)-3-[3-[2-(4-Chloro-phenyl)-ethoxy]-6-(2,6-dichl...)Show SMILES OC(=O)\C=C\c1nc(CSc2c(Cl)cccc2Cl)ccc1OCCc1ccc(Cl)cc1 Show InChI InChI=1S/C23H18Cl3NO3S/c24-16-6-4-15(5-7-16)12-13-30-21-10-8-17(27-20(21)9-11-22(28)29)14-31-23-18(25)2-1-3-19(23)26/h1-11H,12-14H2,(H,28,29)/b11-9+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-LTB4 binding to human neutrophils |
J Med Chem 39: 3837-41 (1996)
Article DOI: 10.1021/jm960248s
BindingDB Entry DOI: 10.7270/Q2VX0FKP |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(BOVINE) | BDBM50021196
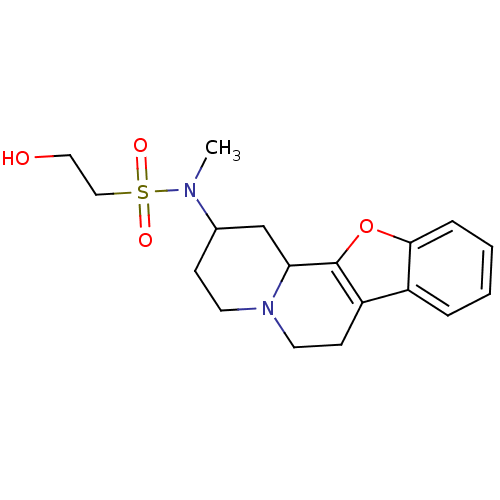
(2-Hydroxy-ethanesulfonic acid (1,3,4,5,6,11b-hexah...)Show SMILES CN(C1CCN2CCc3c(oc4ccccc34)C2C1)S(=O)(=O)CCO Show InChI InChI=1S/C18H24N2O4S/c1-19(25(22,23)11-10-21)13-6-8-20-9-7-15-14-4-2-3-5-17(14)24-18(15)16(20)12-13/h2-5,13,16,21H,6-12H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for its ability to displace [3H]-clonidine from alpha-2 adrenergic receptor of calf cerebral cortex |
J Med Chem 28: 1756-9 (1986)
BindingDB Entry DOI: 10.7270/Q2JW8CWS |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50053353
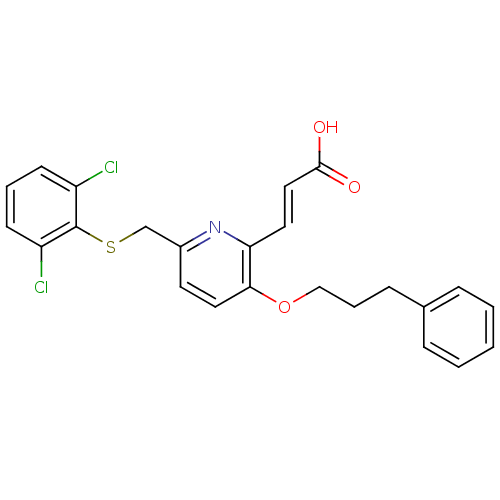
((E)-3-[6-(2,6-Dichloro-phenylsulfanylmethyl)-3-(3-...)Show SMILES OC(=O)\C=C\c1nc(CSc2c(Cl)cccc2Cl)ccc1OCCCc1ccccc1 Show InChI InChI=1S/C24H21Cl2NO3S/c25-19-9-4-10-20(26)24(19)31-16-18-11-13-22(21(27-18)12-14-23(28)29)30-15-5-8-17-6-2-1-3-7-17/h1-4,6-7,9-14H,5,8,15-16H2,(H,28,29)/b14-12+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-LTB4 binding to human neutrophils |
J Med Chem 39: 3837-41 (1996)
Article DOI: 10.1021/jm960248s
BindingDB Entry DOI: 10.7270/Q2VX0FKP |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50074791
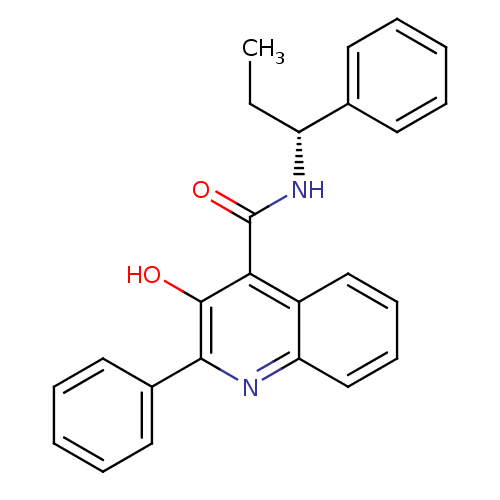
(3-Hydroxy-2-phenyl-quinoline-4-carboxylic acid ((R...)Show SMILES CC[C@@H](NC(=O)c1c(O)c(nc2ccccc12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C25H22N2O2/c1-2-20(17-11-5-3-6-12-17)27-25(29)22-19-15-9-10-16-21(19)26-23(24(22)28)18-13-7-4-8-14-18/h3-16,20,28H,2H2,1H3,(H,27,29)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A.
