 Found 1597 hits with Last Name = 'schultz' and Initial = 'c'
Found 1597 hits with Last Name = 'schultz' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50237140

(CHEMBL4068763)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1n[nH]c2cc(Nc3cccc(F)c3)ccc12 Show InChI InChI=1S/C28H32FN7O2/c1-6-27(37)31-23-16-24(26(38-5)17-25(23)36(4)13-12-35(2)3)32-28-21-11-10-20(15-22(21)33-34-28)30-19-9-7-8-18(29)14-19/h6-11,14-17,30H,1,12-13H2,2-5H3,(H,31,37)(H2,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
Reversible inhibition of wild-type human N-terminal GST-tagged EGFR cytoplasmic domain (669 to 1210 end amino acid residues) expressed in baculovirus... |
J Med Chem 60: 2361-2372 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01626
BindingDB Entry DOI: 10.7270/Q2FB556R |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50104413
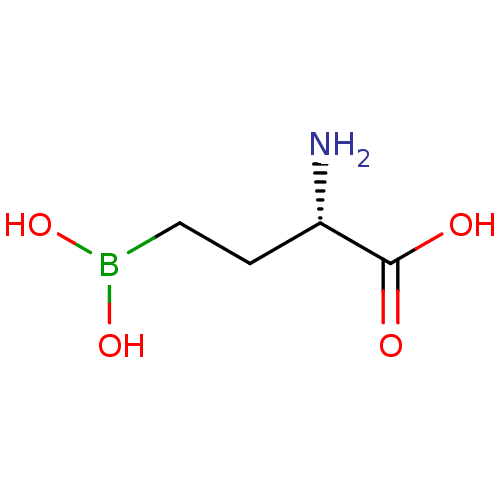
(CHEMBL87796 | Glutamyl-gamma-boronate analogue)Show InChI InChI=1S/C4H10BNO4/c6-3(4(7)8)1-2-5(9)10/h3,9-10H,1-2,6H2,(H,7,8)/t3-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116986
BindingDB Entry DOI: 10.7270/Q29K4GB2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
Reversible inhibition of wild-type human N-terminal GST-tagged EGFR cytoplasmic domain (669 to 1210 end amino acid residues) expressed in baculovirus... |
J Med Chem 60: 2361-2372 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01626
BindingDB Entry DOI: 10.7270/Q2FB556R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50104413

(CHEMBL87796 | Glutamyl-gamma-boronate analogue)Show InChI InChI=1S/C4H10BNO4/c6-3(4(7)8)1-2-5(9)10/h3,9-10H,1-2,6H2,(H,7,8)/t3-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116986
BindingDB Entry DOI: 10.7270/Q29K4GB2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50607251
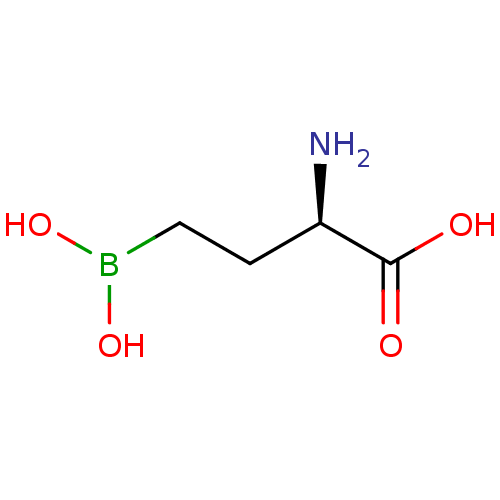
(CHEMBL5219012) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116986
BindingDB Entry DOI: 10.7270/Q29K4GB2 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM149404
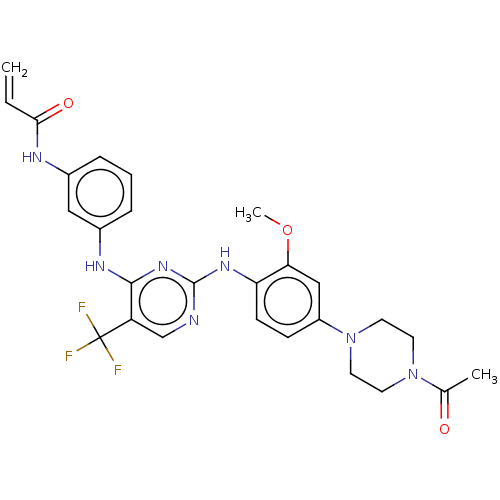
(AVL-301 | CHEMBL3545308 | CNX-419 | CO-1686 | Roci...)Show SMILES COc1cc(ccc1Nc1ncc(c(Nc2cccc(NC(=O)C=C)c2)n1)C(F)(F)F)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C27H28F3N7O3/c1-4-24(39)32-18-6-5-7-19(14-18)33-25-21(27(28,29)30)16-31-26(35-25)34-22-9-8-20(15-23(22)40-3)37-12-10-36(11-13-37)17(2)38/h4-9,14-16H,1,10-13H2,2-3H3,(H,32,39)(H2,31,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
Reversible inhibition of wild-type human N-terminal GST-tagged EGFR cytoplasmic domain (669 to 1210 end amino acid residues) expressed in baculovirus... |
J Med Chem 60: 2361-2372 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01626
BindingDB Entry DOI: 10.7270/Q2FB556R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50607251

