

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM20608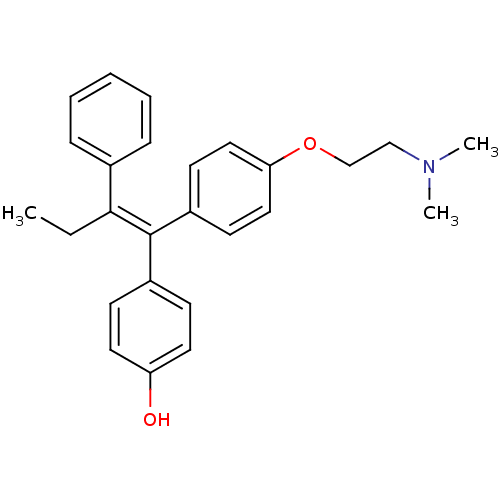 (4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SignalGene Inc. Curated by ChEMBL | Assay Description In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor beta | J Med Chem 46: 1408-18 (2003) Article DOI: 10.1021/jm020536q BindingDB Entry DOI: 10.7270/Q20R9Q5J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM20608 (4-Hydroxytamoxifen | 4-Hydroxytamoxifen (9) | 4-[(...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SignalGene Inc. Curated by ChEMBL | Assay Description In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor alpha | J Med Chem 46: 1408-18 (2003) Article DOI: 10.1021/jm020536q BindingDB Entry DOI: 10.7270/Q20R9Q5J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM20625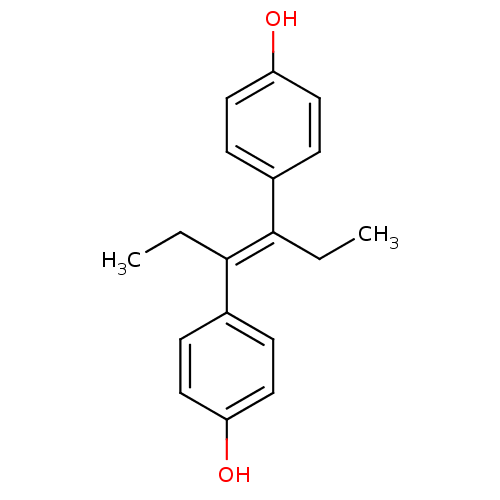 (4-[(3E)-4-(4-hydroxyphenyl)hex-3-en-3-yl]phenol | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SignalGene Inc. Curated by ChEMBL | Assay Description In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor alpha | J Med Chem 46: 1408-18 (2003) Article DOI: 10.1021/jm020536q BindingDB Entry DOI: 10.7270/Q20R9Q5J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM20625 (4-[(3E)-4-(4-hydroxyphenyl)hex-3-en-3-yl]phenol | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SignalGene Inc. Curated by ChEMBL | Assay Description In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor beta | J Med Chem 46: 1408-18 (2003) Article DOI: 10.1021/jm020536q BindingDB Entry DOI: 10.7270/Q20R9Q5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50169743 ((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SignalGene Inc. Curated by ChEMBL | Assay Description In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor alpha | J Med Chem 46: 1408-18 (2003) Article DOI: 10.1021/jm020536q BindingDB Entry DOI: 10.7270/Q20R9Q5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50169743 ((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SignalGene Inc. Curated by ChEMBL | Assay Description In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor beta | J Med Chem 46: 1408-18 (2003) Article DOI: 10.1021/jm020536q BindingDB Entry DOI: 10.7270/Q20R9Q5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50292348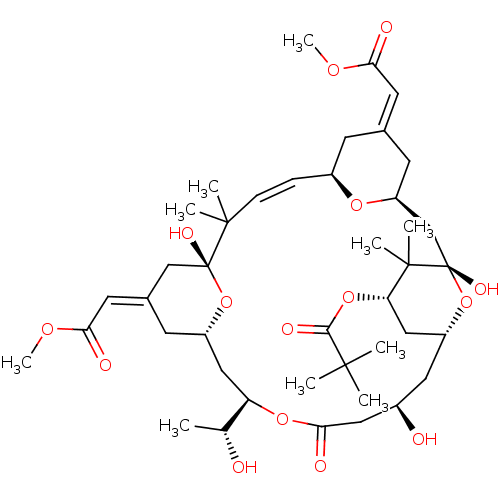 (CHEMBL501262 | bryostatin 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arizona State University Curated by ChEMBL | Assay Description Displacement of [3H]PDBu form PKCalpha | J Nat Prod 59: 286-9 (1997) Article DOI: 10.1021/np960100b BindingDB Entry DOI: 10.7270/Q2FQ9WNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50292349 (CHEMBL505767 | bryostatin 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arizona State University Curated by ChEMBL | Assay Description Displacement of [3H]PDBu form PKCalpha | J Nat Prod 59: 286-9 (1997) Article DOI: 10.1021/np960100b BindingDB Entry DOI: 10.7270/Q2FQ9WNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50126767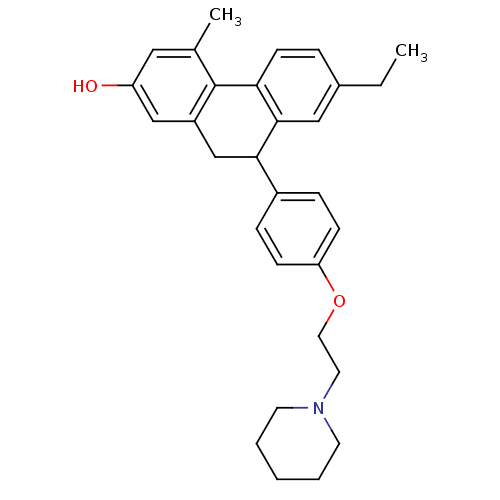 (7-Ethyl-4-methyl-9-[4-(2-piperidin-1-yl-ethoxy)-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 