 Found 1506 hits with Last Name = 'schubert-zsilavecz' and Initial = 'm'
Found 1506 hits with Last Name = 'schubert-zsilavecz' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nuclear receptor subfamily 5 group A member 2
(Homo sapiens (Human)) | BDBM50418303
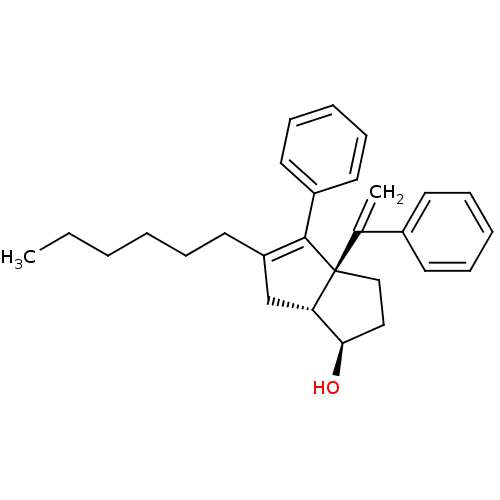
(CHEMBL1765959)Show SMILES CCCCCCC1=C(c2ccccc2)[C@@]2(CC[C@@H](O)[C@@H]2C1)C(=C)c1ccccc1 |r,c:6| Show InChI InChI=1S/C28H34O/c1-3-4-5-8-17-24-20-25-26(29)18-19-28(25,21(2)22-13-9-6-10-14-22)27(24)23-15-11-7-12-16-23/h6-7,9-16,25-26,29H,2-5,8,17-20H2,1H3/t25-,26+,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor subfamily 5 group A member 2
(Homo sapiens (Human)) | CHEMBL5271417

Show SMILES CCOc1nc2ccccc2nc1C(=O)N1CCN(CC1)c1ccc(OC)cc1 Show InChI InChI=1S/C22H24N4O3/c1-3-29-21-20(23-18-6-4-5-7-19(18)24-21)22(27)26-14-12-25(13-15-26)16-8-10-17(28-2)11-9-16/h4-11H,3,12-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50264106

(CHEMBL3818875)Show SMILES CNc1nc(C)nc(N[C@H]2CCC[C@H](C2)C(=O)NCc2ccc(cc2C(F)(F)F)C#N)n1 |r| Show InChI InChI=1S/C21H24F3N7O/c1-12-28-19(26-2)31-20(29-12)30-16-5-3-4-14(9-16)18(32)27-11-15-7-6-13(10-25)8-17(15)21(22,23)24/h6-8,14,16H,3-5,9,11H2,1-2H3,(H,27,32)(H2,26,28,29,30,31)/t14-,16+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561492
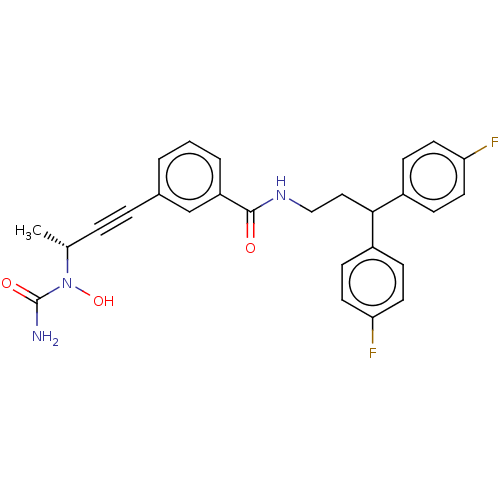
(CHEMBL4800490)Show SMILES C[C@H](C#Cc1cccc(c1)C(=O)NCCC(c1ccc(F)cc1)c1ccc(F)cc1)N(O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561490
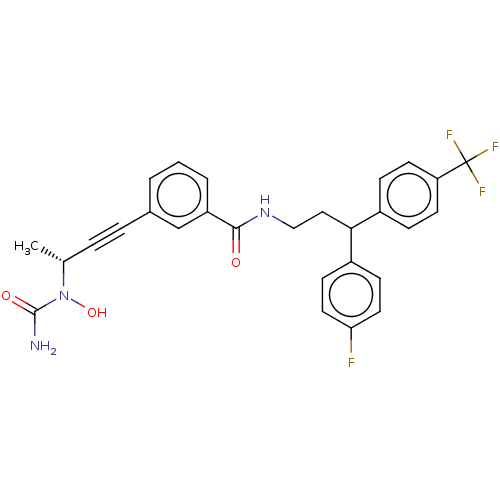
(CHEMBL4746942)Show SMILES C[C@H](C#Cc1cccc(c1)C(=O)NCCC(c1ccc(F)cc1)c1ccc(cc1)C(F)(F)F)N(O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561482
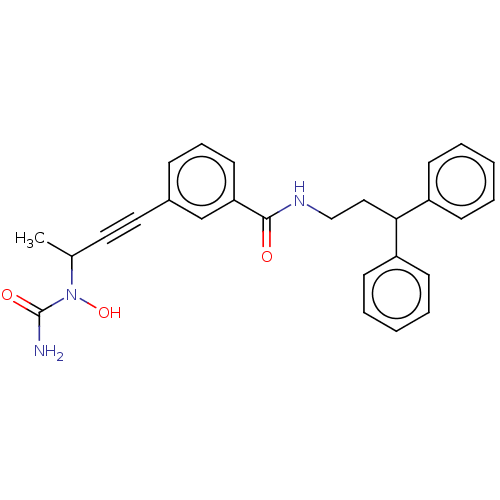
(CHEMBL4764099)Show SMILES CC(C#Cc1cccc(c1)C(=O)NCCC(c1ccccc1)c1ccccc1)N(O)C(N)=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561483

(CHEMBL4795110)Show SMILES CC(C#Cc1cccc(c1)C(=O)NCCC(c1ccccc1)c1ccc(F)cc1)N(O)C(N)=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561486