(CHEMBL5219012) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116986
BindingDB Entry DOI: 10.7270/Q29K4GB2 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50237139

(CHEMBL4089863)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1n[nH]c2ccc(cc12)-c1ccc2ccccc2c1 Show InChI InChI=1S/C32H34N6O2/c1-6-31(39)33-27-19-28(30(40-5)20-29(27)38(4)16-15-37(2)3)34-32-25-18-24(13-14-26(25)35-36-32)23-12-11-21-9-7-8-10-22(21)17-23/h6-14,17-20H,1,15-16H2,2-5H3,(H,33,39)(H2,34,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards 5-hydroxytryptamine 3 receptor was determined |
J Med Chem 60: 2361-2372 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01626
BindingDB Entry DOI: 10.7270/Q2FB556R |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50237153
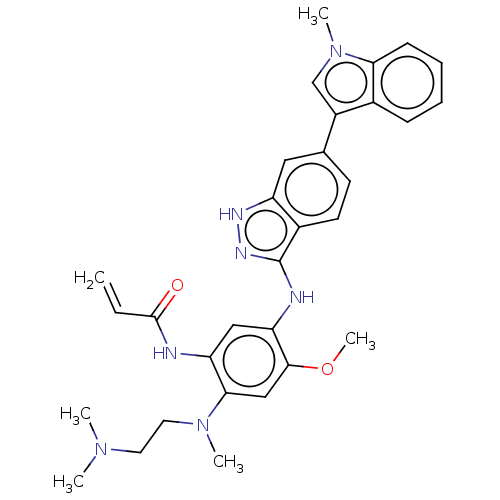
(CHEMBL4098444)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1n[nH]c2cc(ccc12)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C31H35N7O2/c1-7-30(39)32-25-17-26(29(40-6)18-28(25)37(4)15-14-36(2)3)33-31-22-13-12-20(16-24(22)34-35-31)23-19-38(5)27-11-9-8-10-21(23)27/h7-13,16-19H,1,14-15H2,2-6H3,(H,32,39)(H2,33,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
Inhibition of cloned isozyme, human carbonic anhydrase II |
J Med Chem 60: 2361-2372 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01626
BindingDB Entry DOI: 10.7270/Q2FB556R |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50607252

(CHEMBL5219273) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116986
BindingDB Entry DOI: 10.7270/Q29K4GB2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50607253
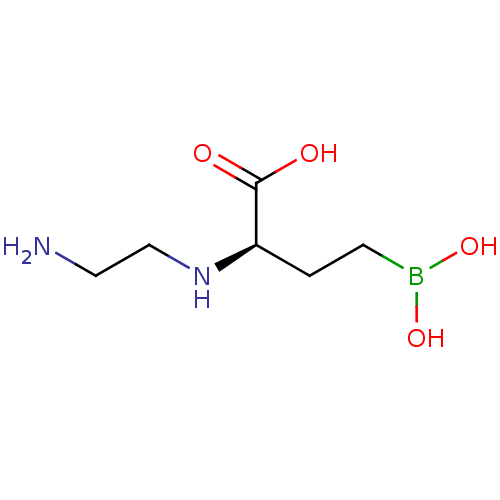
(CHEMBL5220603) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116986
BindingDB Entry DOI: 10.7270/Q29K4GB2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50607257

(CHEMBL5219724) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116986
BindingDB Entry DOI: 10.7270/Q29K4GB2 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50607262

(CHEMBL5218792)Show SMILES N[C@H](CN[C@H](CCB(O)O)C(O)=O)Cc1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116986
BindingDB Entry DOI: 10.7270/Q29K4GB2 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50607252