Curated by ChEMBL
| Assay Description
Binding affinity towards cloned human Tachykinin receptor 3 (hNK-3) expressed in CHO cells using [125I][MePhe7]-NKB |
J Med Chem 42: 1053-65 (1999)
Article DOI: 10.1021/jm980633c
BindingDB Entry DOI: 10.7270/Q2F47PTQ |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50016752

(CHEMBL339943 | N*1*-[1-(1-Carbamoyl-4-guanidino-bu...)Show SMILES CCOc1ccc(C[C@H]2NC(=O)CC3(CCCCC3)SCSC[C@@H](NC(=O)[C@@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)C(C)C)C(=O)N[C@H](CCCNC(N)=N)C(N)=O)cc1 Show InChI InChI=1S/C47H69N11O9S2/c1-4-67-31-17-15-30(16-18-31)23-33-41(62)55-34(22-29-12-7-5-8-13-29)43(64)58-39(28(2)3)45(66)56-35(24-37(48)59)42(63)57-36(44(65)54-32(40(49)61)14-11-21-52-46(50)51)26-68-27-69-47(25-38(60)53-33)19-9-6-10-20-47/h5,7-8,12-13,15-18,28,32-36,39H,4,6,9-11,14,19-27H2,1-3H3,(H2,48,59)(H2,49,61)(H,53,60)(H,54,65)(H,55,62)(H,56,66)(H,57,63)(H,58,64)(H4,50,51,52)/t32-,33-,34-,35-,36-,39+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories
Curated by ChEMBL
| Assay Description
Inhibition constant for vasopressin-stimulated adenylate cyclase (Vasopressin V2 receptor) of medullary membranes of human kidney |
J Med Chem 32: 391-6 (1989)
BindingDB Entry DOI: 10.7270/Q2GM8688 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50053351
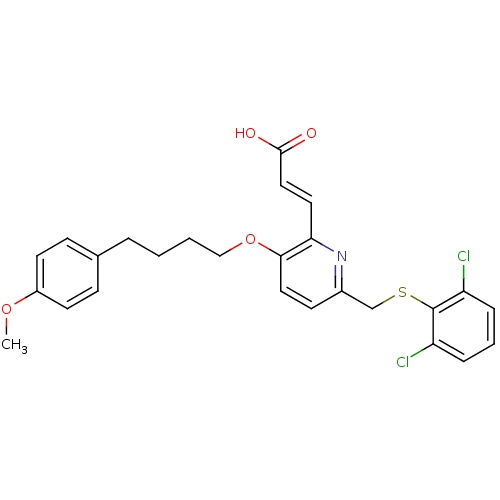