(CHEMBL4759111)Show SMILES CC(C#Cc1cccc(c1)C(=O)NCCC(c1ccccc1)c1ccc(Cl)cc1)N(O)C(N)=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561489

(CHEMBL4745687)Show SMILES C[C@H](C#Cc1cccc(c1)C(=O)NCCC(c1ccccc1)c1ccc(Cl)c(Cl)c1)N(O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561488

(CHEMBL4745452)Show SMILES C[C@H](C#Cc1cccc(c1)C(=O)NCCC(c1ccccc1)c1ccc(cc1)S(N)(=O)=O)N(O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561491
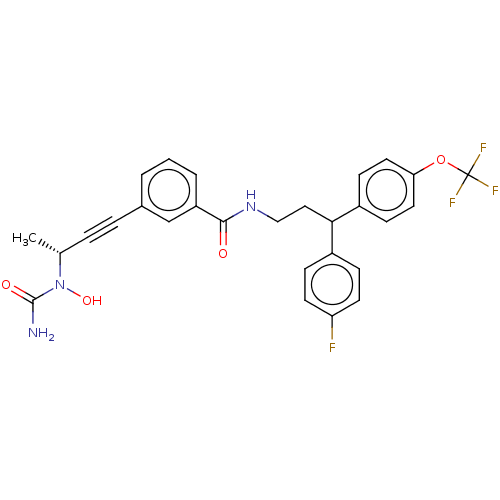
(CHEMBL4755533)Show SMILES C[C@H](C#Cc1cccc(c1)C(=O)NCCC(c1ccc(F)cc1)c1ccc(OC(F)(F)F)cc1)N(O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561484
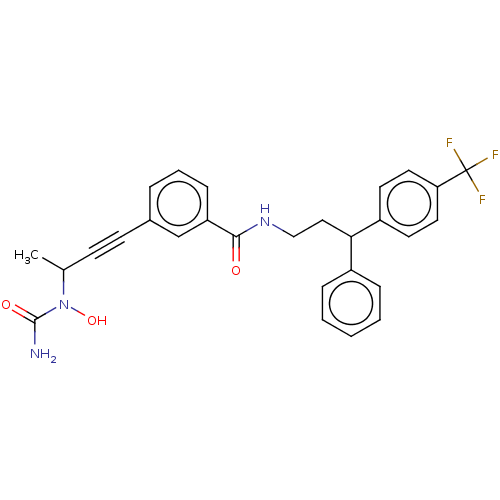
(CHEMBL4778283)Show SMILES CC(C#Cc1cccc(c1)C(=O)NCCC(c1ccccc1)c1ccc(cc1)C(F)(F)F)N(O)C(N)=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50497566
(CHEMBL3344193)Show InChI InChI=1S/C21H21ClN2O2/c1-25-21-12-18(14-24-13-16-8-10-23-11-9-16)4-7-20(21)26-15-17-2-5-19(22)6-3-17/h2-12,24H,13-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann-Wolfgang-Goethe University of Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of Plk1 immunoprecipitated from human HeLa cells using casein substrate after 12 to 26 hrs by autoradiography |
Bioorg Med Chem Lett 24: 5063-9 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.015
BindingDB Entry DOI: 10.7270/Q26Q2171 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561487

(CHEMBL4759652)Show SMILES C[C@H](C#Cc1cccc(c1)C(=O)NCCC(c1ccccc1)c1ccc(NS(C)(=O)=O)cc1)N(O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561495
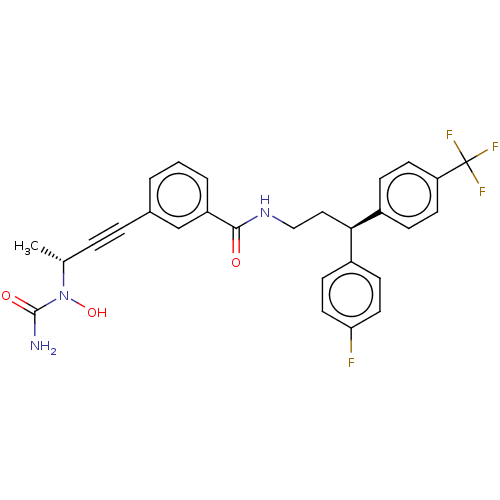
(CHEMBL4794423)Show SMILES C[C@H](C#Cc1cccc(c1)C(=O)NCC[C@H](c1ccc(F)cc1)c1ccc(cc1)C(F)(F)F)N(O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561494
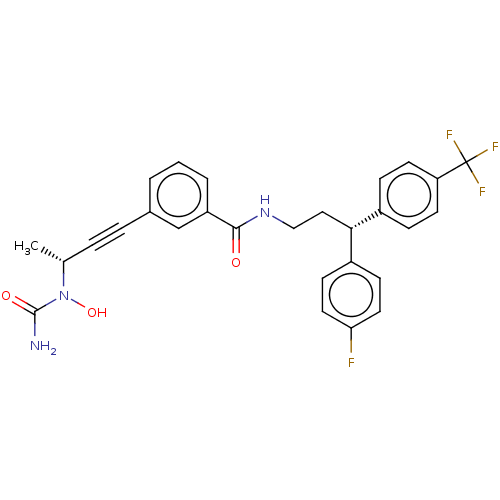
(CHEMBL4751593)Show SMILES C[C@H](C#Cc1cccc(c1)C(=O)NCC[C@@H](c1ccc(F)cc1)c1ccc(cc1)C(F)(F)F)N(O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561480
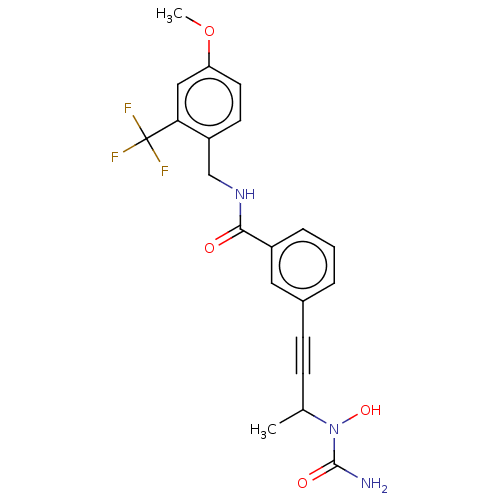
