

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50314131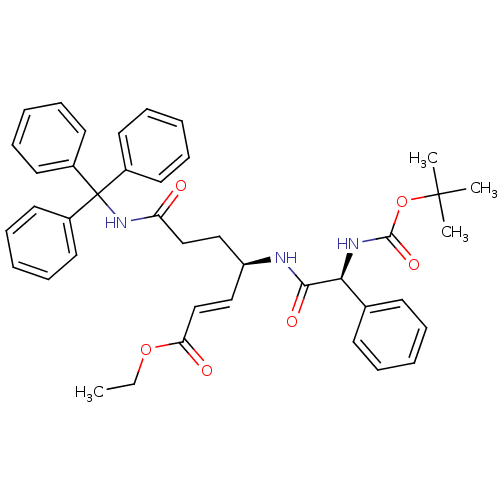 ((S,E)-Ethyl 4-((R)-2-(tert-Butoxycarbonylamino)-2-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense recombinant rhodesain by standard fluorescence assay | J Med Chem 53: 1951-63 (2010) Article DOI: 10.1021/jm900946n BindingDB Entry DOI: 10.7270/Q2V9887Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50314126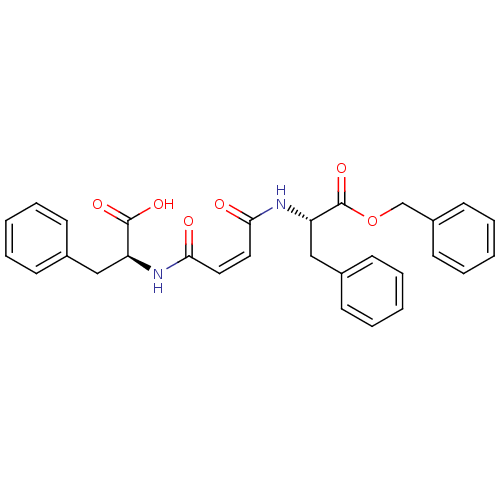 ((S)-Benzyl-2-[(Z)-3-((S)-1-benzyloxycarbonyl-2-phe...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense recombinant rhodesain by standard fluorescence assay | J Med Chem 53: 1951-63 (2010) Article DOI: 10.1021/jm900946n BindingDB Entry DOI: 10.7270/Q2V9887Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50314125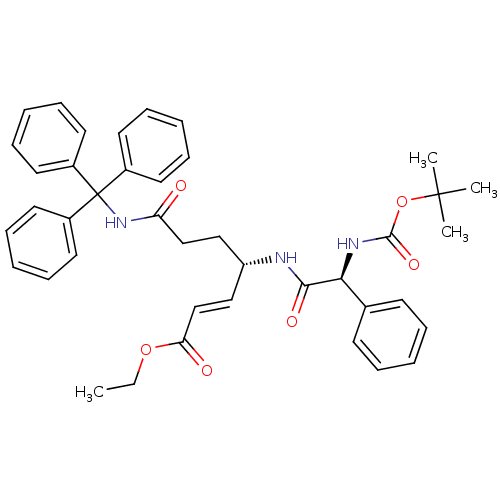 ((S,E)-Ethyl 4-((S)-2-(tert-Butoxycarbonylamino)-2-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense recombinant rhodesain using low concentration of enzyme by standard fluorescence assay in presence of de... | J Med Chem 53: 1951-63 (2010) Article DOI: 10.1021/jm900946n BindingDB Entry DOI: 10.7270/Q2V9887Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50314130 ((S)-2-[(E)-3-((S)-1-Benzyloxycarbonyl-2-phenylethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense recombinant rhodesain by standard fluorescence assay | J Med Chem 53: 1951-63 (2010) Article DOI: 10.1021/jm900946n BindingDB Entry DOI: 10.7270/Q2V9887Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50314125 ((S,E)-Ethyl 4-((S)-2-(tert-Butoxycarbonylamino)-2-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense recombinant rhodesain using high concentration of enzyme by standard fluorescence assay in presence of d... | J Med Chem 53: 1951-63 (2010) Article DOI: 10.1021/jm900946n BindingDB Entry DOI: 10.7270/Q2V9887Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50314125 ((S,E)-Ethyl 4-((S)-2-(tert-Butoxycarbonylamino)-2-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense recombinant rhodesain by standard fluorescence assay | J Med Chem 53: 1951-63 (2010) Article DOI: 10.1021/jm900946n BindingDB Entry DOI: 10.7270/Q2V9887Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50314125 ((S,E)-Ethyl 4-((S)-2-(tert-Butoxycarbonylamino)-2-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense recombinant rhodesain using low concentration of enzyme by standard fluorescence assay | J Med Chem 53: 1951-63 (2010) Article DOI: 10.1021/jm900946n BindingDB Entry DOI: 10.7270/Q2V9887Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50314125 ((S,E)-Ethyl 4-((S)-2-(tert-Butoxycarbonylamino)-2-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense recombinant rhodesain using high concentration of enzyme by standard fluorescence assay | J Med Chem 53: 1951-63 (2010) Article DOI: 10.1021/jm900946n BindingDB Entry DOI: 10.7270/Q2V9887Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50314127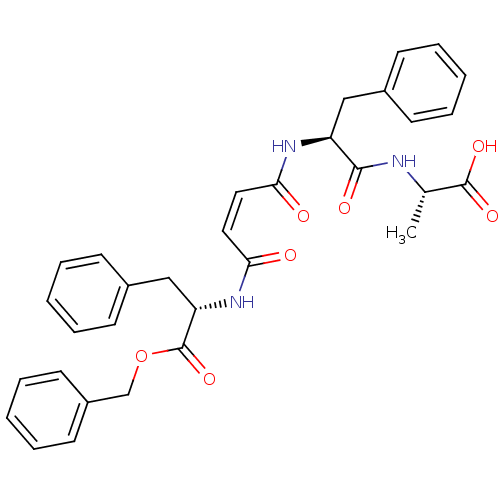 ((S)-Benzyl-2-{(Z)-3-[(S)-1-((S)-1-benzyloxycarbony...