

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50247054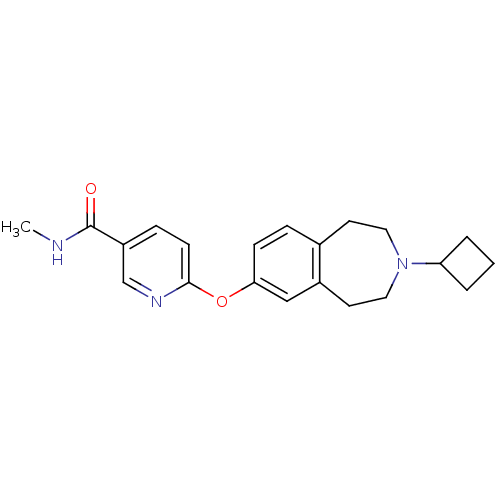 (6-(3-cyclobutyl-2,3,4,5-tetrahydro-1H-benzo[d]azep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to histamine H3 receptor | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50429823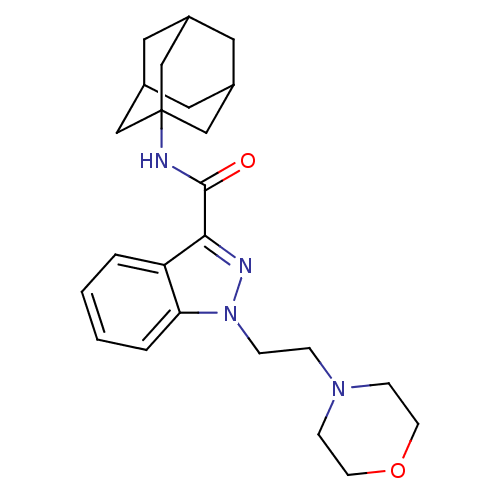 (CHEMBL2338173) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor (unknown origin) | Bioorg Med Chem Lett 23: 1177-81 (2013) Article DOI: 10.1016/j.bmcl.2013.01.044 BindingDB Entry DOI: 10.7270/Q2M32X3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50247053 (1-(3-(3-(4-chlorophenyl)propoxy)propyl)piperidine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human histamine H3 receptor expressed in HEK293 cells by [35S]gammaS binding assay | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50268293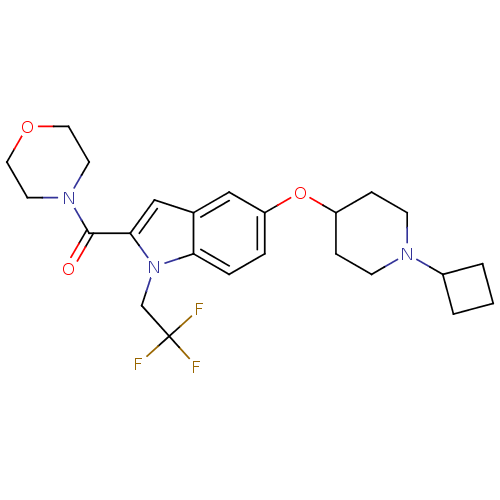 ((5-(1-cyclobutylpiperidin-4-yloxy)-1-(2,2,2-triflu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50268291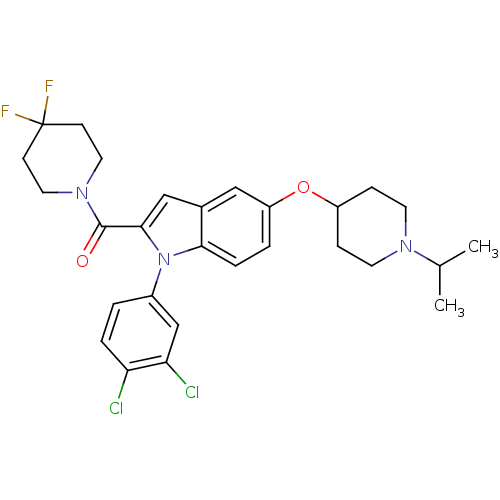 ((1-(3,4-dichlorophenyl)-5-(1-isopropylpiperidin-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50268323 ((5-(1-cyclopropylpiperidin-4-yloxy)-1-(2,2,2-trifl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50139391 ((R)-4-(2-(2-(2-methylpyrrolidin-1-yl)ethyl)benzofu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to histamine H3 receptor | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50077223 (CHEMBL3416881) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from human vasopressin 1a receptor expressed in HEK293 cell membranes after 1 hr by scintillation proximity assay | J Med Chem 58: 2275-89 (2015) Article DOI: 10.1021/jm501745f BindingDB Entry DOI: 10.7270/Q2JW8GK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50159110 (1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to histamine H3 receptor | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50077217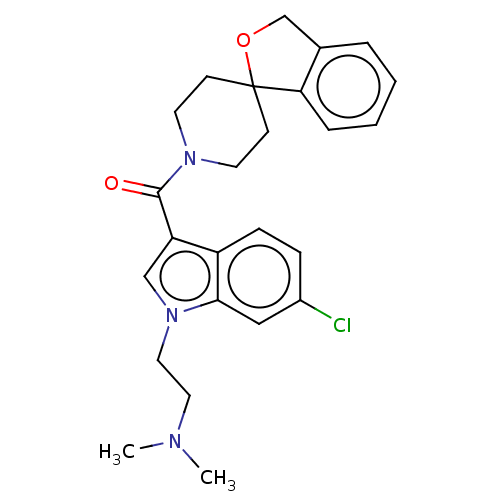 (CHEMBL3416885) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from human vasopressin 1a receptor expressed in HEK293 cell membranes after 1 hr by scintillation proximity assay | J Med Chem 58: 2275-89 (2015) Article DOI: 10.1021/jm501745f BindingDB Entry DOI: 10.7270/Q2JW8GK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50077221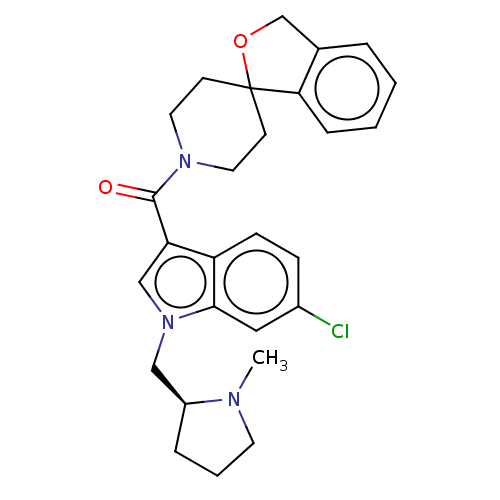 (CHEMBL3416883) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from human vasopressin 1a receptor expressed in HEK293 cell membranes after 1 hr by scintillation proximity assay | J Med Chem 58: 2275-89 (2015) Article DOI: 10.1021/jm501745f BindingDB Entry DOI: 10.7270/Q2JW8GK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50077452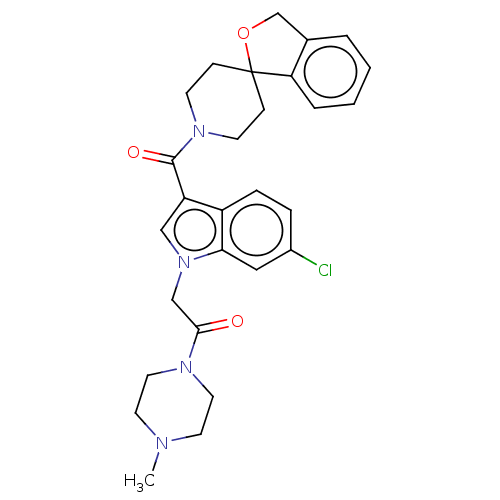 (CHEMBL3416868) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from human vasopressin 