(CHEMBL4791222)Show SMILES COc1ccc(CNC(=O)c2cccc(c2)C#CC(C)N(O)C(N)=O)c(c1)C(F)(F)F | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561493
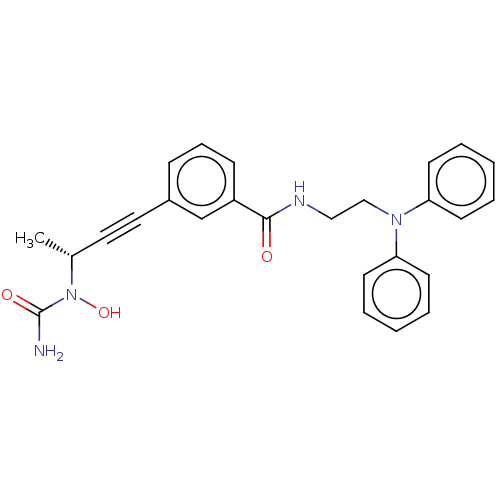
(CHEMBL4747688)Show SMILES C[C@H](C#Cc1cccc(c1)C(=O)NCCN(c1ccccc1)c1ccccc1)N(O)C(N)=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561474
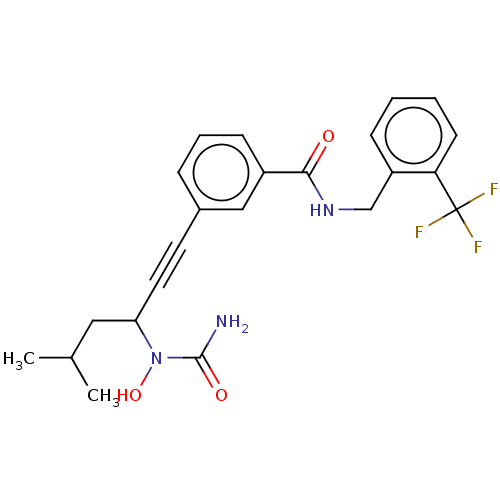
(CHEMBL4746544)Show SMILES CC(C)CC(C#Cc1cccc(c1)C(=O)NCc1ccccc1C(F)(F)F)N(O)C(N)=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
ZAFES/LiFF/Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 after 5 mins |
J Med Chem 53: 4691-700 (2010)
Article DOI: 10.1021/jm1003073
BindingDB Entry DOI: 10.7270/Q2N016PB |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Ovis aries (Sheep)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe-University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX-1 assessed as residual activity by measuring formation of 12-HHT from arachidonic acid by HPLC analysis |
Bioorg Med Chem 19: 5372-82 (2011)
Article DOI: 10.1016/j.bmc.2011.08.003
BindingDB Entry DOI: 10.7270/Q2JQ11D0 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50561485

(CHEMBL4757630)Show SMILES CC(C#Cc1cccc(c1)C(=O)NCCC(c1ccccc1)c1ccc(OC(F)(F)F)cc1)N(O)C(N)=O | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH (1 to 555 residues) expressed in Escherichia coli BL21-(DE3) using PHOME substrate incubated for 30 to 45 mins by fluorescenc... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00561
BindingDB Entry DOI: 10.7270/Q2ST7TJS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50006805

(3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...)Show SMILES CC(C)c1ccc2n(Cc3ccc(Cl)cc3)c(CC(C)(C)C(O)=O)c(SC(C)(C)C)c2c1 Show InChI InChI=1S/C27H34ClNO2S/c1-17(2)19-10-13-22-21(14-19)24(32-26(3,4)5)23(15-27(6,7)25(30)31)29(22)16-18-8-11-20(28)12-9-18/h8-14,17H,15-16H2,1-7H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM22334

(BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...)Show InChI InChI=1S/C17H17NO3/c1-14(19)18(20)12-6-8-15-7-5-11-17(13-15)21-16-9-3-2-4-10-16/h2-11,13,20H,12H2,1H3/b8-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273442
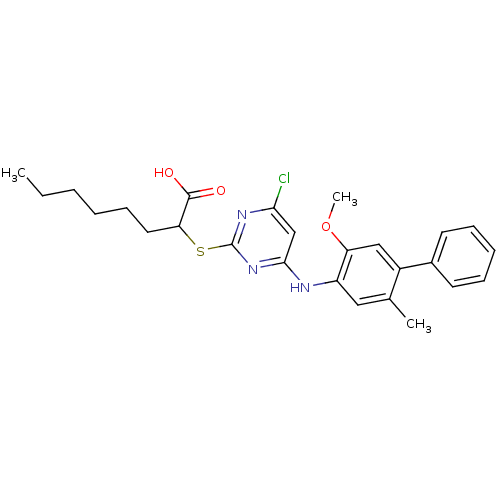
(2-(4-Chloro-6-(5-methoxy-2-methylbiphenyl-4-ylamin...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2cc(C)c(cc2OC)-c2ccccc2)n1)C(O)=O Show InChI InChI=1S/C26H30ClN3O3S/c1-4-5-6-10-13-22(25(31)32)34-26-29-23(27)16-24(30-26)28-20-14-17(2)19(15-21(20)33-3)18-11-8-7-9-12-18/h7-9,11-12,14-16,22H,4-6,10,13H2,1-3H3,(H,31,32)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273441
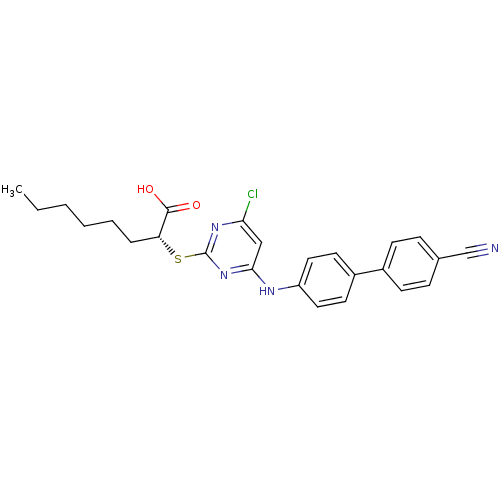
((R)-2-(4-Chloro-6-(4'-cyanobiphenyl-4-ylamino)pyri...)Show SMILES CCCCCC[C@@H](Sc1nc(Cl)cc(Nc2ccc(cc2)-c2ccc(cc2)C#N)n1)C(O)=O |r| Show InChI InChI=1S/C25H25ClN4O2S/c1-2-3-4-5-6-21(24(31)32)33-25-29-22(26)15-23(30-25)28-20-13-11-19(12-14-20)18-9-7-17(16-27)8-10-18/h7-15,21H,2-6H2,1H3,(H,31,32)(H,28,29,30)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273390
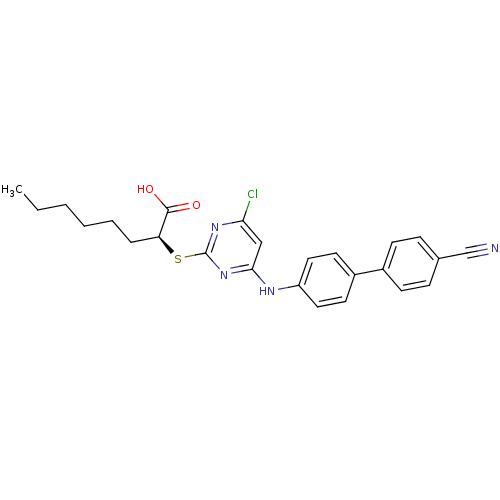
((S)-2-(4-Chloro-6-(4'-cyanobiphenyl-4-ylamino)pyri...)Show SMILES CCCCCC[C@H](Sc1nc(Cl)cc(Nc2ccc(cc2)-c2ccc(cc2)C#N)n1)C(O)=O |r| Show InChI InChI=1S/C25H25ClN4O2S/c1-2-3-4-5-6-21(24(31)32)33-25-29-22(26)15-23(30-25)28-20-13-11-19(12-14-20)18-9-7-17(16-27)8-10-18/h7-15,21H,2-6H2,1H3,(H,31,32)(H,28,29,30)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273389
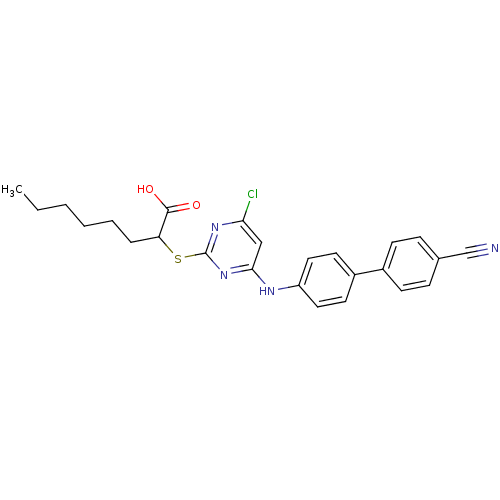
(2-(4-Chloro-6-(4'-cyanobiphenyl-4-ylamino)pyrimidi...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2ccc(cc2)-c2ccc(cc2)C#N)n1)C(O)=O Show InChI InChI=1S/C25H25ClN4O2S/c1-2-3-4-5-6-21(24(31)32)33-25-29-22(26)15-23(30-25)28-20-13-11-19(12-14-20)18-9-7-17(16-27)8-10-18/h7-15,21H,2-6H2,1H3,(H,31,32)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273388
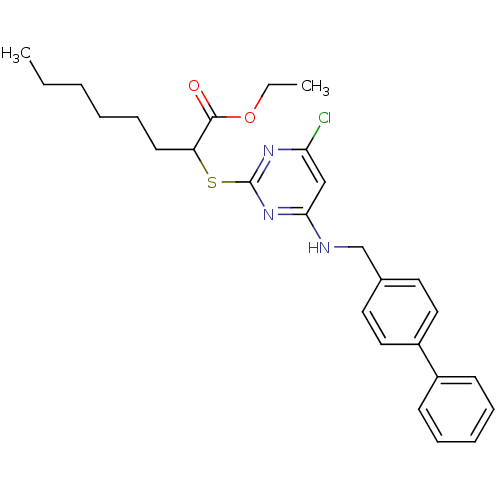