((E)-3-{6-(2,6-Dichloro-phenylsulfanylmethyl)-3-[4-...)Show SMILES COc1ccc(CCCCOc2ccc(CSc3c(Cl)cccc3Cl)nc2\C=C\C(O)=O)cc1 Show InChI InChI=1S/C26H25Cl2NO4S/c1-32-20-11-8-18(9-12-20)5-2-3-16-33-24-14-10-19(29-23(24)13-15-25(30)31)17-34-26-21(27)6-4-7-22(26)28/h4,6-15H,2-3,5,16-17H2,1H3,(H,30,31)/b15-13+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-LTB4 binding to human neutrophils |
J Med Chem 39: 3837-41 (1996)
Article DOI: 10.1021/jm960248s
BindingDB Entry DOI: 10.7270/Q2VX0FKP |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50053355
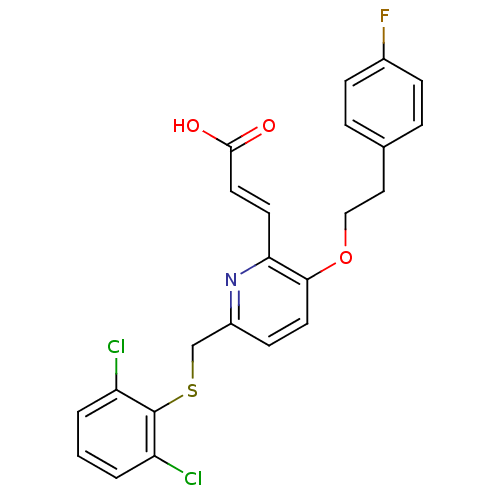
((E)-3-{6-(2,6-Dichloro-phenylsulfanylmethyl)-3-[2-...)Show SMILES OC(=O)\C=C\c1nc(CSc2c(Cl)cccc2Cl)ccc1OCCc1ccc(F)cc1 Show InChI InChI=1S/C23H18Cl2FNO3S/c24-18-2-1-3-19(25)23(18)31-14-17-8-10-21(20(27-17)9-11-22(28)29)30-13-12-15-4-6-16(26)7-5-15/h1-11H,12-14H2,(H,28,29)/b11-9+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-LTB4 binding to human neutrophils |
J Med Chem 39: 3837-41 (1996)
Article DOI: 10.1021/jm960248s
BindingDB Entry DOI: 10.7270/Q2VX0FKP |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50074785
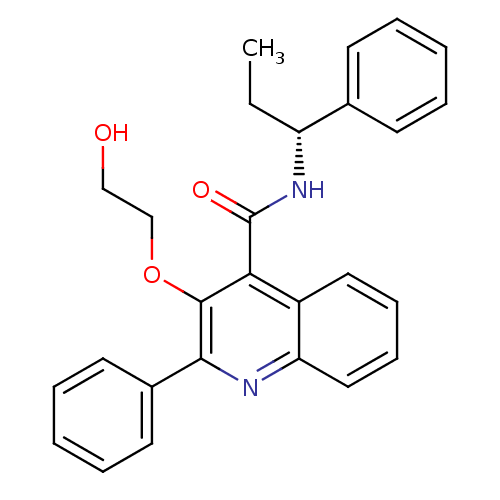
(3-(2-Hydroxy-ethoxy)-2-phenyl-quinoline-4-carboxyl...)Show SMILES CC[C@@H](NC(=O)c1c(OCCO)c(nc2ccccc12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C27H26N2O3/c1-2-22(19-11-5-3-6-12-19)29-27(31)24-21-15-9-10-16-23(21)28-25(26(24)32-18-17-30)20-13-7-4-8-14-20/h3-16,22,30H,2,17-18H2,1H3,(H,29,31)/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A.