(CHEMBL5219273) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 8.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116986
BindingDB Entry DOI: 10.7270/Q29K4GB2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50607258
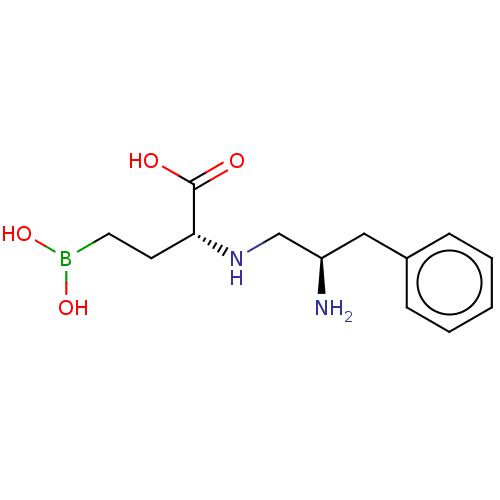
(CHEMBL5218962)Show SMILES N[C@@H](CN[C@H](CCB(O)O)C(O)=O)Cc1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116986
BindingDB Entry DOI: 10.7270/Q29K4GB2 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50607257

(CHEMBL5219724) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116986
BindingDB Entry DOI: 10.7270/Q29K4GB2 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50607261
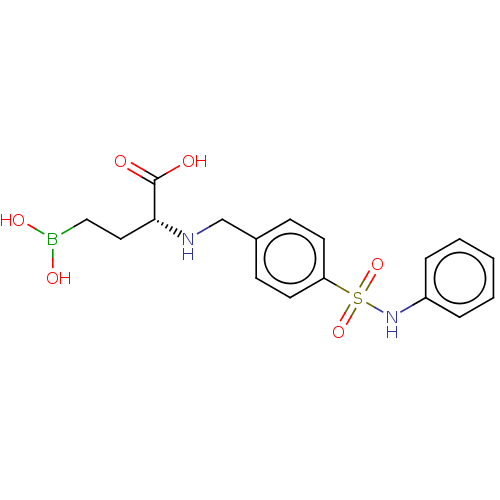
(CHEMBL5218489)Show SMILES OB(O)CC[C@@H](NCc1ccc(cc1)S(=O)(=O)Nc1ccccc1)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.73E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116986
BindingDB Entry DOI: 10.7270/Q29K4GB2 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50607255
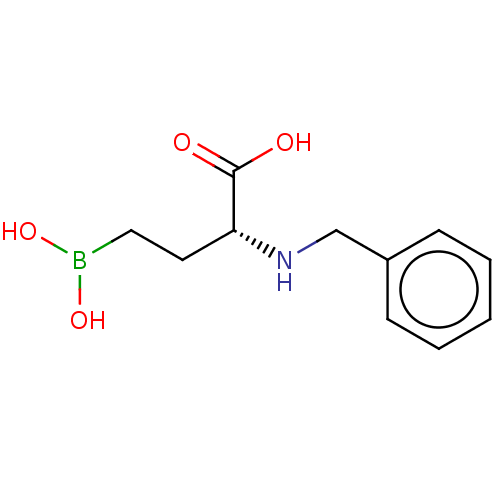
(CHEMBL5219778) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116986
BindingDB Entry DOI: 10.7270/Q29K4GB2 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50607256
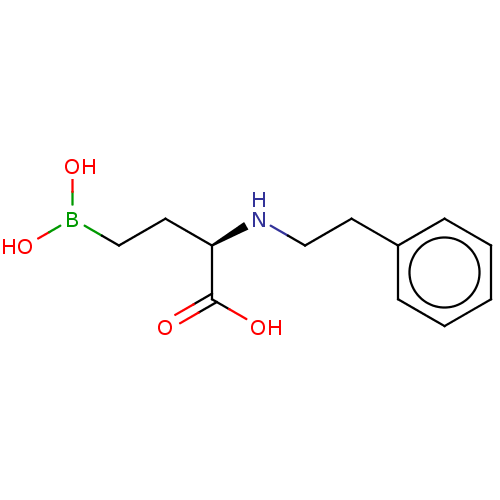
(CHEMBL5220724) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116986
BindingDB Entry DOI: 10.7270/Q29K4GB2 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50607254
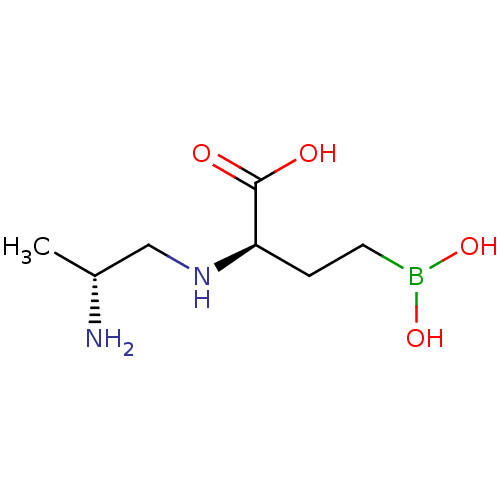
(CHEMBL5219218) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.54E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116986
BindingDB Entry DOI: 10.7270/Q29K4GB2 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50607253