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense recombinant rhodesain by standard fluorescence assay | J Med Chem 53: 1951-63 (2010) Article DOI: 10.1021/jm900946n BindingDB Entry DOI: 10.7270/Q2V9887Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50314128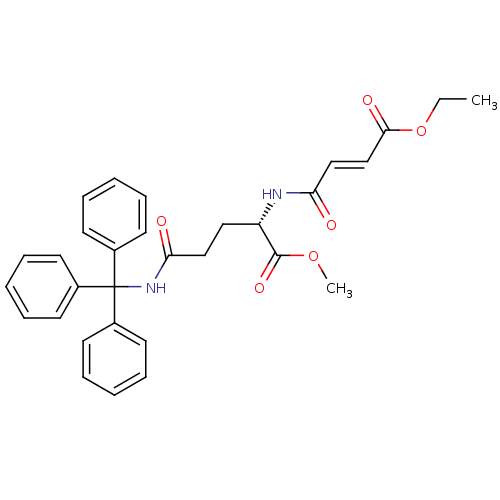 ((S,E)-Methyl 2-(4-Ethoxy-4-oxobut-2-enamido)-5-oxo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense recombinant rhodesain by standard fluorescence assay | J Med Chem 53: 1951-63 (2010) Article DOI: 10.1021/jm900946n BindingDB Entry DOI: 10.7270/Q2V9887Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50314124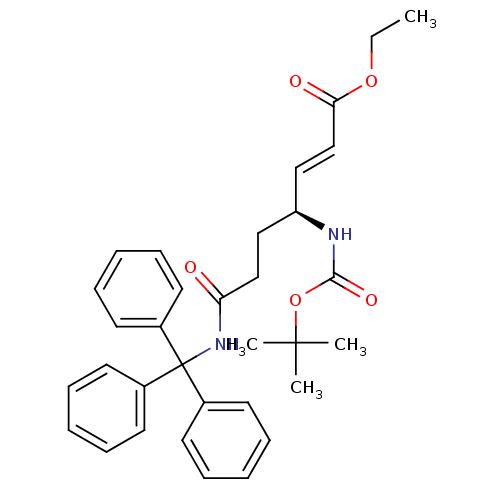 ((S,E)-ethyl 4-(tert-butoxycarbonylamino)-7-oxo-7-(...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense recombinant rhodesain by standard fluorescence assay in presence of DTT | J Med Chem 53: 1951-63 (2010) Article DOI: 10.1021/jm900946n BindingDB Entry DOI: 10.7270/Q2V9887Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50314124 ((S,E)-ethyl 4-(tert-butoxycarbonylamino)-7-oxo-7-(...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense recombinant rhodesain by standard fluorescence assay | J Med Chem 53: 1951-63 (2010) Article DOI: 10.1021/jm900946n BindingDB Entry DOI: 10.7270/Q2V9887Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50175214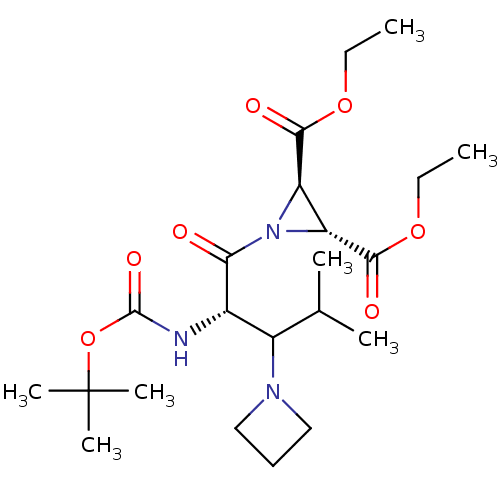 ((2R,3R)-diethyl 1-((2S)-3-(azetidin-1-yl)-2-(tert-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Inhibitory activity against cathepsin L | Bioorg Med Chem Lett 15: 5365-9 (2005) Article DOI: 10.1016/j.bmcl.2005.09.012 BindingDB Entry DOI: 10.7270/Q2XP74HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50314132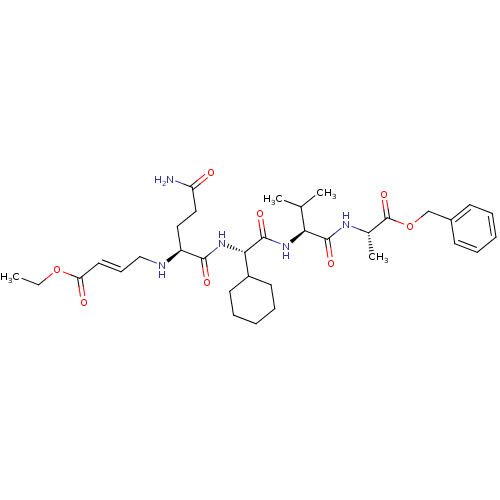 ((2S,5S,8S,11S,E)-1-Benzyl 16-ethyl 11-(3-amino-3-o...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense recombinant rhodesain by standard fluorescence assay | J Med Chem 53: 1951-63 (2010) Article DOI: 10.1021/jm900946n BindingDB Entry DOI: 10.7270/Q2V9887Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50175213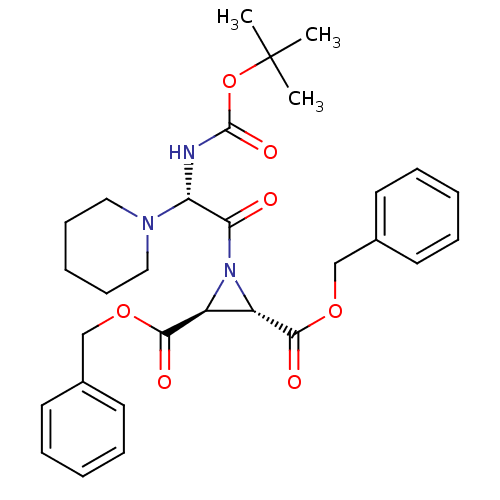 ((2S,3S)-dibenzyl 1-((S)-2-(tert-butoxycarbonyl)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg Curated by ChEMBL | Assay Description Inhibitory activity against cathepsin L | Bioorg Med Chem Lett 15: 5365-9 (2005) Article DOI: 10.1016/j.bmcl.2005.09.012 BindingDB Entry DOI: 10.7270/Q2XP74HX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50314129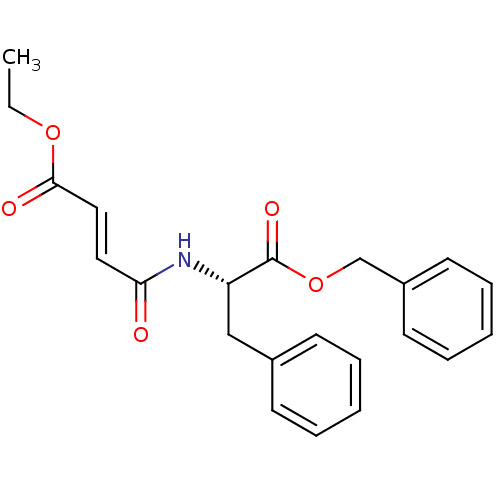 ((S,E)-3-((S)-1-Benzyloxycarbonyl-2-phenylethylcarb...