Curated by ChEMBL
| Assay Description
Binding affinity towards cloned human Tachykinin receptor 3 (hNK-3) expressed in CHO cells using [125I][MePhe7]-NKB |
J Med Chem 42: 1053-65 (1999)
Article DOI: 10.1021/jm980633c
BindingDB Entry DOI: 10.7270/Q2F47PTQ |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor
(RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50021196

(2-Hydroxy-ethanesulfonic acid (1,3,4,5,6,11b-hexah...)Show SMILES CN(C1CCN2CCc3c(oc4ccccc34)C2C1)S(=O)(=O)CCO Show InChI InChI=1S/C18H24N2O4S/c1-19(25(22,23)11-10-21)13-6-8-20-9-7-15-14-4-2-3-5-17(14)24-18(15)16(20)12-13/h2-5,13,16,21H,6-12H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of hog kidney renin |
J Med Chem 28: 1756-9 (1986)
BindingDB Entry DOI: 10.7270/Q2JW8CWS |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(GUINEA PIG) | BDBM85845
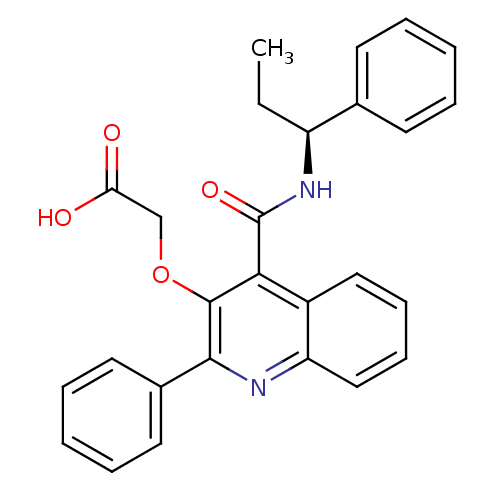
(N-[(S)-1-Phenylpropyl]-2-phenyl-3-(carboxymethoxy)...)Show SMILES CC[C@H](NC(=O)c1c(OCC(O)=O)c(nc2ccccc12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C27H24N2O4/c1-2-21(18-11-5-3-6-12-18)29-27(32)24-20-15-9-10-16-22(20)28-25(19-13-7-4-8-14-19)26(24)33-17-23(30)31/h3-16,21H,2,17H2,1H3,(H,29,32)(H,30,31)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 314-23 (2002)
Article DOI: 10.1124/jpet.300.1.314
BindingDB Entry DOI: 10.7270/Q2SB449D |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50408857
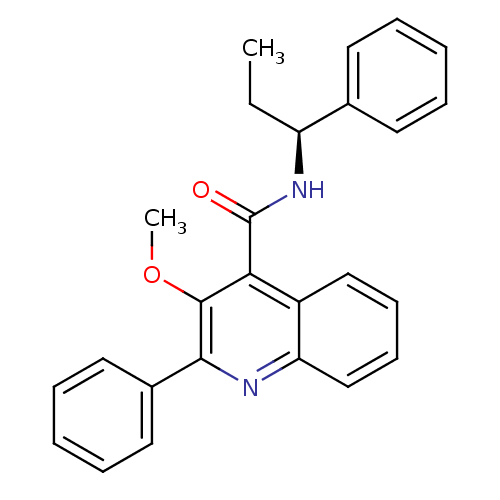
(CHEMBL2113673)Show SMILES CC[C@H](NC(=O)c1c(OC)c(nc2ccccc12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C26H24N2O2/c1-3-21(18-12-6-4-7-13-18)28-26(29)23-20-16-10-11-17-22(20)27-24(25(23)30-2)19-14-8-5-9-15-19/h4-17,21H,3H2,1-2H3,(H,28,29)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A.
Curated by ChEMBL
| Assay Description
Binding affinity towards cloned human Tachykinin receptor 3 (hNK-3) expressed in CHO cells using [125I][MePhe7]-NKB |
J Med Chem 42: 1053-65 (1999)
Article DOI: 10.1021/jm980633c
BindingDB Entry DOI: 10.7270/Q2F47PTQ |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50408858
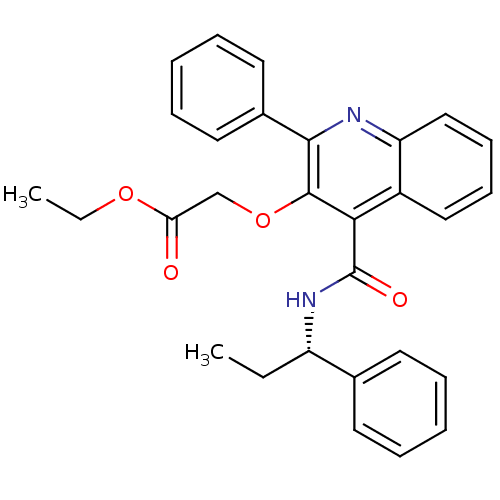
(CHEMBL2113680)Show SMILES CCOC(=O)COc1c(nc2ccccc2c1C(=O)N[C@@H](CC)c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C29H28N2O4/c1-3-23(20-13-7-5-8-14-20)31-29(33)26-22-17-11-12-18-24(22)30-27(21-15-9-6-10-16-21)28(26)35-19-25(32)34-4-2/h5-18,23H,3-4,19H2,1-2H3,(H,31,33)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A.