(CHEMBL5220603) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 2.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116986
BindingDB Entry DOI: 10.7270/Q29K4GB2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50607259
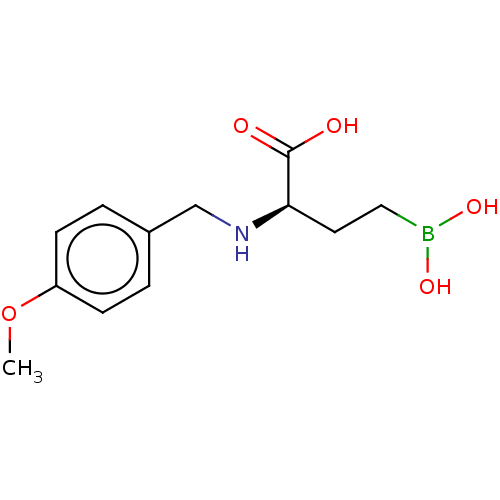
(CHEMBL5220006) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.43E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116986
BindingDB Entry DOI: 10.7270/Q29K4GB2 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50607256

(CHEMBL5220724) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.56E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116986
BindingDB Entry DOI: 10.7270/Q29K4GB2 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50607258

(CHEMBL5218962)Show SMILES N[C@@H](CN[C@H](CCB(O)O)C(O)=O)Cc1ccccc1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116986
BindingDB Entry DOI: 10.7270/Q29K4GB2 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50607260
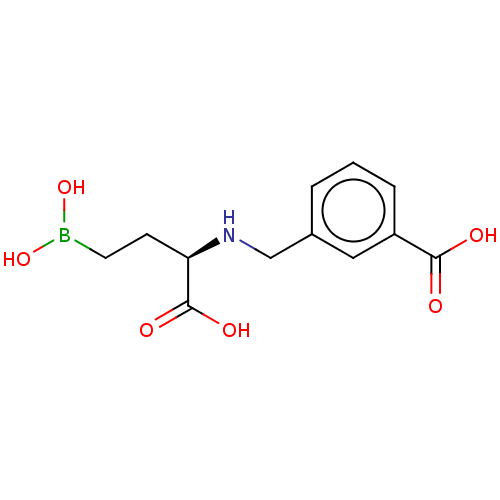
(CHEMBL5218520)Show SMILES OB(O)CC[C@@H](NCc1cccc(c1)C(O)=O)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.89E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116986
BindingDB Entry DOI: 10.7270/Q29K4GB2 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50607254

(CHEMBL5219218) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116986
BindingDB Entry DOI: 10.7270/Q29K4GB2 |
More data for this
Ligand-Target Pair | |
Glutathione hydrolase 1 proenzyme
(Homo sapiens (Human)) | BDBM50607255