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense recombinant rhodesain by standard fluorescence assay | J Med Chem 53: 1951-63 (2010) Article DOI: 10.1021/jm900946n BindingDB Entry DOI: 10.7270/Q2V9887Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-1 (Homo sapiens (Human)) | BDBM50346543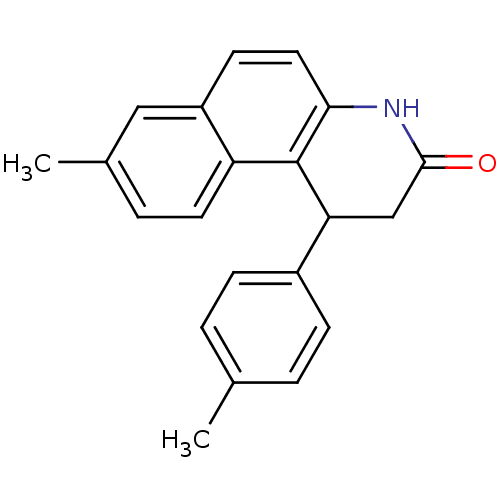 (CHEMBL1797750) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Ernst-Moritz-Arndt-University Greifswald Curated by ChEMBL | Assay Description Inhibition of SIRT1 | Bioorg Med Chem 19: 3669-77 (2011) Article DOI: 10.1016/j.bmc.2011.01.026 BindingDB Entry DOI: 10.7270/Q2XD121S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM3175 (3-[1-[3-(Amidinothio)propyl]-3-indolyl]-4-(1-methy...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Ernst-Moritz-Arndt-University Greifswald Curated by ChEMBL | Assay Description Inhibition of human N-terminally His6-tagged Sirt2 using ZMAL as substrate after 4 hrs by homogeneous fluorescent deacetylase assay | Bioorg Med Chem 19: 3669-77 (2011) Article DOI: 10.1016/j.bmc.2011.01.026 BindingDB Entry DOI: 10.7270/Q2XD121S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50346550 (CHEMBL270110) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ernst-Moritz-Arndt-University Greifswald Curated by ChEMBL | Assay Description Inhibition of SIRT2 | Bioorg Med Chem 19: 3669-77 (2011) Article DOI: 10.1016/j.bmc.2011.01.026 BindingDB Entry DOI: 10.7270/Q2XD121S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-1 (Homo sapiens (Human)) | BDBM3175 (3-[1-[3-(Amidinothio)propyl]-3-indolyl]-4-(1-methy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ernst-Moritz-Arndt-University Greifswald Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminally GST-tagged Sirt1 expressed in Escherichia coli using ZMAL as substrate after 4 hrs by homogeneous fluore... | Bioorg Med Chem 19: 3669-77 (2011) Article DOI: 10.1016/j.bmc.2011.01.026 BindingDB Entry DOI: 10.7270/Q2XD121S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-3, mitochondrial (Homo sapiens (Human)) | BDBM3175 (3-[1-[3-(Amidinothio)propyl]-3-indolyl]-4-(1-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ernst-Moritz-Arndt-University Greifswald Curated by ChEMBL | Assay Description Inhibition of human C-terminally His6-tagged Sirt3 using ZMAL as substrate after 4 hrs by homogeneous fluorescent deacetylase assay | Bioorg Med Chem 19: 3669-77 (2011) Article DOI: 10.1016/j.bmc.2011.01.026 BindingDB Entry DOI: 10.7270/Q2XD121S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50346546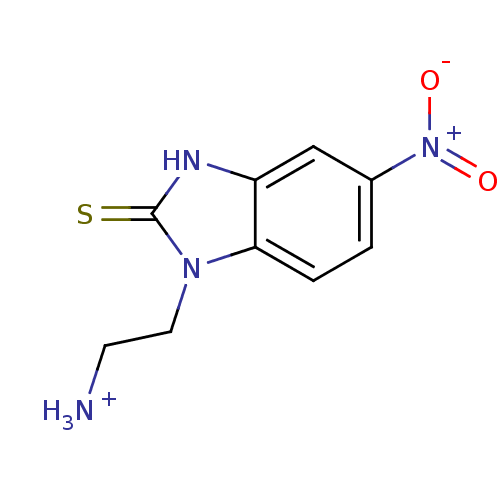 (CHEMBL1797926) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ernst-Moritz-Arndt-University Greifswald Curated by ChEMBL | Assay Description Inhibition of human N-terminally His6-tagged Sirt2 using ZMAL as substrate after 4 hrs by homogeneous fluorescent deacetylase assay | Bioorg Med Chem 19: 3669-77 (2011) Article DOI: 10.1016/j.bmc.2011.01.026 BindingDB Entry DOI: 10.7270/Q2XD121S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-1 (Homo sapiens (Human)) | BDBM50346546 (CHEMBL1797926) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ernst-Moritz-Arndt-University Greifswald Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminally GST-tagged Sirt1 expressed in Escherichia coli using ZMAL as substrate after 4 hrs by homogeneous fluore... | Bioorg Med Chem 19: 3669-77 (2011) Article DOI: 10.1016/j.bmc.2011.01.026 BindingDB Entry DOI: 10.7270/Q2XD121S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-1 (Homo sapiens (Human)) | BDBM74978 (1,3-dihydrobenzo[e]benzimidazole-2-thione | CHEMBL...