Curated by ChEMBL
| Assay Description
Binding affinity towards cloned human Tachykinin receptor 3 (hNK-3) expressed in CHO cells using [125I][MePhe7]-NKB |
J Med Chem 42: 1053-65 (1999)
Article DOI: 10.1021/jm980633c
BindingDB Entry DOI: 10.7270/Q2F47PTQ |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50526216
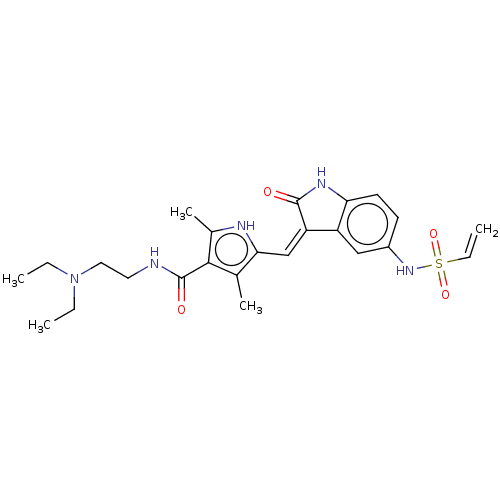
(CHEMBL4553725)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(NS(=O)(=O)C=C)cc23)c1C Show InChI InChI=1S/C24H31N5O4S/c1-6-29(7-2)12-11-25-24(31)22-15(4)21(26-16(22)5)14-19-18-13-17(28-34(32,33)8-3)9-10-20(18)27-23(19)30/h8-10,13-14,26,28H,3,6-7,11-12H2,1-2,4-5H3,(H,25,31)(H,27,30)/b19-14- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Technische Universit£t Darmstadt
Curated by ChEMBL
| Assay Description
Irreversible inhibition of recombinant human FLT3 D835Y mutant (571 to 993 residues) expressed in baculovirus expression system preincubated for 5 to... |
J Med Chem 62: 2428-2446 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01714
BindingDB Entry DOI: 10.7270/Q26113R9 |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50001609
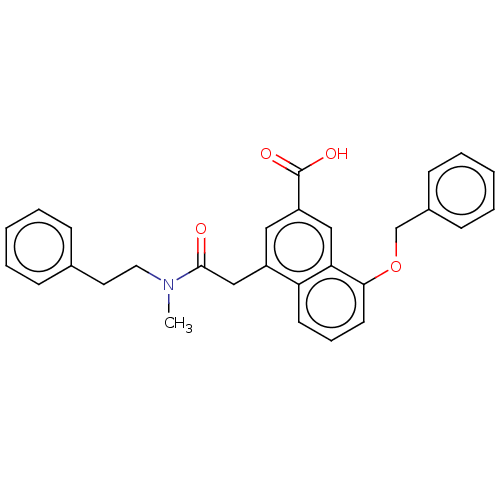
(8-Benzyloxy-4-[(methyl-phenethyl-carbamoyl)-methyl...)Show SMILES CN(CCc1ccccc1)C(=O)Cc1cc(cc2c(OCc3ccccc3)cccc12)C(O)=O Show InChI InChI=1S/C29H27NO4/c1-30(16-15-21-9-4-2-5-10-21)28(31)19-23-17-24(29(32)33)18-26-25(23)13-8-14-27(26)34-20-22-11-6-3-7-12-22/h2-14,17-18H,15-16,19-20H2,1H3,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity against LTB4 receptors in human PMNs using [3H]-LTB4 as radioligand |