(CHEMBL456575 | Ethyl 2-(4-(biphenyl-4-ylmethylamin...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(NCc2ccc(cc2)-c2ccccc2)n1)C(=O)OCC Show InChI InChI=1S/C27H32ClN3O2S/c1-3-5-6-10-13-23(26(32)33-4-2)34-27-30-24(28)18-25(31-27)29-19-20-14-16-22(17-15-20)21-11-8-7-9-12-21/h7-9,11-12,14-18,23H,3-6,10,13,19H2,1-2H3,(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273710
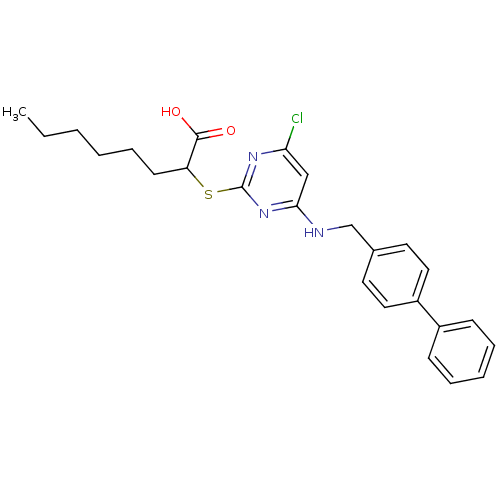
(2-(4-(Biphenyl-4-ylmethylamino)-6-chloropyrimidin-...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(NCc2ccc(cc2)-c2ccccc2)n1)C(O)=O Show InChI InChI=1S/C25H28ClN3O2S/c1-2-3-4-8-11-21(24(30)31)32-25-28-22(26)16-23(29-25)27-17-18-12-14-20(15-13-18)19-9-6-5-7-10-19/h5-7,9-10,12-16,21H,2-4,8,11,17H2,1H3,(H,30,31)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273709
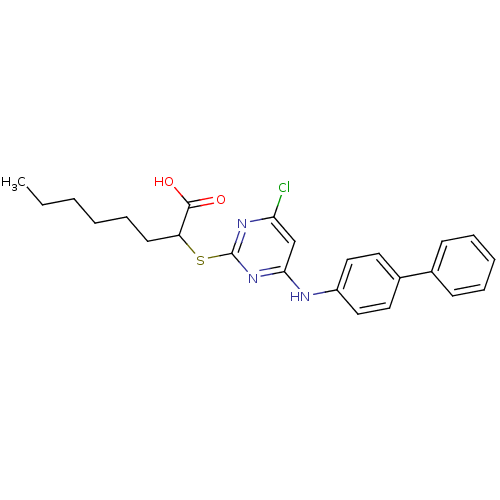
(2-(4-(Biphenyl-4-ylamino)-6-chloropyrimidine-2-ylt...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2ccc(cc2)-c2ccccc2)n1)C(O)=O Show InChI InChI=1S/C24H26ClN3O2S/c1-2-3-4-8-11-20(23(29)30)31-24-27-21(25)16-22(28-24)26-19-14-12-18(13-15-19)17-9-6-5-7-10-17/h5-7,9-10,12-16,20H,2-4,8,11H2,1H3,(H,29,30)(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273708
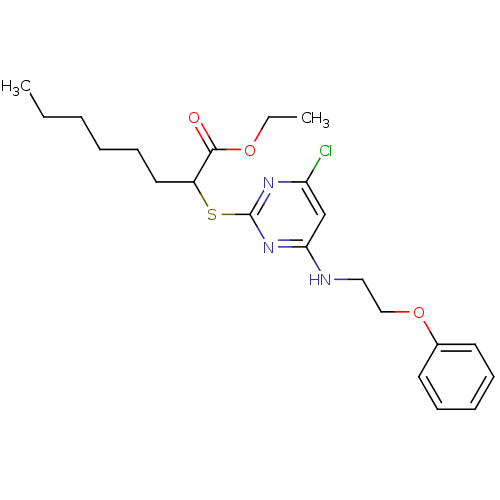
(CHEMBL460615 | Ethyl 2-(4-chloro-6-(2-phenoxyethyl...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(NCCOc2ccccc2)n1)C(=O)OCC Show InChI InChI=1S/C22H30ClN3O3S/c1-3-5-6-10-13-18(21(27)28-4-2)30-22-25-19(23)16-20(26-22)24-14-15-29-17-11-8-7-9-12-17/h7-9,11-12,16,18H,3-6,10,13-15H2,1-2H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273707
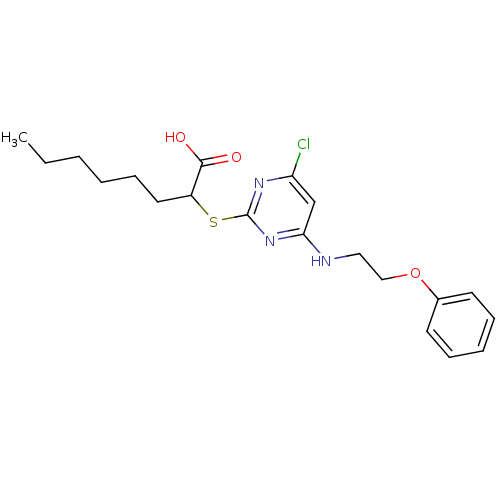
(2-(4-Chloro-6-(2-phenoxyethylamino)pyrimidin-2-ylt...)Show InChI InChI=1S/C20H26ClN3O3S/c1-2-3-4-8-11-16(19(25)26)28-20-23-17(21)14-18(24-20)22-12-13-27-15-9-6-5-7-10-15/h5-7,9-10,14,16H,2-4,8,11-13H2,1H3,(H,25,26)(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273685
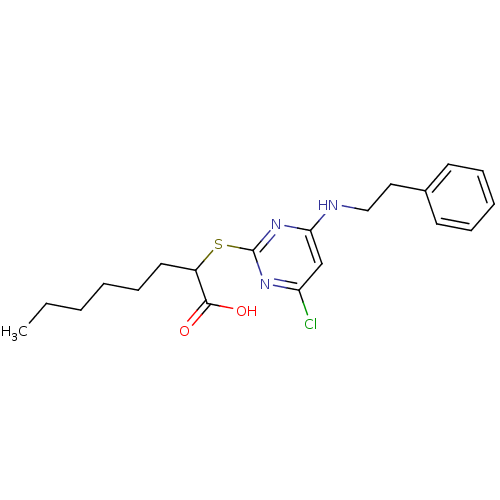
(2-(4-Chloro-6-(phenethylamino)pyrimidin-2-ylthio)o...)Show InChI InChI=1S/C20H26ClN3O2S/c1-2-3-4-8-11-16(19(25)26)27-20-23-17(21)14-18(24-20)22-13-12-15-9-6-5-7-10-15/h5-7,9-10,14,16H,2-4,8,11-13H2,1H3,(H,25,26)(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273684