8.69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SignalGene Inc. Curated by ChEMBL | Assay Description In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor alpha | J Med Chem 46: 1408-18 (2003) Article DOI: 10.1021/jm020536q BindingDB Entry DOI: 10.7270/Q20R9Q5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50267297 (CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) assessed as midazolam 1'- hydroxylation | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50267295 (CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) assessed as midazolam 1'- hydroxylation | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50292351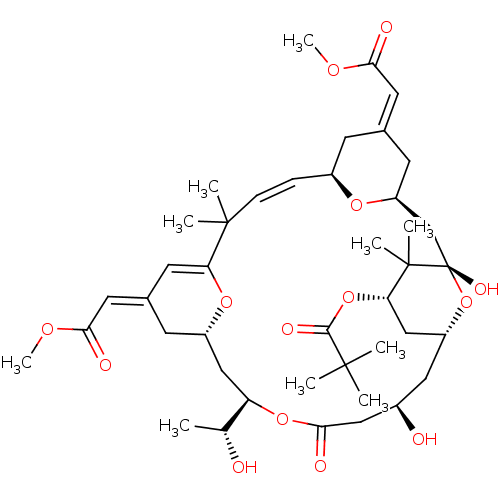 (CHEMBL509516 | bryostatin 16) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arizona State University Curated by ChEMBL | Assay Description Displacement of [3H]PDBu form PKCalpha | J Nat Prod 59: 286-9 (1997) Article DOI: 10.1021/np960100b BindingDB Entry DOI: 10.7270/Q2FQ9WNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50126766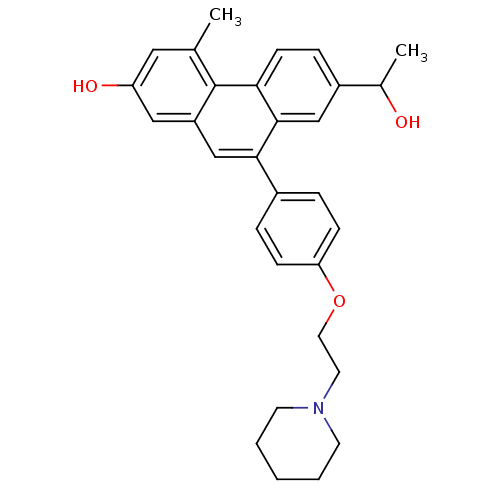 (7-(1-Hydroxy-ethyl)-4-methyl-9-[4-(2-piperidin-1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SignalGene Inc. Curated by ChEMBL | Assay Description In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor alpha | J Med Chem 46: 1408-18 (2003) Article DOI: 10.1021/jm020536q BindingDB Entry DOI: 10.7270/Q20R9Q5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50126772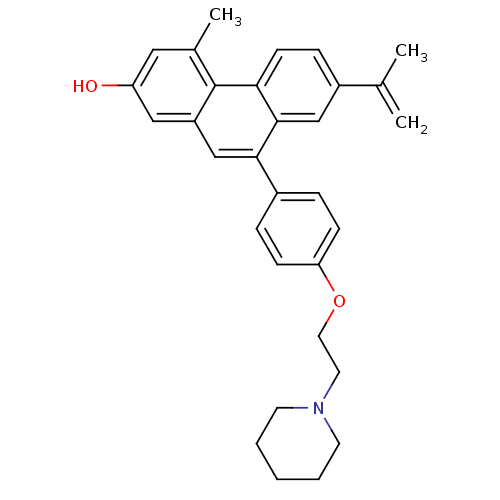 (7-Isopropenyl-4-methyl-9-[4-(2-piperidin-1-yl-etho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SignalGene Inc. Curated by ChEMBL | Assay Description In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor beta | J Med Chem 46: 1408-18 (2003) Article DOI: 10.1021/jm020536q BindingDB Entry DOI: 10.7270/Q20R9Q5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50292350 (CHEMBL452391 | bryostatin 17 | delta 19,20-bryosta...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arizona State University Curated by ChEMBL | Assay Description Displacement of [3H]PDBu form PKCalpha | J Nat Prod 59: 286-9 (1997) Article DOI: 10.1021/np960100b BindingDB Entry DOI: 10.7270/Q2FQ9WNK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50126767 (7-Ethyl-4-methyl-9-[4-(2-piperidin-1-yl-ethoxy)-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SignalGene Inc. Curated by ChEMBL | Assay Description In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor beta | J Med Chem 46: 1408-18 (2003) Article DOI: 10.1021/jm020536q BindingDB Entry DOI: 10.7270/Q20R9Q5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50126766 (7-(1-Hydroxy-ethyl)-4-methyl-9-[4-(2-piperidin-1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SignalGene Inc. Curated by ChEMBL | Assay Description In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor beta | J Med Chem 46: 1408-18 (2003) Article DOI: 10.1021/jm020536q BindingDB Entry DOI: 10.7270/Q20R9Q5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50126770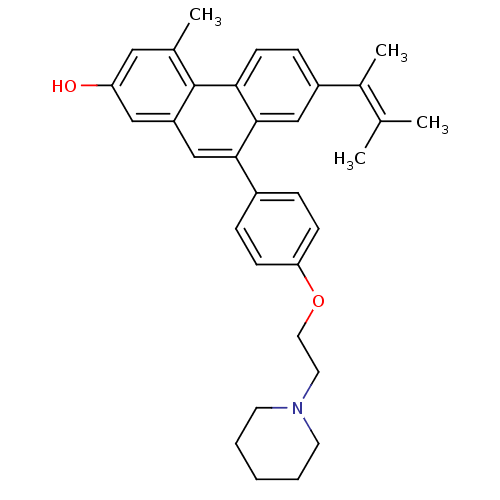 (7-(1,2-Dimethyl-propenyl)-4-methyl-9-[4-(2-piperid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SignalGene Inc. Curated by ChEMBL | Assay Description In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor beta | J Med Chem 46: 1408-18 (2003) Article DOI: 10.1021/jm020536q BindingDB Entry DOI: 10.7270/Q20R9Q5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50126770 (7-(1,2-Dimethyl-propenyl)-4-methyl-9-[4-(2-piperid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SignalGene Inc. Curated by ChEMBL | Assay Description In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor alpha | J Med Chem 46: 1408-18 (2003) Article DOI: 10.1021/jm020536q BindingDB Entry DOI: 10.7270/Q20R9Q5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50126772 (7-Isopropenyl-4-methyl-9-[4-(2-piperidin-1-yl-etho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SignalGene Inc. Curated by ChEMBL | Assay Description In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor alpha | J Med Chem 46: 1408-18 (2003) Article DOI: 10.1021/jm020536q BindingDB Entry DOI: 10.7270/Q20R9Q5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50126768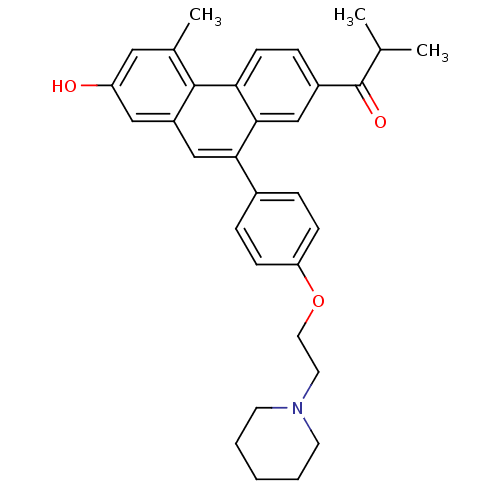 (1-{7-Hydroxy-5-methyl-10-[4-(2-piperidin-1-yl-etho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SignalGene Inc. Curated by ChEMBL | Assay Description In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor alpha | J Med Chem 46: 1408-18 (2003) Article DOI: 10.1021/jm020536q BindingDB Entry DOI: 10.7270/Q20R9Q5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50267295 (CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of cathepsin D (unknown origin) | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50126771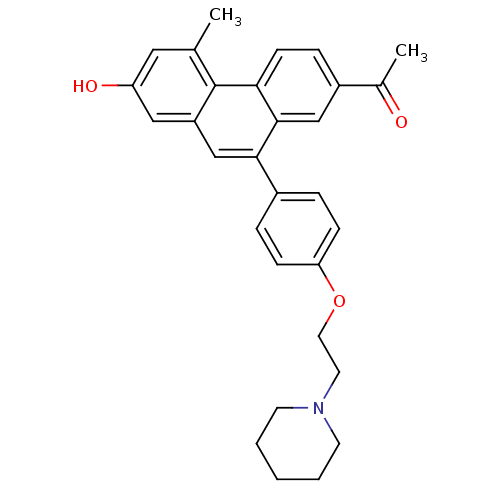 (1-{7-Hydroxy-5-methyl-10-[4-(2-piperidin-1-yl-etho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SignalGene Inc. Curated by ChEMBL | Assay Description In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor beta | J Med Chem 46: 1408-18 (2003) Article DOI: 10.1021/jm020536q BindingDB Entry DOI: 10.7270/Q20R9Q5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50126771 (1-{7-Hydroxy-5-methyl-10-[4-(2-piperidin-1-yl-etho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SignalGene Inc. Curated by ChEMBL | Assay Description In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor alpha | J Med Chem 46: 1408-18 (2003) Article DOI: 10.1021/jm020536q BindingDB Entry DOI: 10.7270/Q20R9Q5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50126768 (1-{7-Hydroxy-5-methyl-10-[4-(2-piperidin-1-yl-etho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SignalGene Inc. Curated by ChEMBL | Assay Description In vitro displacement of 0.5 nM [3H]17-beta-estradiol from human Estrogen receptor beta | J Med Chem 46: 1408-18 (2003) Article DOI: 10.1021/jm020536q BindingDB Entry DOI: 10.7270/Q20R9Q5J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50267297 (CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50267297 (CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of cathepsin D (unknown origin) | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50267295 (CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM19235 (2-{5-[4-({[(2,4-difluorophenyl)methyl](3-methanesu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19234 (4-{4-[3-(4-{[benzyl(3-methanesulfonamido-2-methylp...