J Med Chem 36: 2703-5 (1993)
BindingDB Entry DOI: 10.7270/Q2HQ3XZC |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50074779
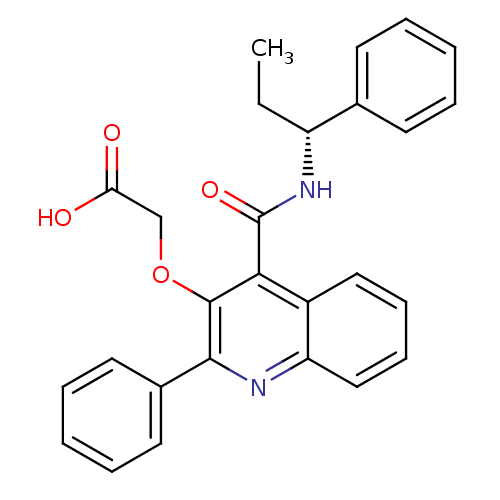
(CHEMBL10303 | [2-Phenyl-4-((R)-1-phenyl-propylcarb...)Show SMILES CC[C@@H](NC(=O)c1c(OCC(O)=O)c(nc2ccccc12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C27H24N2O4/c1-2-21(18-11-5-3-6-12-18)29-27(32)24-20-15-9-10-16-22(20)28-25(19-13-7-4-8-14-19)26(24)33-17-23(30)31/h3-16,21H,2,17H2,1H3,(H,29,32)(H,30,31)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A.
Curated by ChEMBL
| Assay Description
Binding affinity towards cloned human Tachykinin receptor 3 (hNK-3) expressed in CHO cells using [125I][MePhe7]-NKB |
J Med Chem 42: 1053-65 (1999)
Article DOI: 10.1021/jm980633c
BindingDB Entry DOI: 10.7270/Q2F47PTQ |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50074781
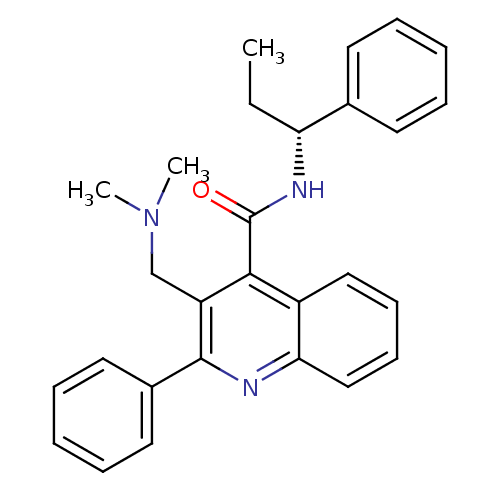
(3-Dimethylaminomethyl-2-phenyl-quinoline-4-carboxy...)Show SMILES CC[C@@H](NC(=O)c1c(CN(C)C)c(nc2ccccc12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H29N3O/c1-4-24(20-13-7-5-8-14-20)30-28(32)26-22-17-11-12-18-25(22)29-27(23(26)19-31(2)3)21-15-9-6-10-16-21/h5-18,24H,4,19H2,1-3H3,(H,30,32)/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A.