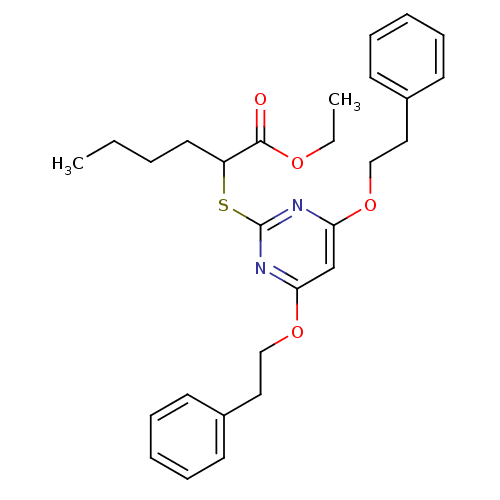
(CHEMBL460184 | Ethyl 2-(4,6-diphenethoxypyrimidin-...)Show SMILES CCCCC(Sc1nc(OCCc2ccccc2)cc(OCCc2ccccc2)n1)C(=O)OCC Show InChI InChI=1S/C28H34N2O4S/c1-3-5-16-24(27(31)32-4-2)35-28-29-25(33-19-17-22-12-8-6-9-13-22)21-26(30-28)34-20-18-23-14-10-7-11-15-23/h6-15,21,24H,3-5,16-20H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273683
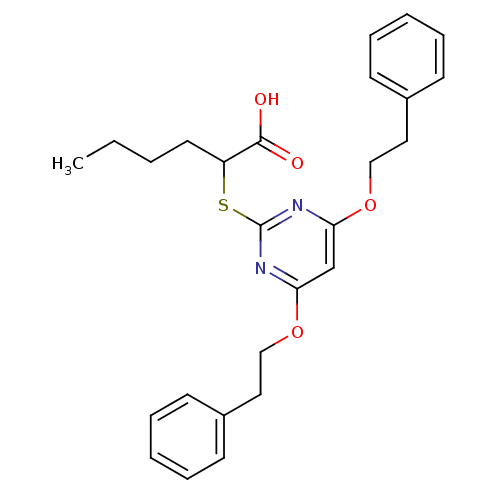
(2-(4,6-Diphenethoxypyrimidin-2-ylthio)hexanoic aci...)Show SMILES CCCCC(Sc1nc(OCCc2ccccc2)cc(OCCc2ccccc2)n1)C(O)=O Show InChI InChI=1S/C26H30N2O4S/c1-2-3-14-22(25(29)30)33-26-27-23(31-17-15-20-10-6-4-7-11-20)19-24(28-26)32-18-16-21-12-8-5-9-13-21/h4-13,19,22H,2-3,14-18H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273682
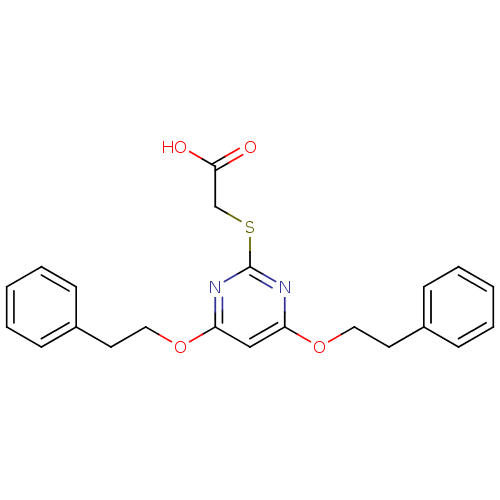
(2-(4,6-Diphenethoxypyrimidin-2-ylthio)acetic acid ...)Show InChI InChI=1S/C22H22N2O4S/c25-21(26)16-29-22-23-19(27-13-11-17-7-3-1-4-8-17)15-20(24-22)28-14-12-18-9-5-2-6-10-18/h1-10,15H,11-14,16H2,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM24565
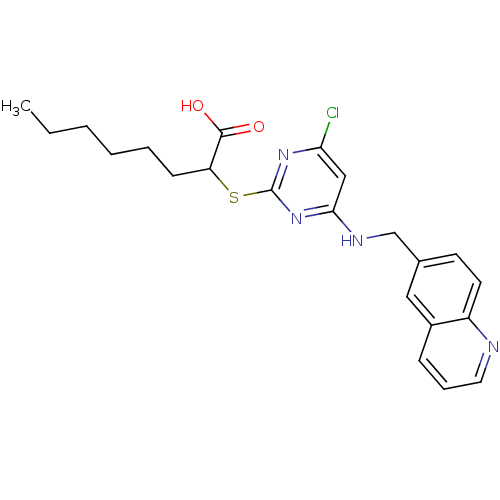
(2-({4-chloro-6-[(quinolin-6-ylmethyl)amino]pyrimid...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(NCc2ccc3ncccc3c2)n1)C(O)=O Show InChI InChI=1S/C22H25ClN4O2S/c1-2-3-4-5-8-18(21(28)29)30-22-26-19(23)13-20(27-22)25-14-15-9-10-17-16(12-15)7-6-11-24-17/h6-7,9-13,18H,2-5,8,14H2,1H3,(H,28,29)(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50253880
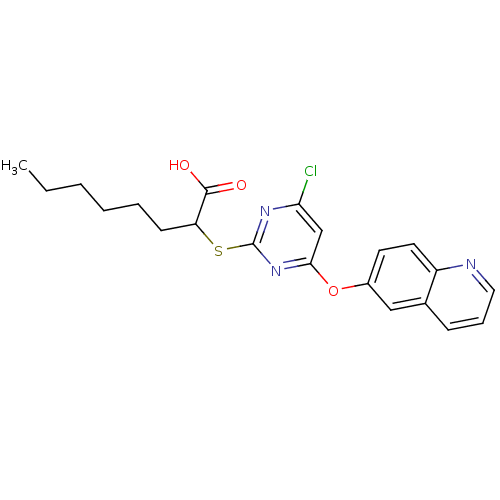
(2-(4-chloro-6-(quinolin-6-yloxy)pyrimidin-2-ylthio...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Oc2ccc3ncccc3c2)n1)C(O)=O Show InChI InChI=1S/C21H22ClN3O3S/c1-2-3-4-5-8-17(20(26)27)29-21-24-18(22)13-19(25-21)28-15-9-10-16-14(12-15)7-6-11-23-16/h6-7,9-13,17H,2-5,8H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM24561
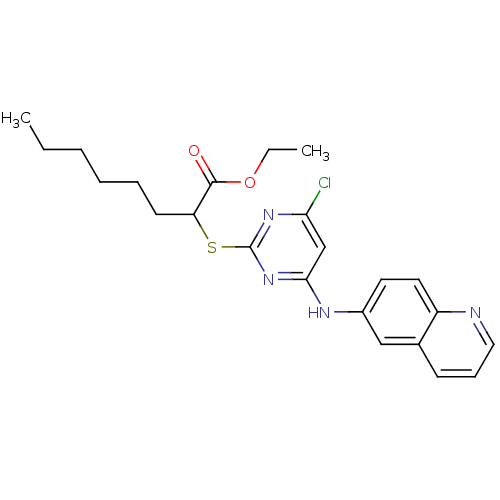