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ernst-Moritz-Arndt-University Greifswald Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminally GST-tagged Sirt1 expressed in Escherichia coli using ZMAL as substrate after 4 hrs by homogeneous fluore... | Bioorg Med Chem 19: 3669-77 (2011) Article DOI: 10.1016/j.bmc.2011.01.026 BindingDB Entry DOI: 10.7270/Q2XD121S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM74978 (1,3-dihydrobenzo[e]benzimidazole-2-thione | CHEMBL...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ernst-Moritz-Arndt-University Greifswald Curated by ChEMBL | Assay Description Inhibition of human N-terminally His6-tagged Sirt2 using ZMAL as substrate after 4 hrs by homogeneous fluorescent deacetylase assay | Bioorg Med Chem 19: 3669-77 (2011) Article DOI: 10.1016/j.bmc.2011.01.026 BindingDB Entry DOI: 10.7270/Q2XD121S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ORF 73 (Human herpesvirus 8) | BDBM50521373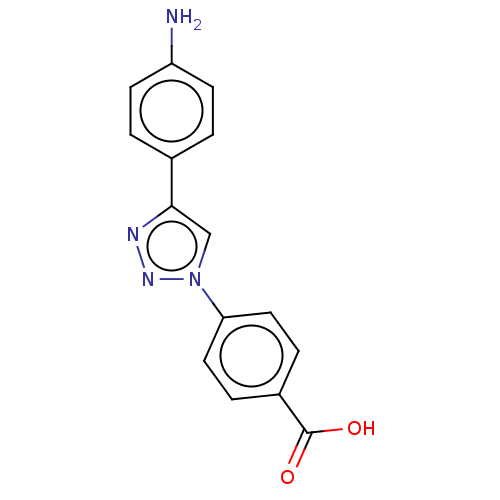 (CHEMBL4573866) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Pharmaceutical Research Saarland (HIPS)-Helmholtz Centre for Infection Research (HZI) Curated by ChEMBL | Assay Description Inhibition of fluorescent labelled LBS3 DNA binding to human herpesvirus 8 C-terminal His-tagged LANA oligomerization deficient mutant DBD (1008 to 1... | J Med Chem 62: 3924-3939 (2019) Article DOI: 10.1021/acs.jmedchem.8b01827 BindingDB Entry DOI: 10.7270/Q2H135D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50346547 (CHEMBL1797919) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ernst-Moritz-Arndt-University Greifswald Curated by ChEMBL | Assay Description Inhibition of human N-terminally His6-tagged Sirt2 using ZMAL as substrate after 4 hrs by homogeneous fluorescent deacetylase assay | Bioorg Med Chem 19: 3669-77 (2011) Article DOI: 10.1016/j.bmc.2011.01.026 BindingDB Entry DOI: 10.7270/Q2XD121S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ORF 73 (Human herpesvirus 8) | BDBM50521373 (CHEMBL4573866) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Pharmaceutical Research Saarland (HIPS)-Helmholtz Centre for Infection Research (HZI) Curated by ChEMBL | Assay Description Inhibition of fluorescent labelled LBS2 DNA binding to human herpesvirus 8 C-terminal His-tagged LANA oligomerization deficient mutant DBD (1008 to 1... | J Med Chem 62: 3924-3939 (2019) Article DOI: 10.1021/acs.jmedchem.8b01827 BindingDB Entry DOI: 10.7270/Q2H135D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50346545 (CHEMBL1797923) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ernst-Moritz-Arndt-University Greifswald Curated by ChEMBL | Assay