Curated by ChEMBL
| Assay Description
Binding affinity towards cloned human Tachykinin receptor 3 (hNK-3) expressed in CHO cells using [125I][MePhe7]-NKB |
J Med Chem 42: 1053-65 (1999)
Article DOI: 10.1021/jm980633c
BindingDB Entry DOI: 10.7270/Q2F47PTQ |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM85845

(N-[(S)-1-Phenylpropyl]-2-phenyl-3-(carboxymethoxy)...)Show SMILES CC[C@H](NC(=O)c1c(OCC(O)=O)c(nc2ccccc12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C27H24N2O4/c1-2-21(18-11-5-3-6-12-18)29-27(32)24-20-15-9-10-16-22(20)28-25(19-13-7-4-8-14-19)26(24)33-17-23(30)31/h3-16,21H,2,17H2,1H3,(H,29,32)(H,30,31)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 300: 314-23 (2002)
Article DOI: 10.1124/jpet.300.1.314
BindingDB Entry DOI: 10.7270/Q2SB449D |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Rhesus) | BDBM81894

(Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@@H]2CCCCN2C(=O)[C@H]2CCC=NN2C1=O |c:55| Show InChI InChI=1S/C39H50N10O6/c1-3-23(2)33-39(55)49-32(13-8-15-43-49)38(54)48-16-7-6-12-30(48)36(52)45-29(19-25-21-40-22-42-25)37(53)47-17-9-14-31(47)35(51)44-28(34(50)46-33)18-24-20-41-27-11-5-4-10-26(24)27/h4-5,10-11,15,20-23,28-33,41H,3,6-9,12-14,16-19H2,1-2H3,(H,40,42)(H,44,51)(H,45,52)(H,46,50)/t23-,28+,29+,30-,31-,32+,33-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50074794

(3-Dimethylcarbamoylmethoxy-2-phenyl-quinoline-4-ca...)Show SMILES CC[C@@H](NC(=O)c1c(OCC(=O)N(C)C)c(nc2ccccc12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H29N3O3/c1-4-23(20-13-7-5-8-14-20)31-29(34)26-22-17-11-12-18-24(22)30-27(21-15-9-6-10-16-21)28(26)35-19-25(33)32(2)3/h5-18,23H,4,19H2,1-3H3,(H,31,34)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A.
Curated by ChEMBL
| Assay Description
Binding affinity towards cloned human Tachykinin receptor 3 (hNK-3) expressed in CHO cells using [125I][MePhe7]-NKB |
J Med Chem 42: 1053-65 (1999)
Article DOI: 10.1021/jm980633c
BindingDB Entry DOI: 10.7270/Q2F47PTQ |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50074819
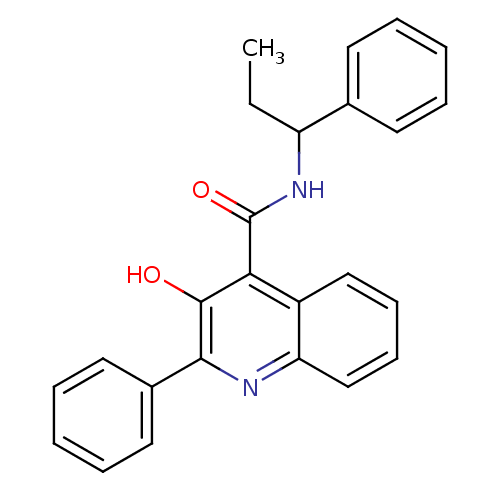
(3-Hydroxy-2-phenyl-quinoline-4-carboxylic acid (1-...)Show SMILES CCC(NC(=O)c1c(O)c(nc2ccccc12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C25H22N2O2/c1-2-20(17-11-5-3-6-12-17)27-25(29)22-19-15-9-10-16-21(19)26-23(24(22)28)18-13-7-4-8-14-18/h3-16,20,28H,2H2,1H3,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A.