(CHEMBL459722 | Pirinixic acid-based compound, 6d |...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2ccc3ncccc3c2)n1)C(=O)OCC Show InChI InChI=1S/C23H27ClN4O2S/c1-3-5-6-7-10-19(22(29)30-4-2)31-23-27-20(24)15-21(28-23)26-17-11-12-18-16(14-17)9-8-13-25-18/h8-9,11-15,19H,3-7,10H2,1-2H3,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM24564
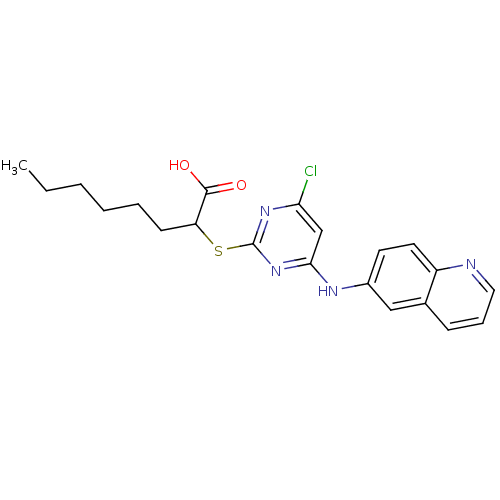
(2-{[4-chloro-6-(quinolin-6-ylamino)pyrimidin-2-yl]...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2ccc3ncccc3c2)n1)C(O)=O Show InChI InChI=1S/C21H23ClN4O2S/c1-2-3-4-5-8-17(20(27)28)29-21-25-18(22)13-19(26-21)24-15-9-10-16-14(12-15)7-6-11-23-16/h6-7,9-13,17H,2-5,8H2,1H3,(H,27,28)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50253803
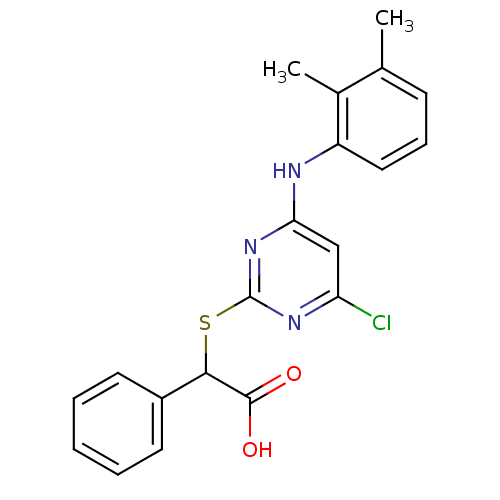
(2-(4-chloro-6-(2,3-dimethylphenylamino)pyrimidin-2...)Show SMILES Cc1cccc(Nc2cc(Cl)nc(SC(C(O)=O)c3ccccc3)n2)c1C Show InChI InChI=1S/C20H18ClN3O2S/c1-12-7-6-10-15(13(12)2)22-17-11-16(21)23-20(24-17)27-18(19(25)26)14-8-4-3-5-9-14/h3-11,18H,1-2H3,(H,25,26)(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50273385

(2-(4-chloro-6-(2,3-dimethylphenylamino)pyrimidin-2...)Show SMILES CCCCCCCCC(Sc1nc(Cl)cc(Nc2cccc(C)c2C)n1)C(O)=O Show InChI InChI=1S/C22H30ClN3O2S/c1-4-5-6-7-8-9-13-18(21(27)28)29-22-25-19(23)14-20(26-22)24-17-12-10-11-15(2)16(17)3/h10-12,14,18H,4-9,13H2,1-3H3,(H,27,28)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50253879
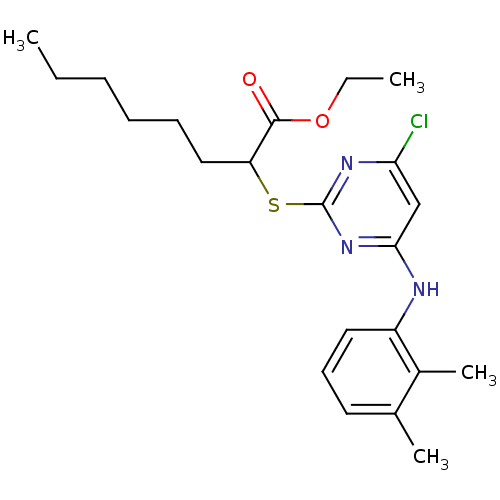
(CHEMBL459720 | ethyl 2-(4-chloro-6-(2,3-dimethylph...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2cccc(C)c2C)n1)C(=O)OCC Show InChI InChI=1S/C22H30ClN3O2S/c1-5-7-8-9-13-18(21(27)28-6-2)29-22-25-19(23)14-20(26-22)24-17-12-10-11-15(3)16(17)4/h10-12,14,18H,5-9,13H2,1-4H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM24560
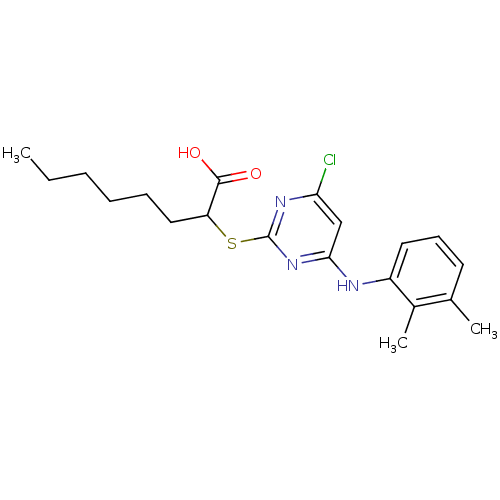