1a receptor expressed in HEK293 cell membranes after 1 hr by scintillation proximity assay | J Med Chem 58: 2275-89 (2015) Article DOI: 10.1021/jm501745f BindingDB Entry DOI: 10.7270/Q2JW8GK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50181073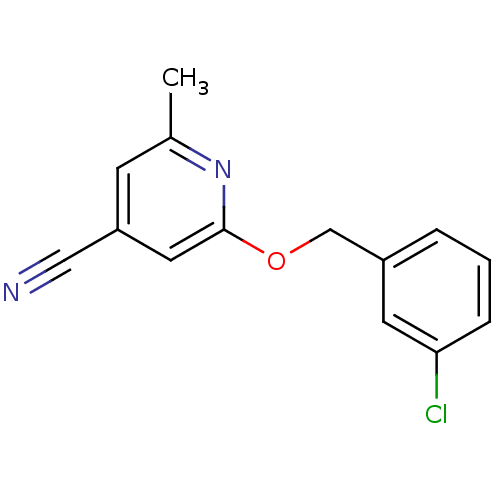 (2-(3-chloro-benzyloxy)-6-methyl-isonicotinonitrile...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffman-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]MPEP from recombinant human mGlu5 receptor | Bioorg Med Chem Lett 16: 1892-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.088 BindingDB Entry DOI: 10.7270/Q2ZG6RT8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50263570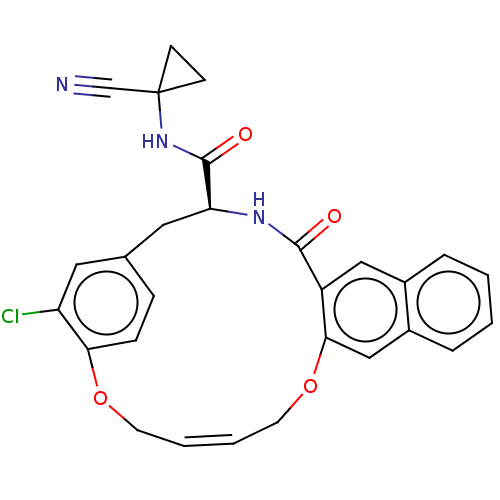 (CHEMBL4066422) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50268291 ((1-(3,4-dichlorophenyl)-5-(1-isopropylpiperidin-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50268293 ((5-(1-cyclobutylpiperidin-4-yloxy)-1-(2,2,2-triflu...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50269053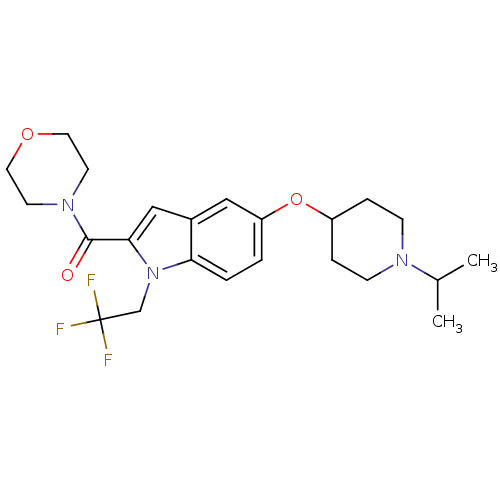 ((5-(1-isopropylpiperidin-4-yloxy)-1-(2,2,2-trifluo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50269054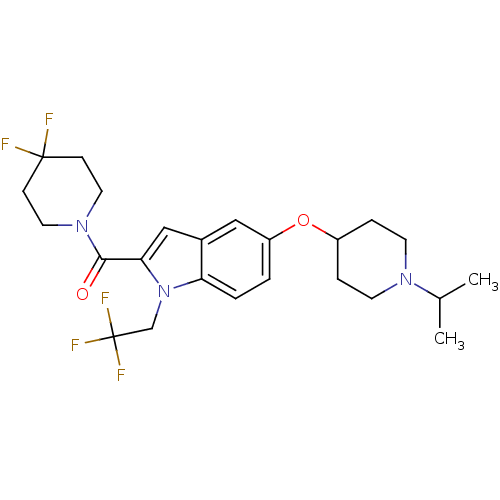 ((4,4-difluoropiperidin-1-yl)(5-(1-isopropylpiperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50263570 (CHEMBL4066422) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of human CatL