(CHEMBL5219778) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.15E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.116986
BindingDB Entry DOI: 10.7270/Q29K4GB2 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50306267
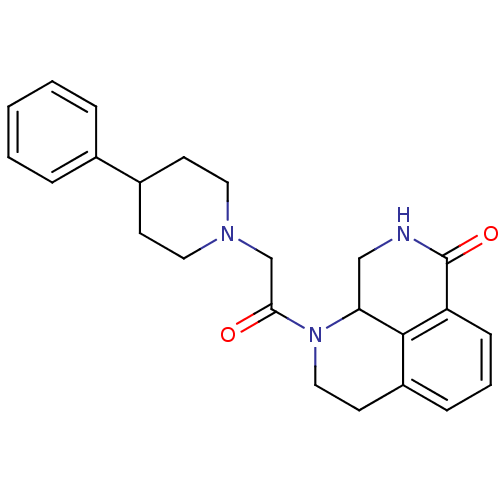
(1-(2-(4-phenylpiperidin-1-yl)acetyl)-2,3,9,9a-tetr...)Show SMILES O=C(CN1CCC(CC1)c1ccccc1)N1CCc2cccc3C(=O)NCC1c23 Show InChI InChI=1S/C24H27N3O2/c28-22(16-26-12-9-18(10-13-26)17-5-2-1-3-6-17)27-14-11-19-7-4-8-20-23(19)21(27)15-25-24(20)29/h1-8,18,21H,9-16H2,(H,25,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 by scintillation proximity assay |
Bioorg Med Chem Lett 20: 448-52 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.002
BindingDB Entry DOI: 10.7270/Q2GT5N91 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Gallus gallus (Chicken)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Technical University of Dortmund
Curated by ChEMBL
| Assay Description
Inhibition of chicken wild-type cSRC (251 to 533)-mediated phosphorylation of biotinylated poly-Glu-Tyr expressed in Escherichia coli BL21(DE3) prein... |
J Med Chem 56: 5757-72 (2014)
Article DOI: 10.1021/jm4004076
BindingDB Entry DOI: 10.7270/Q2W37XPH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50306257
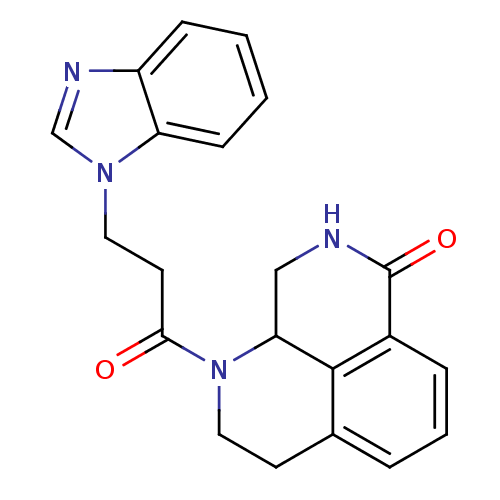
(1-(3-(1H-benzo[d]imidazol-1-yl)propanoyl)-2,3,9,9a...)Show SMILES O=C(CCn1cnc2ccccc12)N1CCc2cccc3C(=O)NCC1c23 Show InChI InChI=1S/C21H20N4O2/c26-19(9-10-24-13-23-16-6-1-2-7-17(16)24)25-11-8-14-4-3-5-15-20(14)18(25)12-22-21(15)27/h1-7,13,18H,8-12H2,(H,22,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 by scintillation proximity assay |
Bioorg Med Chem Lett 20: 448-52 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.002
BindingDB Entry DOI: 10.7270/Q2GT5N91 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50258974
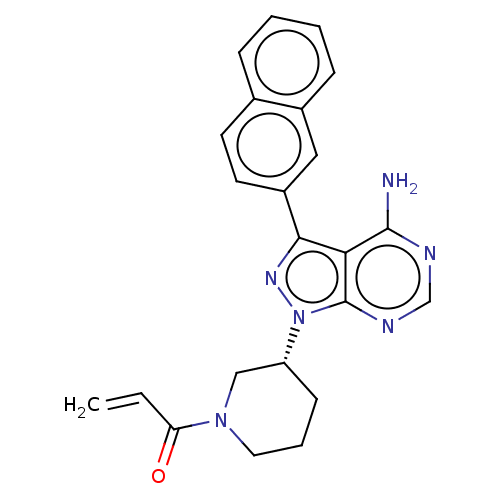
(CHEMBL4090601)Show SMILES Nc1ncnc2n(nc(-c3ccc4ccccc4c3)c12)[C@@H]1CCCN(C1)C(=O)C=C |r| Show InChI InChI=1S/C23H22N6O/c1-2-19(30)28-11-5-8-18(13-28)29-23-20(22(24)25-14-26-23)21(27-29)17-10-9-15-6-3-4-7-16(15)12-17/h2-4,6-7,9-10,12,14,18H,1,5,8,11,13H2,(H2,24,25,26)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged EGFR L858R mutant expressed in baculovirus expression system preincubated for 30 mins followed by ATP and ... |
J Med Chem 60: 7725-7744 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00515
BindingDB Entry DOI: 10.7270/Q28W3GS9 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM50306253