Description Inhibition of human N-terminally His6-tagged Sirt2 using ZMAL as substrate after 4 hrs by homogeneous fluorescent deacetylase assay | Bioorg Med Chem 19: 3669-77 (2011) Article DOI: 10.1016/j.bmc.2011.01.026 BindingDB Entry DOI: 10.7270/Q2XD121S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ORF 73 (Human herpesvirus 8) | BDBM50521371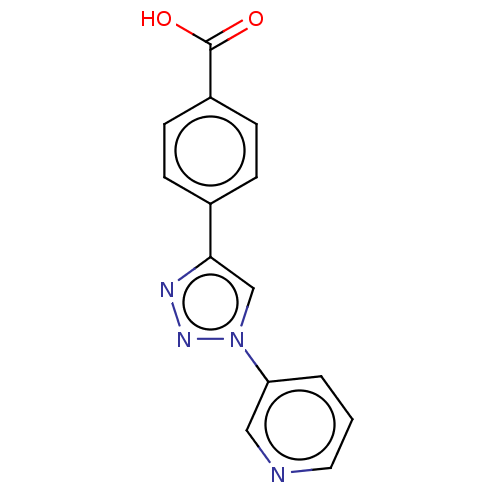 (CHEMBL4569451) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Pharmaceutical Research Saarland (HIPS)-Helmholtz Centre for Infection Research (HZI) Curated by ChEMBL | Assay Description Inhibition of fluorescent labelled LBS2 DNA binding to human herpesvirus 8 C-terminal His-tagged LANA oligomerization deficient mutant DBD (1008 to 1... | J Med Chem 62: 3924-3939 (2019) Article DOI: 10.1021/acs.jmedchem.8b01827 BindingDB Entry DOI: 10.7270/Q2H135D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ORF 73 (Human herpesvirus 8) | BDBM50521372 (CHEMBL4468809) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Pharmaceutical Research Saarland (HIPS)-Helmholtz Centre for Infection Research (HZI) Curated by ChEMBL | Assay Description Inhibition of fluorescent labelled LBS2 DNA binding to human herpesvirus 8 C-terminal His-tagged LANA oligomerization deficient mutant DBD (1008 to 1... | J Med Chem 62: 3924-3939 (2019) Article DOI: 10.1021/acs.jmedchem.8b01827 BindingDB Entry DOI: 10.7270/Q2H135D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ORF 73 (Human herpesvirus 8) | BDBM50521371 (CHEMBL4569451) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Pharmaceutical Research Saarland (HIPS)-Helmholtz Centre for Infection Research (HZI) Curated by ChEMBL | Assay Description Inhibition of fluorescent labelled LBS3 DNA binding to human herpesvirus 8 C-terminal His-tagged LANA oligomerization deficient mutant DBD (1008 to 1... | J Med Chem 62: 3924-3939 (2019) Article DOI: 10.1021/acs.jmedchem.8b01827 BindingDB Entry DOI: 10.7270/Q2H135D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ORF 73 (Human herpesvirus 8) | BDBM50521373 (CHEMBL4573866) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Pharmaceutical Research Saarland (HIPS)-Helmholtz Centre for Infection Research (HZI) Curated by ChEMBL | Assay Description Inhibition of fluorescent labelled LBS1 DNA binding to human herpesvirus 8 C-terminal His-tagged LANA oligomerization deficient mutant DBD (1008 to 1... | J Med Chem 62: 3924-3939 (2019) Article DOI: 10.1021/acs.jmedchem.8b01827 BindingDB Entry DOI: 10.7270/Q2H135D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ORF 73 (Human herpesvirus 8) | BDBM50521369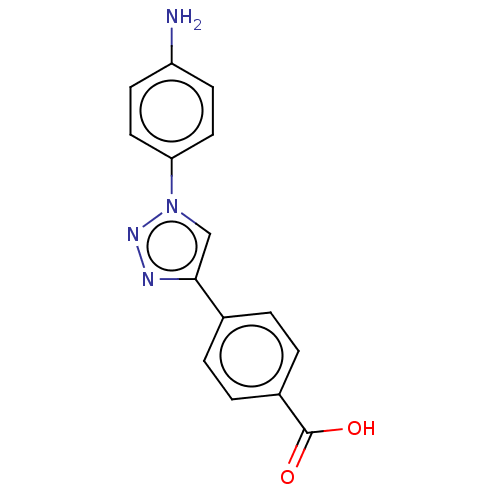 (CHEMBL4439456) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Pharmaceutical Research Saarland (HIPS)-Helmholtz Centre for Infection Research (HZI) Curated by ChEMBL | Assay Description Inhibition of fluorescent labelled LBS2 DNA binding to human herpesvirus 8 C-terminal His-tagged LANA oligomerization deficient mutant DBD (1008 to 1... | J Med Chem 62: 3924-3939 (2019) Article DOI: 10.1021/acs.jmedchem.8b01827 BindingDB Entry DOI: 10.7270/Q2H135D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ORF 73 (Human herpesvirus 8) | BDBM50521371 (CHEMBL4569451) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Pharmaceutical Research Saarland (HIPS)-Helmholtz Centre for Infection Research (HZI) Curated by ChEMBL | Assay Description Inhibition of fluorescent labelled LBS1 DNA binding to human herpesvirus 8 C-terminal His-tagged LANA oligomerization deficient mutant DBD (1008 to 1... | J Med Chem 62: 3924-3939 (2019) Article DOI: 10.1021/acs.jmedchem.8b01827 BindingDB Entry DOI: 10.7270/Q2H135D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-1 (Homo sapiens (Human)) | BDBM50346545 (CHEMBL1797923) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ernst-Moritz-Arndt-University Greifswald Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminally GST-tagged Sirt1 expressed in Escherichia coli using ZMAL as substrate after 4 hrs by homogeneous fluore... | Bioorg Med Chem 19: 3669-77 (2011) Article DOI: 10.1016/j.bmc.2011.01.026 BindingDB Entry DOI: 10.7270/Q2XD121S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ORF 73 (Human herpesvirus 8) | BDBM50521370 (CHEMBL4468972) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Pharmaceutical Research Saarland (HIPS)-Helmholtz Centre for Infection Research (HZI) Curated by ChEMBL | Assay Description Inhibition of fluorescent labelled LBS1 DNA binding to human herpesvirus 8 C-terminal His-tagged LANA oligomerization deficient mutant DBD (1008 to 1... | J Med Chem 62: 3924-3939 (2019) Article DOI: 10.1021/acs.jmedchem.8b01827 BindingDB Entry DOI: 10.7270/Q2H135D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ORF 73 (Human herpesvirus 8) | BDBM50521372 (CHEMBL4468809) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Pharmaceutical Research Saarland (HIPS)-Helmholtz Centre for Infection Research (HZI) Curated by ChEMBL | Assay Description Inhibition of fluorescent labelled LBS1 DNA binding to human herpesvirus 8 C-terminal His-tagged LANA oligomerization deficient mutant DBD (1008 to 1... | J Med Chem 62: 3924-3939 (2019) Article DOI: 10.1021/acs.jmedchem.8b01827 BindingDB Entry DOI: 10.7270/Q2H135D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ORF 73 (Human herpesvirus 8) | BDBM50521372 (CHEMBL4468809) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Pharmaceutical Research Saarland (HIPS)-Helmholtz Centre for Infection Research (HZI) Curated by ChEMBL | Assay Description Inhibition of fluorescent labelled LBS3 DNA binding to human herpesvirus 8 C-terminal His-tagged LANA oligomerization deficient mutant DBD (1008 to 1... | J Med Chem 62: 3924-3939 (2019) Article DOI: 10.1021/acs.jmedchem.8b01827 BindingDB Entry DOI: 10.7270/Q2H135D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ORF 73 (Human herpesvirus 8) | BDBM50521370 (CHEMBL4468972) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Pharmaceutical