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | 86 | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM19234 (4-{4-[3-(4-{[benzyl(3-methanesulfonamido-2-methylp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 1.10 | n/a | 4.80 | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (RAT) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19236 (2-{2-bromo-5-[4-({[(2,4-difluorophenyl)methyl](3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | 330 | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM19238 (2-{2-chloro-5-[4-({[(2,4-difluorophenyl)methyl](3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM19237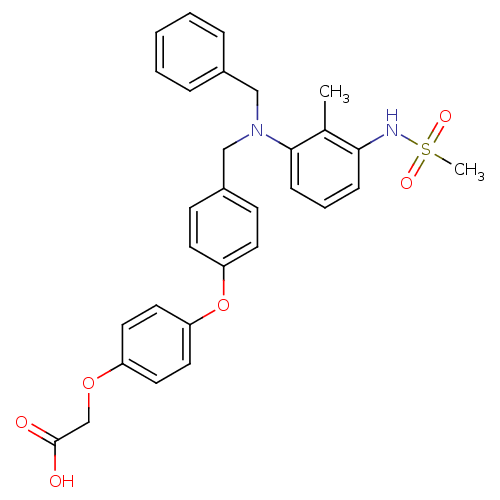 (2-[4-(4-{[benzyl(3-methanesulfonamido-2-methylphen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (RAT) | BDBM19233 ((2R,10S,11S,14S,15S)-14-hydroxy-15-methyl-2-[(4-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19233 ((2R,10S,11S,14S,15S)-14-hydroxy-15-methyl-2-[(4-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | 43 | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19238 (2-{2-chloro-5-[4-({[(2,4-difluorophenyl)methyl](3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | 500 | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19235 (2-{5-[4-({[(2,4-difluorophenyl)methyl](3-methanesu...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | 440 | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM19237 (2-[4-(4-{[benzyl(3-methanesulfonamido-2-methylphen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40 | n/a | 255 | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the 3(H)-dexamethasone from the receptor an IC50 value (the concentration required to inhibit 50% of the binding of 3(... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM19234 (4-{4-[3-(4-{[benzyl(3-methanesulfonamido-2-methylp...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM19236 (2-{2-bromo-5-[4-({[(2,4-difluorophenyl)methyl](3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM19238 (2-{2-chloro-5-[4-({[(2,4-difluorophenyl)methyl](3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM19235 (2-{5-[4-({[(2,4-difluorophenyl)methyl](3-methanesu...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM19234 (4-{4-[3-(4-{[benzyl(3-methanesulfonamido-2-methylp...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM19237 (2-[4-(4-{[benzyl(3-methanesulfonamido-2-methylphen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM19238 (2-{2-chloro-5-[4-({[(2,4-difluorophenyl)methyl](3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 4 |
Abbott Laboratories | Assay Description For compounds able to displace the radiolabeled ligand (competitor) from the receptor an IC50 value (the concentration required to inhibit 50% of the... | J Med Chem 48: 5295-304 (2005) Article DOI: 10.1021/jm050205o BindingDB Entry DOI: 10.7270/Q21C1V5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 222 total ) | Next | Last >> |