(1-(2-(pyridin-3-yl)acetyl)-2,3,9,9a-tetrahydro-1H-...)Show InChI InChI=1S/C18H17N3O2/c22-16(9-12-3-2-7-19-10-12)21-8-6-13-4-1-5-14-17(13)15(21)11-20-18(14)23/h1-5,7,10,15H,6,8-9,11H2,(H,20,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Inhibition of PARP2 |
Bioorg Med Chem Lett 20: 448-52 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.002
BindingDB Entry DOI: 10.7270/Q2GT5N91 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 2
(Homo sapiens (Human)) | BDBM50306256
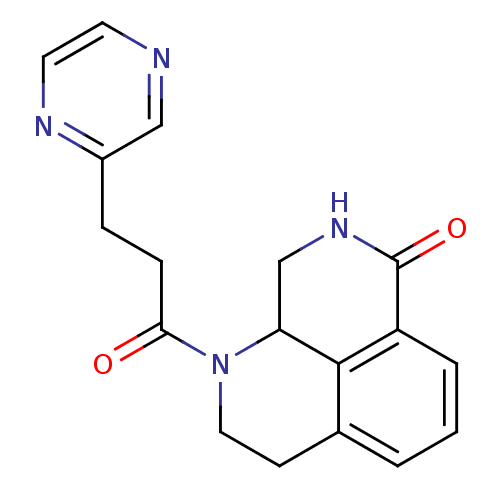
(1-(3-(pyrazin-2-yl)propanoyl)-2,3,9,9a-tetrahydro-...)Show InChI InChI=1S/C18H18N4O2/c23-16(5-4-13-10-19-7-8-20-13)22-9-6-12-2-1-3-14-17(12)15(22)11-21-18(14)24/h1-3,7-8,10,15H,4-6,9,11H2,(H,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Inhibition of PARP2 |
Bioorg Med Chem Lett 20: 448-52 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.002
BindingDB Entry DOI: 10.7270/Q2GT5N91 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
Inhibition of wild type EGFR (unknown origin) expressed in Sf9 cells pre-incubated for 30 mins before substrate and ATP addition by homogeneous time-... |
J Med Chem 58: 6844-63 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01082
BindingDB Entry DOI: 10.7270/Q2WM1G59 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50306253

(1-(2-(pyridin-3-yl)acetyl)-2,3,9,9a-tetrahydro-1H-...)Show InChI InChI=1S/C18H17N3O2/c22-16(9-12-3-2-7-19-10-12)21-8-6-13-4-1-5-14-17(13)15(21)11-20-18(14)23/h1-5,7,10,15H,6,8-9,11H2,(H,20,23) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 by scintillation proximity assay |
Bioorg Med Chem Lett 20: 448-52 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.002
BindingDB Entry DOI: 10.7270/Q2GT5N91 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
Inhibition of wild type N-terminal GST-fused human EGFR cytoplasmic domain expressed in baculovirus expression system preincubated for 30 mins follow... |
J Med Chem 60: 7725-7744 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00515
BindingDB Entry DOI: 10.7270/Q28W3GS9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50383274
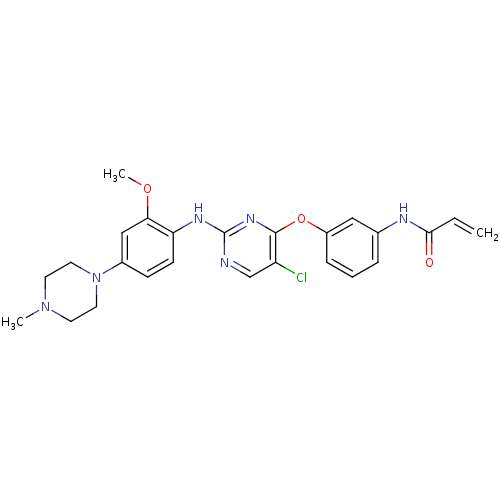
(CHEMBL1229592 | US10167264, WZ4002 | US9670213, WZ...)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Oc2cccc(NC(=O)C=C)c2)n1)N1CCN(C)CC1 Show InChI InChI=1S/C25H27ClN6O3/c1-4-23(33)28-17-6-5-7-19(14-17)35-24-20(26)16-27-25(30-24)29-21-9-8-18(15-22(21)34-3)32-12-10-31(2)11-13-32/h4-9,14-16H,1,10-13H2,2-3H3,(H,28,33)(H,27,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
Inhibition of wild type N-terminal GST-fused human EGFR cytoplasmic domain expressed in baculovirus expression system preincubated for 30 mins follow... |
J Med Chem 60: 7725-7744 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00515
BindingDB Entry DOI: 10.7270/Q28W3GS9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50258974

(CHEMBL4090601)Show SMILES Nc1ncnc2n(nc(-c3ccc4ccccc4c3)c12)[C@@H]1CCCN(C1)C(=O)C=C |r| Show InChI InChI=1S/C23H22N6O/c1-2-19(30)28-11-5-8-18(13-28)29-23-20(22(24)25-14-26-23)21(27-29)17-10-9-15-6-3-4-7-16(15)12-17/h2-4,6-7,9-10,12,14,18H,1,5,8,11,13H2,(H2,24,25,26)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
Inhibition of wild type N-terminal GST-fused human EGFR cytoplasmic domain expressed in baculovirus expression system preincubated for 30 mins follow... |
J Med Chem 60: 7725-7744 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00515
BindingDB Entry DOI: 10.7270/Q28W3GS9 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards 5-hydroxytryptamine 3 receptor was determined |
J Med Chem 60: 2361-2372 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01626
BindingDB Entry DOI: 10.7270/Q2FB556R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50237140