Research Saarland (HIPS)-Helmholtz Centre for Infection Research (HZI) Curated by ChEMBL | Assay Description Inhibition of fluorescent labelled LBS3 DNA binding to human herpesvirus 8 C-terminal His-tagged LANA oligomerization deficient mutant DBD (1008 to 1... | J Med Chem 62: 3924-3939 (2019) Article DOI: 10.1021/acs.jmedchem.8b01827 BindingDB Entry DOI: 10.7270/Q2H135D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50346548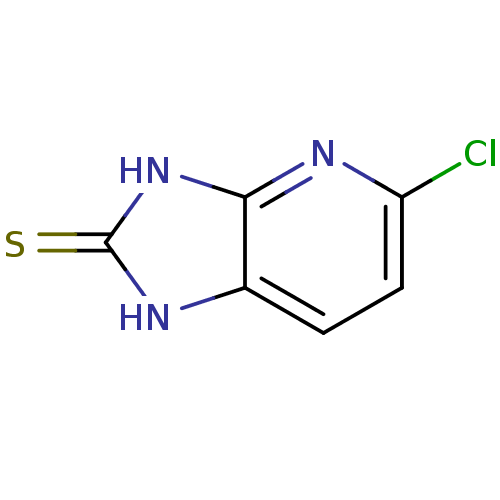 (CHEMBL1797922) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ernst-Moritz-Arndt-University Greifswald Curated by ChEMBL | Assay Description Inhibition of human N-terminally His6-tagged Sirt2 using ZMAL as substrate after 4 hrs by homogeneous fluorescent deacetylase assay | Bioorg Med Chem 19: 3669-77 (2011) Article DOI: 10.1016/j.bmc.2011.01.026 BindingDB Entry DOI: 10.7270/Q2XD121S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ORF 73 (Human herpesvirus 8) | BDBM50521382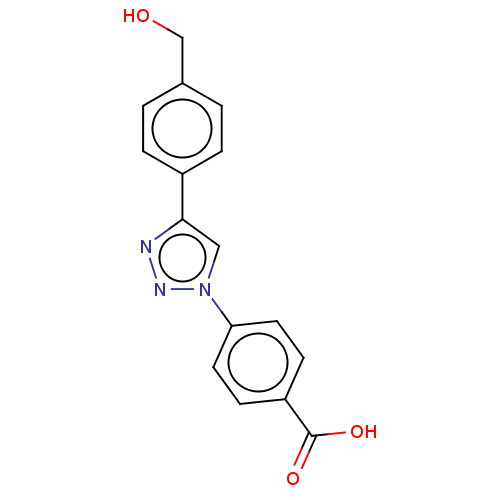 (CHEMBL4548393) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Pharmaceutical Research Saarland (HIPS)-Helmholtz Centre for Infection Research (HZI) Curated by ChEMBL | Assay Description Inhibition of fluorescent labelled LBS2 DNA binding to human herpesvirus 8 C-terminal His-tagged LANA oligomerization deficient mutant DBD (1008 to 1... | J Med Chem 62: 3924-3939 (2019) Article DOI: 10.1021/acs.jmedchem.8b01827 BindingDB Entry DOI: 10.7270/Q2H135D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ORF 73 (Human herpesvirus 8) | BDBM50521370 (CHEMBL4468972) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Pharmaceutical Research Saarland (HIPS)-Helmholtz Centre for Infection Research (HZI) Curated by ChEMBL | Assay Description Inhibition of fluorescent labelled LBS2 DNA binding to human herpesvirus 8 C-terminal His-tagged LANA oligomerization deficient mutant DBD (1008 to 1... | J Med Chem 62: 3924-3939 (2019) Article DOI: 10.1021/acs.jmedchem.8b01827 BindingDB Entry DOI: 10.7270/Q2H135D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-1 (Homo sapiens (Human)) | BDBM50346544 (CHEMBL1797920) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ernst-Moritz-Arndt-University Greifswald Curated by ChEMBL | Assay Description Inhibition of human recombinant N-terminally GST-tagged Sirt1 expressed in Escherichia coli using ZMAL as substrate after 4 hrs by homogeneous fluore... | Bioorg Med Chem 19: 3669-77 (2011) Article DOI: 10.1016/j.bmc.2011.01.026 BindingDB Entry DOI: 10.7270/Q2XD121S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ORF 73 (Human herpesvirus 8) | BDBM50328901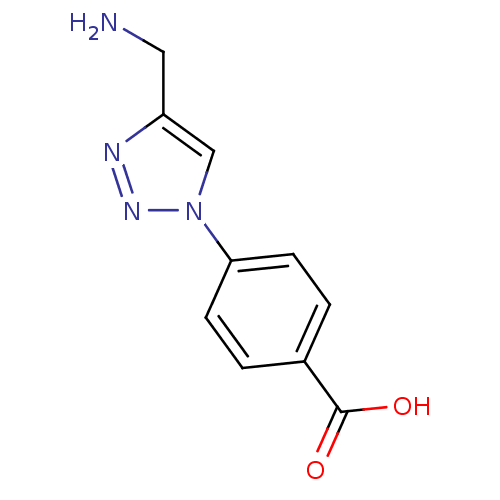 (4-(4-(Aminomethyl)-1H-1,2,3-triazol-1-yl)benzoic a...