using Cbz-Phe-Arg-AMC as substrate measured over 30 mins by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50077222 (CHEMBL3416882) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from human vasopressin 1a receptor expressed in HEK293 cell membranes after 1 hr by scintillation proximity assay | J Med Chem 58: 2275-89 (2015) Article DOI: 10.1021/jm501745f BindingDB Entry DOI: 10.7270/Q2JW8GK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50077310 (CHEMBL3416869) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from human vasopressin 1a receptor expressed in HEK293 cell membranes after 1 hr by scintillation proximity assay | J Med Chem 58: 2275-89 (2015) Article DOI: 10.1021/jm501745f BindingDB Entry DOI: 10.7270/Q2JW8GK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50268997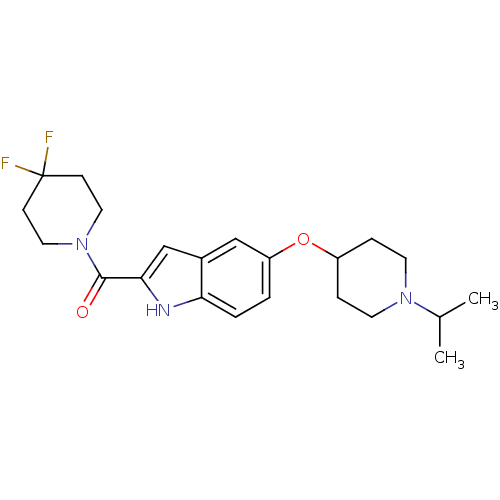 ((4,4-Difluoropiperidin-1-yl)[5-(1-isopropyl-piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50077213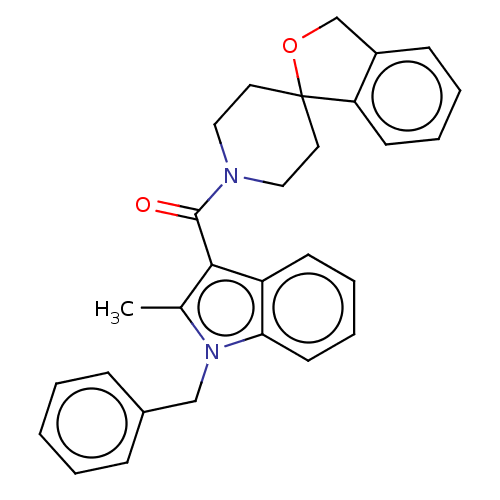 (CHEMBL3416860) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from human vasopressin 1a receptor expressed in HEK293 cell membranes after 1 hr by scintillation proximity assay | J Med Chem 58: 2275-89 (2015) Article DOI: 10.1021/jm501745f BindingDB Entry DOI: 10.7270/Q2JW8GK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50268996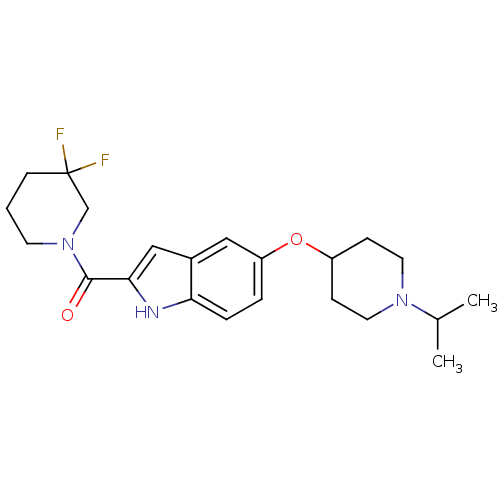 ((3,3-difluoropiperidin-1-yl)(5-(1-isopropylpiperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50268947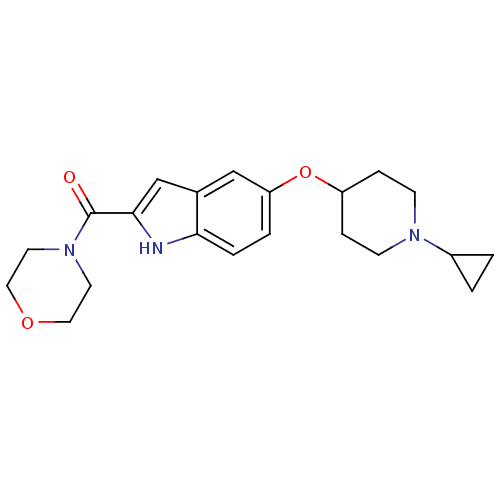 ((5-(1-cyclopropylpiperidin-4-yloxy)-1H-indol-2-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50370569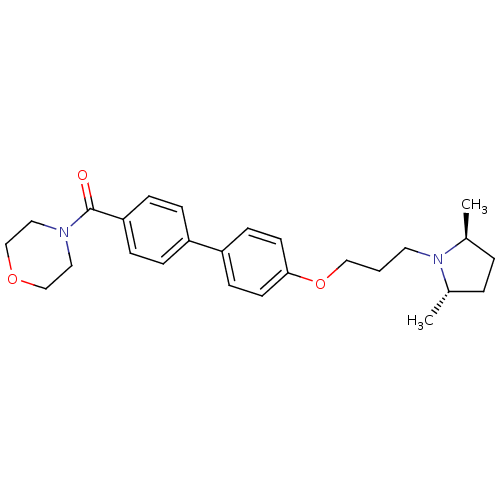 (A-349,821 | A-349821) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to histamine H3 receptor | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50268815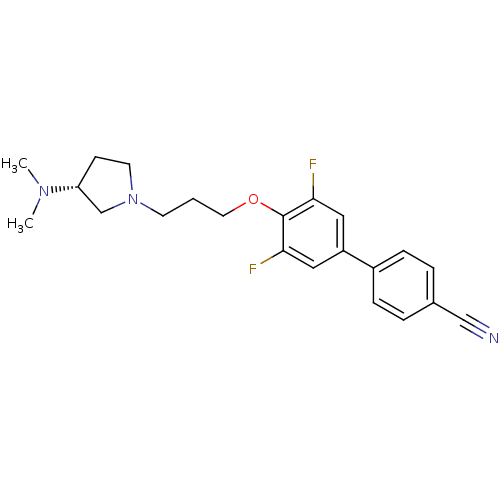 ((R)-4'-(3-(3-(dimethylamino)pyrrolidin-1-yl)propox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Binding affinity to histamine H3 receptor | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50263634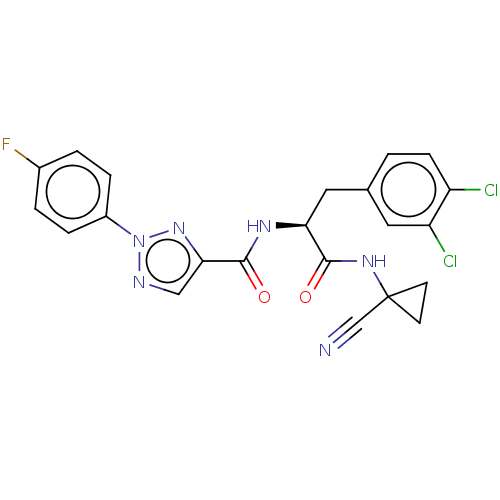 (CHEMBL4073014) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate by fluorimetric method | J Med Chem 61: 3370-3388 (2018) Article DOI: 10.1021/acs.jmedchem.7b01870 BindingDB Entry DOI: 10.7270/Q2MC92GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50263572 (CHEMBL4092050) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50269098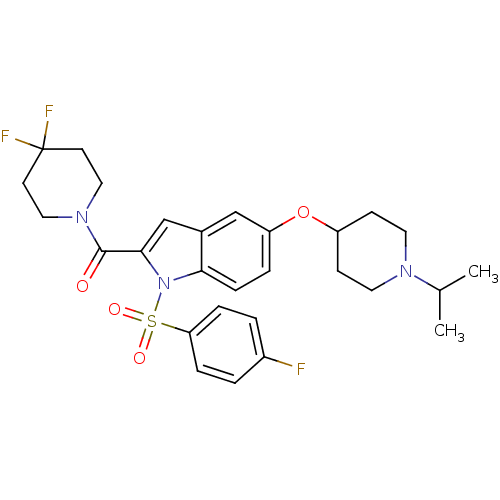 ((4,4-difluoropiperidin-1-yl)(1-(4-fluorophenylsulf...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50268323 ((5-(1-cyclopropylpiperidin-4-yloxy)-1-(2,2,2-trifl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50268451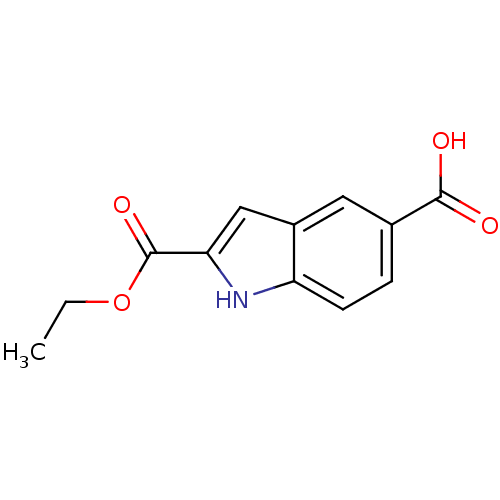 (2-(ethoxycarbonyl)-1H-indole-5-carboxylic acid | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50268947 ((5-(1-cyclopropylpiperidin-4-yloxy)-1H-indol-2-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50269099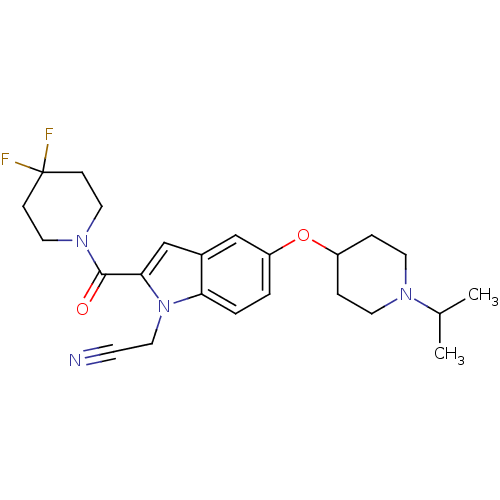 (2-(2-(4,4-difluoropiperidine-1-carbonyl)-5-(1-isop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50269055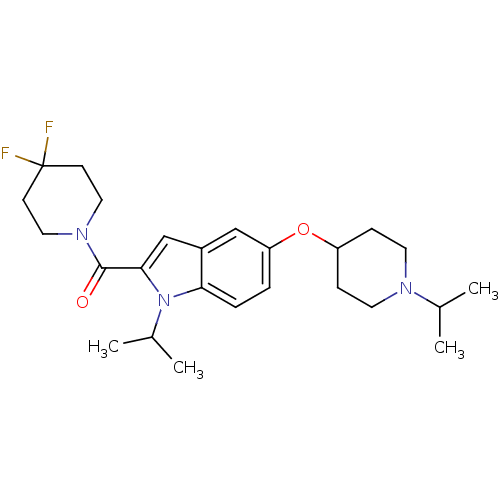 ((4,4-Difluoropiperidin-1-yl)[1-isopropyl-5-(1-isop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-RAMH from human histamine H3 receptor | Bioorg Med Chem Lett 20: 5713-7 (2010) Article DOI: 10.1016/j.bmcl.2010.08.009 BindingDB Entry DOI: 10.7270/Q2571C7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50327479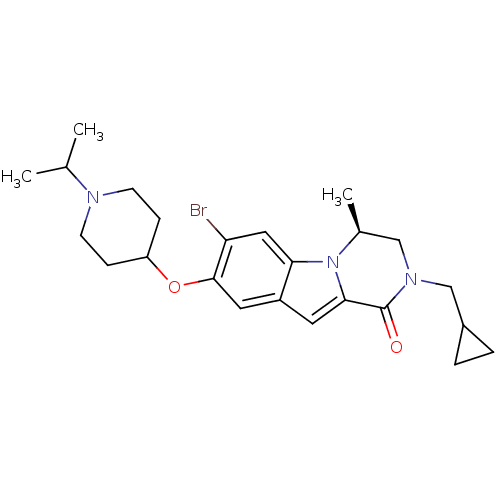 ((S)-7-bromo-2-(cyclopropylmethyl)-8-(1-isopropylpi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Displacement of [3H]-RAMH from human histamine H3 receptor | Bioorg Med Chem Lett 20: 5713-7 (2010) Article DOI: 10.1016/j.bmcl.2010.08.009 BindingDB Entry DOI: 10.7270/Q2571C7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50263577 (CHEMBL4099651) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of human CatL using Cbz-Phe-Arg-AMC as substrate measured over 30 mins by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210850 (US9290467, 20) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of human CatL using Cbz-Phe-Arg-AMC as substrate measured over 30 mins