(CHEMBL4068763)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1n[nH]c2cc(Nc3cccc(F)c3)ccc12 Show InChI InChI=1S/C28H32FN7O2/c1-6-27(37)31-23-16-24(26(38-5)17-25(23)36(4)13-12-35(2)3)32-28-21-11-10-20(15-22(21)33-34-28)30-19-9-7-8-18(29)14-19/h6-11,14-17,30H,1,12-13H2,2-5H3,(H,31,37)(H2,32,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
In vitro Binding affinity towards 5-hydroxytryptamine 3 receptor was determined |
J Med Chem 60: 2361-2372 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01626
BindingDB Entry DOI: 10.7270/Q2FB556R |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50383274

(CHEMBL1229592 | US10167264, WZ4002 | US9670213, WZ...)Show SMILES COc1cc(ccc1Nc1ncc(Cl)c(Oc2cccc(NC(=O)C=C)c2)n1)N1CCN(C)CC1 Show InChI InChI=1S/C25H27ClN6O3/c1-4-23(33)28-17-6-5-7-19(14-17)35-24-20(26)16-27-25(30-24)29-21-9-8-18(15-22(21)34-3)32-12-10-31(2)11-13-32/h4-9,14-16H,1,10-13H2,2-3H3,(H,28,33)(H,27,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged EGFR L858R mutant expressed in baculovirus expression system preincubated for 30 mins followed by ATP and ... |
J Med Chem 60: 7725-7744 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00515
BindingDB Entry DOI: 10.7270/Q28W3GS9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM5447

(CHEMBL939 | GEFITINIB | Iressa | N-(3-Chloro-4-flu...)Show SMILES COc1cc2ncnc(Nc3ccc(F)c(Cl)c3)c2cc1OCCCN1CCOCC1 Show InChI InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant GST-tagged EGFR L858R mutant expressed in baculovirus expression system preincubated for 30 mins followed by ATP and ... |
J Med Chem 60: 7725-7744 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00515
BindingDB Entry DOI: 10.7270/Q28W3GS9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50029668

(AZD-9291 | Osimertinib | US10085983, Compound AZD-...)Show SMILES COc1cc(N(C)CCN(C)C)c(NC(=O)C=C)cc1Nc1nccc(n1)-c1cn(C)c2ccccc12 Show InChI InChI=1S/C28H33N7O2/c1-7-27(36)30-22-16-23(26(37-6)17-25(22)34(4)15-14-33(2)3)32-28-29-13-12-21(31-28)20-18-35(5)24-11-9-8-10-19(20)24/h7-13,16-18H,1,14-15H2,2-6H3,(H,30,36)(H,29,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TU Dortmund University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged EGFR L858R/T790M double mutant expressed in baculovirus expression system preincubated for 30 mins followe... |
J Med Chem 60: 7725-7744 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00515
BindingDB Entry DOI: 10.7270/Q28W3GS9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50258539
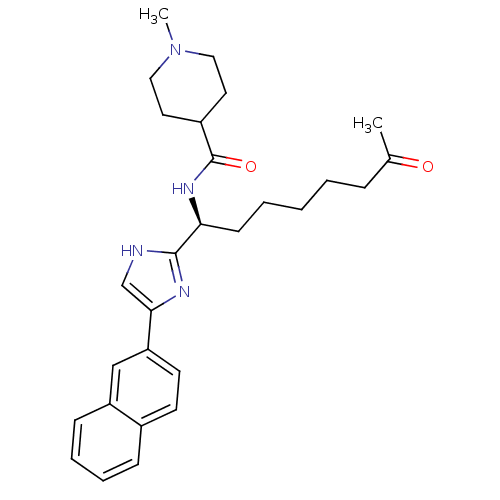
((S)-1-methyl-N-(1-(5-(naphthalen-2-yl)-1H-imidazol...)Show SMILES CN1CCC(CC1)C(=O)N[C@@H](CCCCCC(C)=O)c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C28H36N4O2/c1-20(33)8-4-3-5-11-25(31-28(34)22-14-16-32(2)17-15-22)27-29-19-26(30-27)24-13-12-21-9-6-7-10-23(21)18-24/h6-7,9-10,12-13,18-19,22,25H,3-5,8,11,14-17H2,1-2H3,(H,29,30)(H,31,34)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM25146
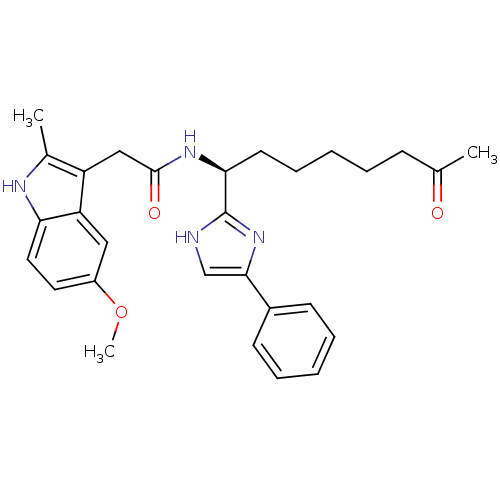
(2-(5-methoxy-2-methyl-1H-indol-3-yl)-N-[(1S)-7-oxo...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(C)=O)c3nc(c[nH]3)-c3ccccc3)c2c1 |r| Show InChI InChI=1S/C29H34N4O3/c1-19(34)10-6-4-9-13-26(29-30-18-27(33-29)21-11-7-5-8-12-21)32-28(35)17-23-20(2)31-25-15-14-22(36-3)16-24(23)25/h5,7-8,11-12,14-16,18,26,31H,4,6,9-10,13,17H2,1-3H3,(H,30,33)(H,32,35)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50306262