Curated by ChEMBL
| Assay Description
Binding affinity towards cloned human Tachykinin receptor 3 (hNK-3) expressed in CHO cells using [125I][MePhe7]-NKB |
J Med Chem 42: 1053-65 (1999)
Article DOI: 10.1021/jm980633c
BindingDB Entry DOI: 10.7270/Q2F47PTQ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(RAT) | BDBM81894

(Cyclo[L-Pro-D-Trp-L-Ile-1,6-didehydro-D-Pyz-L-Pip-...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](Cc2cnc[nH]2)NC(=O)[C@@H]2CCCCN2C(=O)[C@H]2CCC=NN2C1=O |c:55| Show InChI InChI=1S/C39H50N10O6/c1-3-23(2)33-39(55)49-32(13-8-15-43-49)38(54)48-16-7-6-12-30(48)36(52)45-29(19-25-21-40-22-42-25)37(53)47-17-9-14-31(47)35(51)44-28(34(50)46-33)18-24-20-41-27-11-5-4-10-26(24)27/h4-5,10-11,15,20-23,28-33,41H,3,6-9,12-14,16-19H2,1-2H3,(H,40,42)(H,44,51)(H,45,52)(H,46,50)/t23-,28+,29+,30-,31-,32+,33-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 256: 304-8 (1991)
BindingDB Entry DOI: 10.7270/Q2028Q1G |
More data for this
Ligand-Target Pair | |
Leukotriene B4 receptor 1
(Homo sapiens (Human)) | BDBM50053356
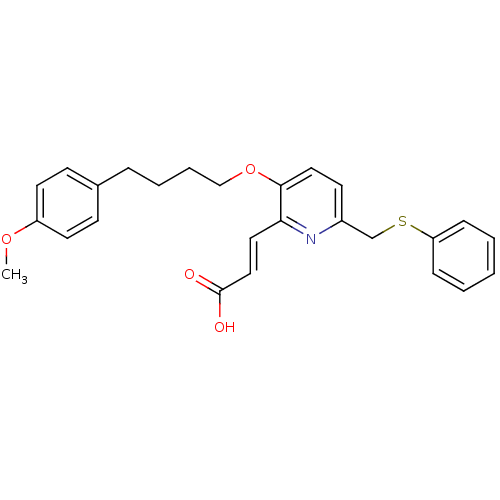
((E)-3-{3-[4-(4-Methoxy-phenyl)-butoxy]-6-phenylsul...)Show SMILES COc1ccc(CCCCOc2ccc(CSc3ccccc3)nc2\C=C\C(O)=O)cc1 Show InChI InChI=1S/C26H27NO4S/c1-30-22-13-10-20(11-14-22)7-5-6-18-31-25-16-12-21(27-24(25)15-17-26(28)29)19-32-23-8-3-2-4-9-23/h2-4,8-17H,5-7,18-19H2,1H3,(H,28,29)/b17-15+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-LTB4 binding to human neutrophils |
J Med Chem 39: 3837-41 (1996)
Article DOI: 10.1021/jm960248s
BindingDB Entry DOI: 10.7270/Q2VX0FKP |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50001450

((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...)Show SMILES [#6]-[#16]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccccc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O |r| Show InChI InChI=1S/C63H98N18O13S/c1-37(2)33-45(57(89)74-41(53(68)85)27-32-95-3)73-52(84)36-72-54(86)46(34-38-15-6-4-7-16-38)78-58(90)47(35-39-17-8-5-9-18-39)79-56(88)42(23-25-50(66)82)75-55(87)43(24-26-51(67)83)76-59(91)49-22-14-31-81(49)62(94)44(20-10-11-28-64)77-60(92)48-21-13-30-80(48)61(93)40(65)19-12-29-71-63(69)70/h4-9,15-18,37,40-49H,10-14,19-36,64-65H2,1-3H3,(H2,66,82)(H2,67,83)(H2,68,85)(H,72,86)(H,73,84)(H,74,89)(H,75,87)(H,76,91)(H,77,92)(H,78,90)(H,79,88)(H4,69,70,71)/t40-,41-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 281: 1303-11 (1997)
BindingDB Entry DOI: 10.7270/Q2K35S61 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50050650

((S)-N-{(S)-1-[(S)-1-({[(S)-1-((S)-1-Carbamoyl-3-me...)Show SMILES CSCCC(NC(=O)C(CC(C)C)NC(=O)CNC(=O)C(Cc1ccccc1)N(C)C(=O)C(Cc1ccccc1)NC(=O)C(CC(O)=O)NC(=O)CCC(O)=O)C(N)=O Show InChI InChI=1S/C40H55N7O11S/c1-24(2)19-28(37(55)45-27(36(41)54)17-18-59-4)44-33(49)23-42-39(57)31(21-26-13-9-6-10-14-26)47(3)40(58)30(20-25-11-7-5-8-12-25)46-38(56)29(22-35(52)53)43-32(48)15-16-34(50)51/h5-14,24,27-31H,15-23H2,1-4H3,(H2,41,54)(H,42,57)(H,43,48)(H,44,49)(H,45,55)(H,46,56)(H,50,51)(H,52,53) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 281: 1303-11 (1997)
BindingDB Entry DOI: 10.7270/Q2K35S61 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data