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Pharmaceutical Research Saarland (HIPS)-Helmholtz Centre for Infection Research (HZI) Curated by ChEMBL | Assay Description Inhibition of fluorescent labelled LBS2 DNA binding to human herpesvirus 8 C-terminal His-tagged LANA oligomerization deficient mutant DBD (1008 to 1... | J Med Chem 62: 3924-3939 (2019) Article DOI: 10.1021/acs.jmedchem.8b01827 BindingDB Entry DOI: 10.7270/Q2H135D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50346549 (CHEMBL1797925) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ernst-Moritz-Arndt-University Greifswald Curated by ChEMBL | Assay Description Inhibition of human N-terminally His6-tagged Sirt2 using ZMAL as substrate after 4 hrs by homogeneous fluorescent deacetylase assay | Bioorg Med Chem 19: 3669-77 (2011) Article DOI: 10.1016/j.bmc.2011.01.026 BindingDB Entry DOI: 10.7270/Q2XD121S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NAD-dependent protein deacetylase sirtuin-2 (Homo sapiens (Human)) | BDBM50346544 (CHEMBL1797920) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ernst-Moritz-Arndt-University Greifswald Curated by ChEMBL | Assay Description Inhibition of human N-terminally His6-tagged Sirt2 using ZMAL as substrate after 4 hrs by homogeneous fluorescent deacetylase assay | Bioorg Med Chem 19: 3669-77 (2011) Article DOI: 10.1016/j.bmc.2011.01.026 BindingDB Entry DOI: 10.7270/Q2XD121S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ORF 73 (Human herpesvirus 8) | BDBM50521381 (CHEMBL4436463) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Pharmaceutical Research Saarland (HIPS)-Helmholtz Centre for Infection Research (HZI) Curated by ChEMBL | Assay Description Inhibition of fluorescent labelled LBS3 DNA binding to human herpesvirus 8 C-terminal His-tagged LANA oligomerization deficient mutant DBD (1008 to 1... | J Med Chem 62: 3924-3939 (2019) Article DOI: 10.1021/acs.jmedchem.8b01827 BindingDB Entry DOI: 10.7270/Q2H135D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ORF 73 (Human herpesvirus 8) | BDBM50521379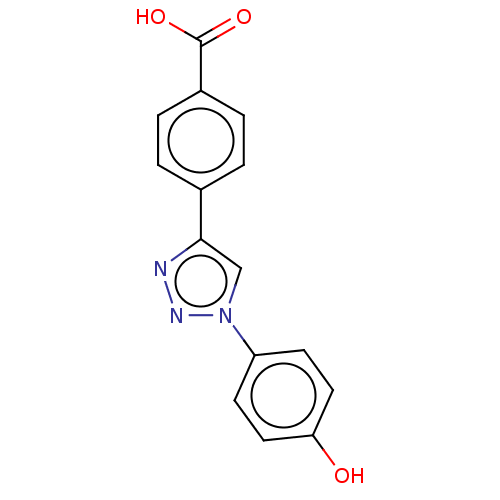 (CHEMBL4449651) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Pharmaceutical Research Saarland (HIPS)-Helmholtz Centre for Infection Research (HZI) Curated by ChEMBL | Assay Description Inhibition of fluorescent labelled LBS2 DNA binding to human herpesvirus 8 C-terminal His-tagged LANA oligomerization deficient mutant DBD (1008 to 1... | J Med Chem 62: 3924-3939 (2019) Article DOI: 10.1021/acs.jmedchem.8b01827 BindingDB Entry DOI: 10.7270/Q2H135D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ORF 73 (Human herpesvirus 8) | BDBM50521383 (CHEMBL4559370) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Pharmaceutical Research Saarland (HIPS)-Helmholtz Centre for Infection Research (HZI) Curated by ChEMBL | Assay Description Inhibition of fluorescent labelled LBS2 DNA binding to human herpesvirus 8 C-terminal His-tagged LANA oligomerization deficient mutant DBD (1008 to 1... | J Med Chem 62: 3924-3939 (2019) Article DOI: 10.1021/acs.jmedchem.8b01827 BindingDB Entry DOI: 10.7270/Q2H135D6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 65 total ) | Next | Last >> |