(1-(pyrrolidine-3-carbonyl)-2,3,9,9a-tetrahydro-1H-...)Show InChI InChI=1S/C16H19N3O2/c20-15-12-3-1-2-10-5-7-19(13(9-18-15)14(10)12)16(21)11-4-6-17-8-11/h1-3,11,13,17H,4-9H2,(H,18,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 by scintillation proximity assay |
Bioorg Med Chem Lett 20: 448-52 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.002
BindingDB Entry DOI: 10.7270/Q2GT5N91 |
More data for this
Ligand-Target Pair | |
Poly [ADP-ribose] polymerase 1
(Homo sapiens (Human)) | BDBM50306263
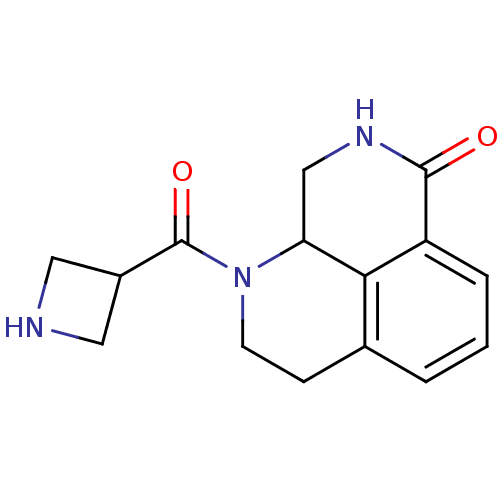
(1-(azetidine-3-carbonyl)-2,3,9,9a-tetrahydro-1H-be...)Show InChI InChI=1S/C15H17N3O2/c19-14-11-3-1-2-9-4-5-18(12(8-17-14)13(9)11)15(20)10-6-16-7-10/h1-3,10,12,16H,4-8H2,(H,17,19) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Inhibition of human PARP1 by scintillation proximity assay |
Bioorg Med Chem Lett 20: 448-52 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.002
BindingDB Entry DOI: 10.7270/Q2GT5N91 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM25146

(2-(5-methoxy-2-methyl-1H-indol-3-yl)-N-[(1S)-7-oxo...)Show SMILES COc1ccc2[nH]c(C)c(CC(=O)N[C@@H](CCCCCC(C)=O)c3nc(c[nH]3)-c3ccccc3)c2c1 |r| Show InChI InChI=1S/C29H34N4O3/c1-19(34)10-6-4-9-13-26(29-30-18-27(33-29)21-11-7-5-8-12-21)32-28(35)17-23-20(2)31-25-15-14-22(36-3)16-24(23)25/h5,7-8,11-12,14-16,18,26,31H,4,6,9-10,13,17H2,1-3H3,(H,30,33)(H,32,35)/t26-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC4 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50258539

((S)-1-methyl-N-(1-(5-(naphthalen-2-yl)-1H-imidazol...)Show SMILES CN1CCC(CC1)C(=O)N[C@@H](CCCCCC(C)=O)c1nc(c[nH]1)-c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C28H36N4O2/c1-20(33)8-4-3-5-11-25(31-28(34)22-14-16-32(2)17-15-22)27-29-19-26(30-27)24-13-12-21-9-6-7-10-23(21)18-24/h6-7,9-10,12-13,18-19,22,25H,3-5,8,11,14-17H2,1-2H3,(H,29,30)(H,31,34)/t25-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HDAC4 |
Bioorg Med Chem Lett 18: 5528-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.003
BindingDB Entry DOI: 10.7270/Q24T6K81 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data