(2-({4-chloro-6-[(2,3-dimethylphenyl)amino]pyrimidi...)Show SMILES CCCCCCC(Sc1nc(Cl)cc(Nc2cccc(C)c2C)n1)C(O)=O Show InChI InChI=1S/C20H26ClN3O2S/c1-4-5-6-7-11-16(19(25)26)27-20-23-17(21)12-18(24-20)22-15-10-8-9-13(2)14(15)3/h8-10,12,16H,4-7,11H2,1-3H3,(H,25,26)(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
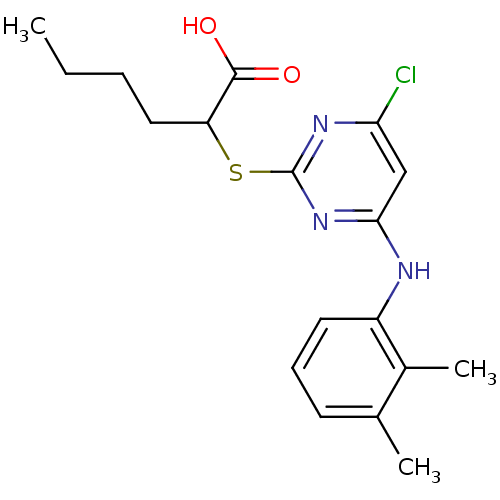
(Homo sapiens (Human)) | BDBM50273681
(2-(4-chloro-6-(2,3-dimethylphenylamino)pyrimidin-2...)Show InChI InChI=1S/C18H22ClN3O2S/c1-4-5-9-14(17(23)24)25-18-21-15(19)10-16(22-18)20-13-8-6-7-11(2)12(13)3/h6-8,10,14H,4-5,9H2,1-3H3,(H,23,24)(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
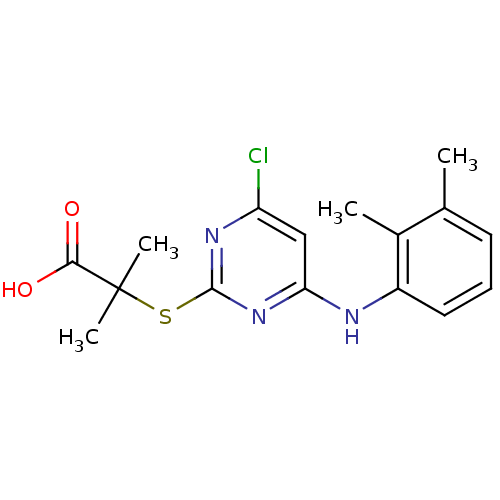
(Homo sapiens (Human)) | BDBM50273680
(2-(4-chloro-6-(2,3-dimethylphenylamino)pyrimidin-2...)Show InChI InChI=1S/C16H18ClN3O2S/c1-9-6-5-7-11(10(9)2)18-13-8-12(17)19-15(20-13)23-16(3,4)14(21)22/h5-8H,1-4H3,(H,21,22)(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
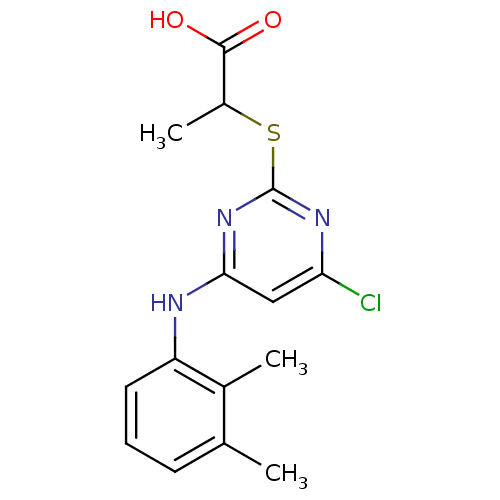
(Homo sapiens (Human)) | BDBM50253802
(2-(4-chloro-6-(2,3-dimethylphenylamino)pyrimidin-2...)Show InChI InChI=1S/C15H16ClN3O2S/c1-8-5-4-6-11(9(8)2)17-13-7-12(16)18-15(19-13)22-10(3)14(20)21/h4-7,10H,1-3H3,(H,20,21)(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
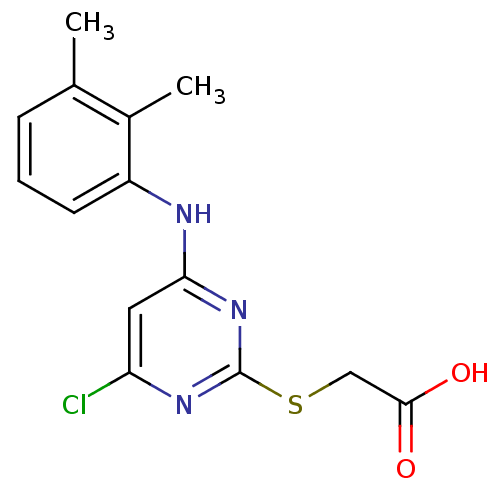
(Ovis aries (Sheep)) | BDBM24566
(2-({4-chloro-6-[(2,3-dimethylphenyl)amino]pyrimidi...)Show InChI InChI=1S/C14H14ClN3O2S/c1-8-4-3-5-10(9(8)2)16-12-6-11(15)17-14(18-12)21-7-13(19)20/h3-6H,7H2,1-2H3,(H,19,20)(H,16,17,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of ovine COX1 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM24566

(2-({4-chloro-6-[(2,3-dimethylphenyl)amino]pyrimidi...)Show InChI InChI=1S/C14H14ClN3O2S/c1-8-4-3-5-10(9(8)2)16-12-6-11(15)17-14(18-12)21-7-13(19)20/h3-6H,7H2,1-2H3,(H,19,20)(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard-Karls-University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant COX2 |
J Med Chem 51: 8068-76 (2008)
Article DOI: 10.1021/jm801085s
BindingDB Entry DOI: 10.7270/Q2639QN4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data