by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM210854 (US9290467, 24 | US9290467, 25 | US9290467, 26) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50263578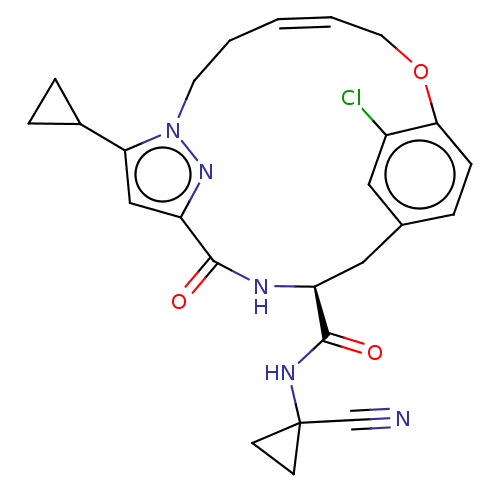 (CHEMBL4064172) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of human CatL using Cbz-Phe-Arg-AMC as substrate measured over 30 mins by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50263632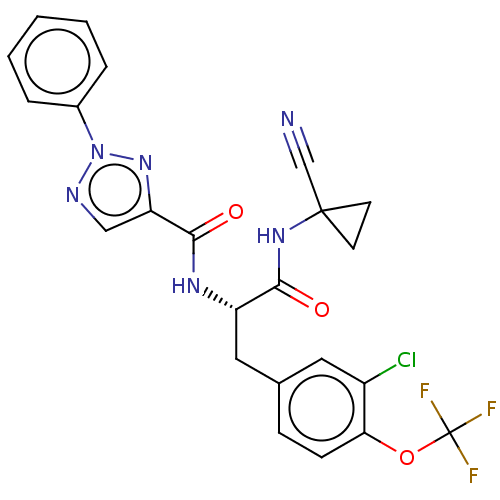 (CHEMBL4080286) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate by fluorimetric method | J Med Chem 61: 3370-3388 (2018) Article DOI: 10.1021/acs.jmedchem.7b01870 BindingDB Entry DOI: 10.7270/Q2MC92GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50263647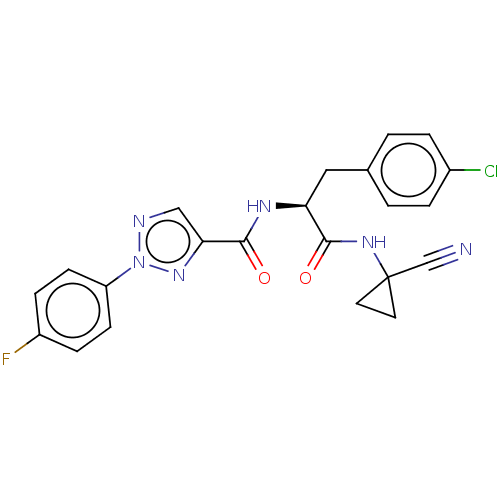 (CHEMBL4099905) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate by fluorimetric method | J Med Chem 61: 3370-3388 (2018) Article DOI: 10.1021/acs.jmedchem.7b01870 BindingDB Entry DOI: 10.7270/Q2MC92GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50269099 (2-(2-(4,4-difluoropiperidine-1-carbonyl)-5-(1-isop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM210859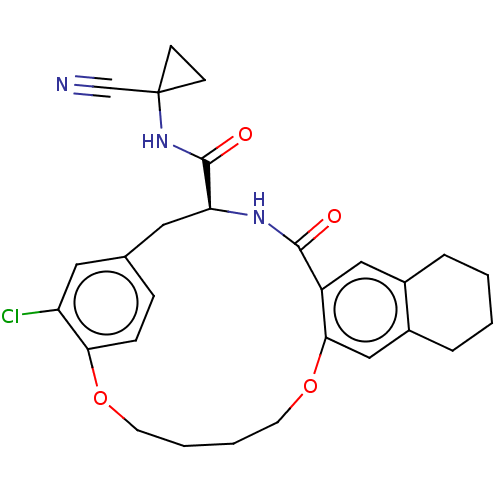 (US9290467, 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of human CatL using Cbz-Phe-Arg-AMC as substrate measured over 30 mins by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50263571 (CHEMBL4062591) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratorium für Organische Chemie , ETH Zurich , Vladimir-Prelog-Weg 3 , 8093 Zürich , Switzerland. Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-AMC as substrate by fluorimetric method | J Med Chem 61: 3350-3369 (2018) Article DOI: 10.1021/acs.jmedchem.7b01869 BindingDB Entry DOI: 10.7270/Q2R49T79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50269097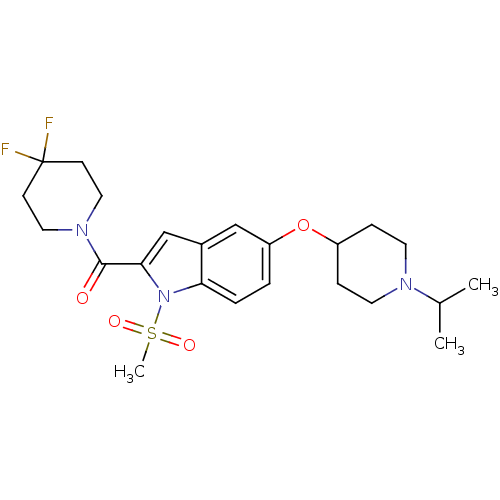 ((4,4-difluoropiperidin-1-yl)(5-(1-isopropylpiperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50268996 ((3,3-difluoropiperidin-1-yl)(5-(1-isopropylpiperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Rattus norvegicus (rat)) | BDBM50268997 ((4,4-Difluoropiperidin-1-yl)[5-(1-isopropyl-piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from rat recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50269053 ((5-(1-isopropylpiperidin-4-yloxy)-1-(2,2,2-trifluo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50269054 ((4,4-difluoropiperidin-1-yl)(5-(1-isopropylpiperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. Curated by ChEMBL | Assay Description Displacement of [3H](R)-alpha-methylhistamine from human recombinant histamine H3 receptor expressed in CHO cells by liquid scintillation counting | J Med Chem 52: 3855-68 (2009) Article DOI: 10.1021/jm900409x BindingDB Entry DOI: 10.7270/Q2SB46NQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 667 total ) | Next | Last >> |